Abstract
Social well-being reflects the perception of one’s social functioning, which plays an important role in physical and psychological health. However, the exact neuroanatomical substrate for social well-being remains unclear. To address the issue, we employed the voxel-based morphometry method to probe the neuroanatomical basis of individual variation in social well-being in young healthy adults (n = 136). The results revealed a significant negative association between social well-being and regional gray matter density (rGMD) in an anatomical cluster that mainly includes the left orbitofrontal cortex (OFC) that has been involved in emotion regulation and social cognition. Furthermore, a balanced 4-fold cross-validation using the machine learning approach revealed that rGMD in the left OFC could be reliably related to social well-being. More importantly, the multiple mediation analysis revealed that neuroticism and dispositional forgiveness independently mediated the association between rGMD in the left OFC and social well-being. In addition, all these results remained stable when subjective socioeconomic status was controlled. Together, our results provide the initial evidence that the OFC is a neuroanatomical substrate for social well-being and demonstrate that the OFC is a crucial neural site linking neuroticism and dispositional forgiveness to social well-being.
Keywords: social well-being, dispositional forgiveness, personality, individual differences, orbitofrontal cortex, voxel-based morphometry
Introduction
In the past decades, more attention has been paid to well-being in private life (i.e. individual well-being), but fewer studies have been concerned with social well-being. Social well-being reflects an individual’s perception toward social functioning including social integration, acceptance of others, contribution to society, social coherence and social actualization (Keyes, 1998; Kong et al., 2015c). Several studies have demonstrated that social well-being and individual well-being are two related but different dimensions of well-being using the bivariate correlation and confirmatory factor analysis (Gallagher et al., 2009; Lamers et al., 2011; Li et al., 2015). Despite the growing interest in this topic from researchers, limited research has investigated it from a neuroscientific perspective. In addition, social well-being has been shown to play a crucial role in physical and psychological health (Van Lente et al., 2012; Zhang et al., 2011), so exploring its neural basis can provide new insights into how to achieve a healthy life.
Functional magnetic resonance imaging (fMRI) (such as resting state fMRI) can examine the relationship between neural activity and social well-being, but the method cannot identify whether individual variation in social well-being can be reliably inferred from more basic structural measurements of the human brain. As we know, a trait or an ability that is related to the function of a particular brain system is not necessarily linked with the structure of the system (Kanai and Rees, 2011). However, the link of the trait or the ability with the structure of a given brain system can provide evidence that the brain system is relevant to the trait or the ability. Thus, it is necessary and important to investigate the structural basis of individual variation in social well-being. In addition, previous studies have demonstrated that individual variation in cognition, personality and behavior can be reflected in the structural anatomy of the brain (DeYoung et al., 2010; Kanai and Rees, 2011; van Gaal et al., 2011; Kanai et al., 2012; Vijayakumar et al., 2014; Schmidt et al., 2018). Together, here we tried to use structural MRI to explore the neuroanatomical basis of individual variation in social well-being.
There are few studies that have investigated the relationship between brain anatomy and individual variability in social well-being. To our knowledge, only one study to date has addressed this issue and reported a negative relationship between social well-being and the left dorsolateral prefrontal cortex (PFC) volume. However, as Kong et al. (2015c) mentioned, this study did not find any correlation of social well-being with the orbitofrontal cortex (OFC) and medial PFC (mPFC) that have been demonstrated to be the important hubs in brain networks that support the social life (Blakemore, 2008; Skuse and Gallagher, 2009). Furthermore, The OFC/mPFC has also been shown to have an important role in regulation of emotion (Ochsner et al., 2002; Ochsner et al., 2004; Krueger et al., 2009; Mak et al., 2009; Welborn et al., 2009; Kanske et al., 2010; Golkar et al., 2012), which is crucial for the acquisition of one’s social functioning. In addition, previous behavioral studies have reported that social well-being is closely associated with emotion-related traits like neuroticism and extraversion (Hill et al., 2012; Lamers et al., 2012; Kong et al., 2015c). Therefore, the OFC/mPFC should be a neural neuroanatomical site for social well-being.
However, in the study by Kong et al. (2015c), the authors tested the relationship between brain anatomy and individual variability in social well-being using a whole-brain voxel-wise analysis, which might increase the severity of correction for multiple tests. To control for Type I error, our study attempted to use a regions of interest (ROI) voxel-wise analysis to examine its neuroanatomical basis. We conjectured that regional gray matter density (rGMD) in the OFC/mPFC might be associated with social well-being. In addition, prior studies have shown a link between the OFC/ventromedial prefrontal cortex (vmPFC) and individual well-being measured via self-report questionnaires (Kong et al., 2014; Kong et al., 2015b; Kong et al., 2016), so we further hypothesized that the OFC/vmPFC might be a common neural underpinning of both types of well-being.
Recently, researchers have started to concern with the association between personality traits and social well-being. In the literature on social well-being, personality traits are one of the most studied variables that relate to the construct (Howell et al., 2011; Hill et al., 2012; Kong et al., 2015c). Several studies reported that social well-being was significantly related to all five general personality traits (i.e. agreeableness, extraversion, neuroticism, openness and conscientiousness; Hill et al., 2012; Lamers et al., 2012; Kong et al., 2015c), suggesting the Big Five personality factors are crucial for social well-being. Furthermore, several studies also explored the association of specific personality traits such as dispositional forgiveness with social well-being and found that the tendency to forgive (TTF) others (i.e. dispositional forgiveness) was positively associated with measures related to social well-being (Lawler-Row and Piferi, 2006). All these findings suggest that personality traits play an important role in social well-being. In addition, although it is hard to determine the direction of the relation between brain structures and behavior, some evidence has supported the view of a directional association from brain anatomy to psychological functions (Kanai and Rees, 2011; Lewis et al., 2011; van Gaal et al., 2011; Kanai et al., 2012; Gilaie-Dotan et al., 2014; Vijayakumar et al., 2014; Schmidt et al., 2018). Moreover, prior research has demonstrated that some brain stimulation techniques such as transcranial direct current stimulation can effectively modulate cognitive, affective and social functions (Fregni et al., 2005; Cattaneo et al., 2011; Riva et al., 2015; Sellaro et al., 2016), suggesting a potential causal relationship between the brain and behavior. Given the important associations of the mPFC/OFC with neuroticism (Wright et al., 2006; DeYoung et al., 2010; Lewis et al., 2014), extraversion (Rauch et al., 2005; Deckersbach et al., 2006; Wright et al., 2006; DeYoung et al., 2010), agreeableness (Sampaio et al., 2014), conscientiousness (Jackson et al., 2011) and dispositional forgiveness (Farrow et al., 2001; Hayashi et al., 2010), we conjectured that the association of structural differences in OFC/mPFC with social well-being might be mediated by these personality traits.
To address the issues, we first employed well-validated self-report measurements to measure participants’ social well-being and personality traits. Second, we carried out an ROI voxel-wise analysis to test the relationship of structural differences in the OFC/mPFC with social well-being using the voxel-based morphometry (VBM) approach. The approach can be adopted to probe the neuroanatomical correlates of behavior performance (e.g. intelligence and personality; Deng et al., 2014; Kong et al., 2014; Takeuchi et al., 2014; Eres et al., 2015; Kong et al., 2015a; Chung et al., 2017; Wang et al., 2017). In light of the important role of the OFC/mPFC in social cognition, we expected that social well-being would be related to rGMD in the OFC/mPFC. Finally, we used a multiple mediation analysis to determine which personality traits can explain the relationship of structural differences in the OFC/mPFC with social well-being. Given the links of personality traits with social well-being (e.g. Lawler-Row and Piferi, 2006; Lamers et al., 2012; Kong et al., 2015c) and that of the OFC/mPFC with neuroticism, extraversion, agreeableness, conscientiousness and dispositional forgiveness (Farrow et al., 2001; Rauch et al., 2005; Deckersbach et al., 2006; Wright et al., 2006; DeYoung et al., 2010; Hayashi et al., 2010; Jackson et al., 2011; Sampaio et al., 2014), we hypothesized that these personality traits might independently mediate the relationship between rGMD in the OFC/mPFC and social well-being.
Methods
Participants
One hundred and thirty-six right-handed Chinese university students (81 women; 21.03 ± 2.10 years old) from South China Normal University participated in the study. In Kong et al.’s (2015c) study, all participants were recruited from Beijing Normal University, so there were non-overlapping participants between these two studies. None of the participants reported history of neurological or psychiatric disorders. Three participants were excluded because of lacking data on social well-being. The questionnaires were filled within a month after the neuroimaging scan. The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of South China Normal University. All participants gave written informed consent.
Measures
Social well-being scale. We used the social well-being scale (SWBS; Keyes, 1998) to measure social well-being. The SWBS includes 15 items such as ‘My community is a source of comfort’ (social integration), ‘People who do a favor expect nothing in return’ (social acceptance), ‘I have something valuable to give to the world’ (social contribution), ‘Society isn’t improving for people like me’ (social actualization) and ‘I find it easy to predict what will happen next in society’ (social coherence). Each item is answered on a 7-point Likert scale with values ranging from ‘strongly disagree’ to ‘strongly agree’. The Chinese version of the SWBS has good reliability and validity (Li et al., 2015). The Cronbach alpha coefficient in our data set was 0.79.
NEO Personality Inventory–Revised. Personality was assessed by an adapted version of the NEO Personality Inventory—Revised (NEO-PI-R; Costa and McCrae, 1992). The scale includes 120 items and measures agreeableness, extraversion, neuroticism, openness and conscientiousness. Each item is answered on a 5-point Likert scale with values ranging from ‘strongly disagree’ to ‘strongly agree’. The Chinese version of the NEO-PI-R has good reliability and validity (Kong et al., 2015c). The Cronbach alpha coefficients for neuroticism, extraversion, openness, agreeableness and conscientiousness in our data set were 0.77, 0.63, 0.62, 0.72 and 0.75, respectively.
Tendency to forgive scale. Dispositional forgiveness was measured by the TTF (Brown, 2003). The scale includes four items (e.g. ‘I tend to get over it quickly when someone hurts my feelings’). Each item is answered on a 7-point Likert scale with values ranging from ‘strongly disagree’ to ‘strongly agree’. The Chinese version of the TTF has good reliability and validity (Zhu, 2015). The Cronbach alpha coefficient in our data set was 0.72.
Subjective socioeconomic status scale. To test whether our findings are specific to social well-being, we measured the participants’ subjective socioeconomic status (SSS) using a picture of a 10 rung ladder. Participants were instructed to think of a ladder with 10 steps representing where people stand in China and indicated their socioeconomic position on this ladder (Adler et al., 2000; Huang et al., 2017). The Chinese version of the measure has shown to be reliable and valid (Wang et al., 2016; Huang et al., 2017).
MRI data acquisition
All participants completed one MRI scan. Anatomical MRI images were obtained using a 3.0 T Siemens Trio scanner with a 12-channel head coil. A 3D magnetization-prepared rapid gradient echo sequence was employed to obtain T1-weighted images with the following parameters: Repetition time (TR) = 1900 ms; Echo time (TE) = 2.52 ms; Flip angle = 9°; Resolution matrix = 256 × 256; FOV = 230 × 230 mm2; Slice thickness = 1.0 mm; Voxel size = 1 × 1 × 1 mm3.
Data pre-processing
We carried out MRI data pre-processing using the Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK) following the VBM protocol in SPM8 from previous studies (Kanai and Rees, 2011; Kraus et al., 2014; Kong et al., 2015a; Wang et al., 2017). First, for better image registration, set image origin to the anterior commissure. Then, the anatomical images were segmented into gray matter (GM), white matter and cerebrospinal fluid using the new segmentation method. This method is an improved version of the unified segmentation method (Ashburner and Friston, 2005). The registration and normalization processes were conducted through DARTEL (Ashburner, 2007). Specifically, the GM images were aligned and resampled to 1.5 mm × 1.5 mm × 1.5 mm and then normalized to a study-specific template in the MNI152 space. Finally, we smoothed images by convolving them with a Gaussian kernel of 8 mm full width at half maximum.
In this study, we employed regional GM volume (rGMV) reflecting the relative GM concentration but not rGMV reflecting the absolute GM volume as the GM index. Despite that the difference between rGMD and rGMV remains unclear, these two measures have been widely used in structural neuroimaging studies and their results are usually similar (Good et al., 2001; Lochhead et al., 2004). Here there are two reasons for using rGMD. First, using participants with the same characteristics as those used in our study, rGMD is associated with factors related to social adaption such as achievement motivation, emotional intelligence and empathy (Takeuchi et al., 2011; Takeuchi et al., 2014; Eres et al., 2015). This study is concerned with the perception of one’s social functioning, so rGMD might be largely associated with social well-being. Second, rGMD is more frequently employed in developmental research relative to rGMV in that the GM thinning in some regions (e.g. the PFC) occurs as one ages (Sowell et al., 1999; 2001). Given that our participants were in the period of late adolescence, we selected rGMD as the GM index.
Statistical analysis
To examine brain regions in which rGMD is associated with social well-being, an ROI multiple regression analysis was carried out using the scores of the SWBS as the variable of interest. Age, sex and total brain volume (TBV) were treated as covariates. We created a combined mask of the mPFC and OFC using the WFU Pickatlas tool based on the Automated Anatomical Labeling (AAL) template (Maldjian et al., 2003). Frontal_Sup_Medial, Rectus, Cingulum_Ant, Frontal_Sup_Orb, Frontal_Mid_Orb, Frontal_Inf_Orb and Frontal_Med_Orb from the AAL template were selected. To correct for non-isotropic smoothness of VBM data, we used a non-stationary cluster correction (Hayasaka et al., 2004), which has been widely applied in the studies on VBM (Arnone et al., 2013; Gilaie-Dotan et al., 2013; Chung et al., 2017; Kitayama et al., 2017). A threshold of corrected cluster P < 0.05 (single voxel P < 0.005) with an extent threshold of k = 358 voxels was set.
Confirmatory cross-validation analysis
To test if the link between rGMD and social well-being is robust, we used a machine-learning method with balanced 4-fold cross-validation. This new method has been widely applied in prior neuroimaging research (Supekar et al., 2013; Qin et al., 2014; Evans et al., 2015; Kong et al., 2015a; Li et al., 2016; Liu et al., 2016; Kong et al., 2018a). We established a linear regression model (LRM), using rGMD in left OFC as an independent variable (IV) and social well-being as a dependent variable (DV), and then divided data into 4-folds under the restriction that there are no significant differences between the distributions of the data. An LRM was built using 3-folds, leaving out 1-fold. Then, we computed the r(predicted, observed) (i.e. the correlation of the observed values with the predicted values) based on the average of four repetitions of this procedure. In order to gain statistical stability, the entire procedure of the balanced 4-fold cross-validation was repeated 100 times. Finally, non-parametric testing procedures were used to test the significance of the model by generating 1000 surrogate data sets.
Multiple mediation analysis
To confirm if personality traits explain the influence of brain anatomy on social well-being, we carried out a multiple mediation analysis with the PROCESS macro for SPSS (Hayes, 2013). In the multiple mediation model, we included social well-being as the DV, brain structure as the IV and personality traits as the mediator variables (MV). By convention, the indirect effect is a product of path A (the relation of the IV with the MV) and path B (the relation of the MV with the DV after controlling for the IV). Bootstrapping procedures confirm the statistical significance of the specific indirect effect. Here we generate a 95% confidence interval (CI) using 5000 samplings. If a 95% CI fails to contain zero, the indirect effect is significant (P < 0.05).
Results
The structural basis of social well-being
As showed in Table 1, the absolute value of the kurtosis and skewness of all the scores was <1, indicating the normality of the data. The Cronbach alpha coefficients for the questionnaires ranged from 0.63 to 0.79, suggesting that they have acceptable internal consistence reliability. A mean SWBS score of 73.14 suggested that the sample had an above-average level of social well-being, which is in accordance with the study by Li et al. (2015) that found a mean of 72.14 in adults aged from 17 to 55 years (n = 630). There was no significant difference in the scores between males and females [t (133) = 0.49; P > 0.05]. The scores of social well-being had no significant correlations with age (r = 0.06; P > 0.05) and TBV (r = −0.09; P > 0.05). Next, we investigated the structural correlates of social well-being.
Table 1.
Descriptive statistics for all measures
| Variables | Mean | s.d. | Range | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Age | 21.02 | 2.11 | 18–26 | 0.60 | −0.58 |
| SSS scale | 5.02 | 1.2 | 3–8 | 0.08 | −0.69 |
| SWBS | 73.14 | 9.36 | 44–98 | −0.19 | 0.16 |
| NEO-PI-R neuroticism scale | 71.80 | 9.87 | 50–98 | 0.20 | −0.32 |
| NEO-PI-R extraversion scale | 76.87 | 7.69 | 54–97 | −0.21 | 0.71 |
| NEO-PI-R openness scale | 82.08 | 8.33 | 61–108 | 0.07 | 0.26 |
| NEO-PI-R agreeableness scale | 83.98 | 8.25 | 62–104 | −0.14 | 0.20 |
| NEO-PI-R conscientiousness scale | 79.97 | 8.96 | 60–101 | 0.01 | −0.42 |
| TTF | 16.01 | 4.20 | 7–25 | −0.07 | −0.68 |
To detect the relationship between social well-being and rGMD, we conducted an ROI multiple regression analysis. Social well-being had a negative association with rGMD in an anatomical cluster that mainly included the left OFC [Montreal Neurological Institute (MNI) coordinate: −15, 30, −14; t = −3.88; Cluster size, 358 voxels; P < 0.05] after controlling for age, sex and TBV (Figure 1; Table 2). No other significant cluster was found. To test the robustness of the association of rGMD with social well-being, we extracted rGMD values of the clusters obtained in the previous analysis and then performed a balanced 4-fold cross-validation using the machine learning approach. The results revealed that the correlation between the observed values in our data and the predicted values obtained using the balanced 4-fold cross-validation was significant (r(predicted, observed) = 0.38; P < 0.001), suggesting that rGMD in the OFC could be reliably related to social well-being.
Fig. 1.
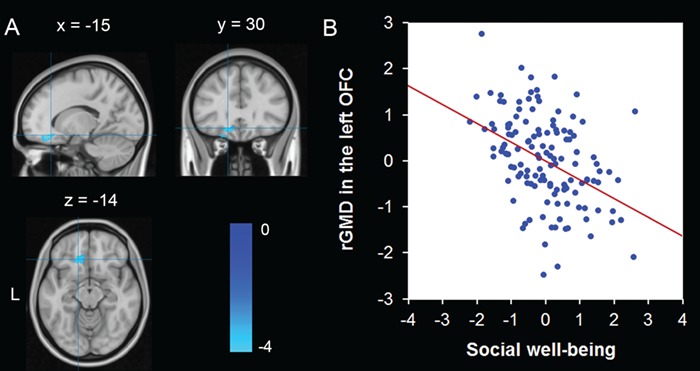
GM structures that correlated with social well-being. (A) rGMD in the left OFC was negatively associated with social well-being. The coordinate is shown in the MNI stereotactic space. (B) A scatterplot between rGMD in the left OFC and social well-being is shown for illustration purpose only. The values on the horizontal and vertical axes represent the standardized residuals of the SWBS scores and rGMD values in the left OFC after regressing out age, sex and TBV.
Table 2.
GM structures that correlated with social well-being
| Region | Side | MNI coordinate x y z |
Peak t-value |
Cluster size (k) | ||
|---|---|---|---|---|---|---|
| OFC | L | −15 | 30 | −14 | −3.88 | 358* |
Note: L means left; *P (corrected) < 0.05.
Brain structure linking personality traits to social well-being
To test which personality traits mediate the relation between the brain and social well-being, we employed the NEO-PI-R and TTF to evaluate Big Five personality traits and dispositional forgiveness in our sample. Behaviorally, the associations of all five personality traits and dispositional forgiveness with social well-being (r = 0.26–0.48; Ps < 0.01) were confirmed in our sample (Table 3). Even after adjusting for age, sex and TBV, the associations remained significant (r = 0.26–0.48; Ps < 0.01). Then, we tested the independent effects of these personality traits on social well-being via the multiple regression analysis. The results found that only neuroticism (β = −0.26; P = 0.002), extraversion (β = 0.23; P = 0.011) and dispositional forgiveness (β = 0.22; P = 0.006) were related to social well-being, and these traits explained an additional 18.8% of the variance in social well-being, indicating that neuroticism, extraversion and dispositional forgiveness have a more important association with social well-being.
Table 3.
Correlations of all measures collected in the study
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Age | 1.00 | |||||||
| 2. SSS | −0.03 | 1.00 | ||||||
| 3. Social well-being | 0.06 | 0.16* | 1.00 | |||||
| 4. Neuroticism | −0.07 | −0.20* | −0.45** | 1.00 | ||||
| 5. Extraversion | −0.01 | 0.10 | 0.48** | −0.39** | 1.00 | |||
| 6. Openness | 0.05 | 0.10 | 0.27** | −0.06 | 0.40** | 1.00 | ||
| 7. Agreeableness | 0.24** | 0.06 | 0.26** | −0.13 | 0.19* | 0.08 | 1.00 | |
| 8. Conscientiousness | 0.08 | 0.02 | 0.36** | −0.41** | 0.39** | .23** | 0.30** | 1.00 |
| 9. Forgiveness | 0.11 | −0.01 | 0.43** | −0.34** | 0.36** | 0.08 | 0.33** | 0.28** |
Note: *P < 0.05; **P < 0.01.
Next, we checked whether rGMD in the clusters obtained in the previous analysis could be related to personality traits. Density of the left OFC was found to be correlated with neuroticism (r = 0.24; P = 0.006) and dispositional forgiveness (r = −0.32; P < 0.001), even after adjusting for age, sex and TBV. To test the robustness of the association of rGMD with neuroticism and dispositional forgiveness, we performed a cross-validation analysis. The results revealed that rGMD in the region could be reliably related to neuroticism (r(predicted, observed) = 0.16; P = 0.013) and dispositional forgiveness (r(predicted, observed) = 0.26; P < 0.001). Together, these results indicate rGMD in the OFC, the personality traits of neuroticism, and dispositional forgiveness, and social well-being are closely related to each other.
To explore whether these personality traits (i.e. neuroticism and dispositional forgiveness) may mediate the link of rGMD in the OFC with social well-being, we performed a multiple mediation analysis. Interestingly, we found that neuroticism (indirect effect, −0.05; 95% CI [−0.13, −0.01]; P < 0.05) and dispositional forgiveness (indirect effect, −0.05; 95% CI [−0.13, −0.01]; P < 0.05) independently mediated the link of rGMD in the region with social well-being, even when age, sex and TBV were adjusted for (Figure 2). In addition, due to multiple mediators tested in our model, we used false discovery rate (FDR) to adjust for the multiple comparisons. We found that all indirect effects were significant (P(neuroticism, corrected) = 0.04; P(neuroticism, corrected) = 0.03).
Fig. 2.
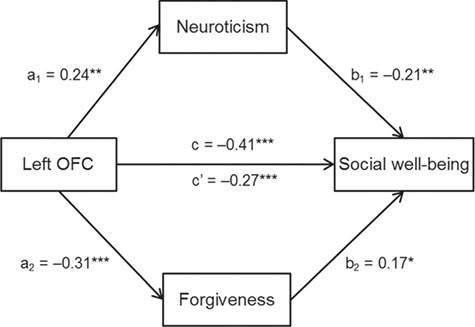
Personality traits mediate the influence of rGMD in the left OFC on social well-being. Depicted is the path diagram of the mediation analysis in which neuroticism and dispositional forgiveness mediate the association between the OFC and social well-being. All path coefficients are standard regression coefficients. Note: *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary analyses
Given that SSS is associated with social well-being (Kong et al., 2015c), we checked if our results were influenced by SSS. First, behaviorally, we replicated a significant correlation between social well-being and SSS (r = 0.16; P = 0.035). Second, when controlling for age, sex, TBV and SSS, social well-being had a negative association with rGMD in the left OFC (r = −0.40; P < 0.001), and the confirmatory cross-validation analysis revealed that rGMD in the left OFC could be reliably related to social well-being (r(predicted, observed) = 0.36; P < 0.001). Third, rGMD in the left OFC was correlated with neuroticism (r = 0.23; P = 0.010) and dispositional forgiveness (r = −0.32; P < 0.001), even after adjusting for age, sex, TBV and SSS. The further confirmatory cross-validation analysis revealed that rGMD in the left OFC could be reliably related to neuroticism (r(predicted, observed) = 0.15; P = 0.019) and dispositional forgiveness (r(predicted, observed) = 0.27; P < 0.001). Finally, the mediation analysis found that after controlling for age, sex, TBV and SSS, neuroticism (indirect effect, −0.04; 95% CI [−0.13, −0.01]; P < 0.05) and dispositional forgiveness (indirect effect, −0.06; 95% CI [−0.13, −0.01]; P < 0.05) could mediate the association of rGMD in the left OFC with social well-being. Taken together, our findings were not influenced by SSS.
Discussion
The aim of this study is to examine the relationship between social well-being and GM structures in young healthy adults. The VBM and confirmatory cross-validation analyses revealed that rGMD in the left OFC could be reliably related to social well-being. Importantly, neuroticism and dispositional forgiveness could dependently mediate the link of rGMD in the region with social well-being. Furthermore, even when controlling for SSS, these findings remained significant, revealing that the findings are specific to social well-being. Thus, these results provide the initial evidence that OFC is a neuroanatomical substrate for social well-being and demonstrate that the left OFC is a crucial neural region linking neuroticism and dispositional forgiveness to social well-being.
Confirming our first hypothesis, we found a negative relationship between rGMD in the OFC and social well-being. This is in accordance with prior studies reporting a link of the OFC with individual well-being. For instance, using VBM, the rGMV in the vmPFC including medial OFC was found to be negatively correlated with individual well-being (Kong et al., 2014). Using resting state fMRI, the neural activity in the OFC was found to be correlated with individual well-being (Kong et al., 2015b; Kong et al., 2016; Kong et al., 2018b). These results indicate that the OFC might be a key neural site for both types of well-being. The OFC occupies the ventral region of the PFC and has been known for its important function in regulation of emotion (Ochsner et al., 2002; 2004; Krueger et al., 2009; Mak et al., 2009; Welborn et al., 2009; Kanske et al., 2010; Golkar et al., 2012), which is consistent with previous behavioral studies reporting an association between social well-being and emotion-related traits like neuroticism and extraversion (Hill et al., 2012; Lamers et al., 2012; Kong et al., 2015c). Furthermore, as a core node of the social brain networks (Skuse and Gallagher, 2009), this region is also found to play an important role in a series of social cognitive processes such as emotion recognition from faces and music (Kringelbach and Rolls, 2004; Heberlein et al., 2008; Omar et al., 2011), theory of mind (Sabbagh, 2004; Viskontas et al., 2007; Shamay-Tsoory et al., 2010; Abu-Akel and Shamay-Tsoory, 2011) and social decision-making such as decisions about trust, reciprocity, altruism and social norm conformity (Sanfey, 2007; Lee, 2008; Rilling et al., 2008; Rilling and Sanfey, 2011). All of these social cognitive processes are crucial for the acquisition of one’s social functioning. In addition, the negative relationship of GM structure and social well-being is not surprising because many VBM studies have found negative correlations between rGMV/rGMD in the PFC and behaviors such as intelligence (Kong et al., 2015a), emotional intelligence (Takeuchi et al., 2011) and achievement motivation (Takeuchi et al., 2014). These negative relationships may be attributed to synaptic pruning of excess neurons during development, which is believed to facilitate a more efficient cognitive process (Paus, 2005; Kanai and Rees, 2011; Takeuchi et al., 2011). Therefore, the synaptic pruning in the OFC may contribute to a series of OFC-related functions, which might lead to higher social well-being. However, further studies are necessary to test which OFC-related functions play an important role in social well-being.
More importantly, we discovered that, although Big Five personality traits and dispositional forgiveness were correlated with social well-being, only neuroticism, extraversion and dispositional forgiveness could independently influence social well-being and explain an additional 18.8% of the variance in social well-being. This indicates that neuroticism, extraversion and dispositional forgiveness are the important personal resources for the acquisition of social well-being. However, only neuroticism and dispositional forgiveness independently mediated the relationship between rGMD in the left OFC and social well-being, which is consistent with previous studies exploring the neural correlates of neuroticism and forgiveness. Prior neuroimaging research has found that neuroticism is significantly associated with the activity (Mobbs et al., 2005; Fujiwara et al., 2008; Madsen et al., 2015) and morphometry of the OFC (Wright et al., 2006; Jackson et al., 2011). In addition, prior fMRI research has shown an association between the OFC and forgiveness (Farrow et al., 2001; Hayashi et al., 2010). For example, when making forgivability judgments based on social scenarios, the OFC was activated (Farrow et al., 2001). Furthermore, the OFC was found to be implicated in forgiveness for moral transgressions involving deception (Hayashi et al., 2010). Together with our results, these findings suggest that the OFC is a crucial neural site of neuroticism and dispositional forgiveness. Given the role of the OFC in emotion regulation and social cognitive processing such as theory of mind and social decision-making, the involvement of the OFC might help individuals develop better emotion regulation that contributes to greater emotional stability (i.e. low neuroticism), recognize others’ intentions and make a decision for forgiveness to gain a peaceful social environment, all of which are crucial for improving one’s social functioning. In short, our findings confirm that neuroticism and dispositional forgiveness can serve as possible mechanisms that explain the impact of rGMD in the OFC on social well-being.
In spite of these strengths, our study also has several potential limitations. First, in spite of their good psychometric properties, all instruments relied on self-report. We also tried to control the response bias (e.g. social desirability) by including some self-report measures such as the SSS scale as covariates, but further studies still need to use other approaches to exclude the influence of the bias. Second, these results were obtained based on a sample of young healthy adults, so more studies should be conducted to test the applicability of these results to other age groups. Third, the current study found the partially mediating role of neuroticism and dispositional forgiveness in the link between the OFC and social well-being, so further studies need to identify other possible mediators such as depressive symptoms and emotion intelligence. Fourth, our VBM protocol in SPM8 has been used widely to uncover the structural basis of behavior (Kanai and Rees, 2011; Kraus et al., 2014; Kong et al., 2015a; Wang et al., 2017), but the default segmentation approach in SPM12 is a slightly modified version of ‘New Segment’ in SPM8. Therefore, further studies are still necessary to examine whether there are significant differences for the present findings using SPM8 and SPM12; although a recent study found that when a cluster-based FDR of 0.05 was used to correct for multiple comparisons, the significant clusters (i.e. left insula and superior/medial frontal gyrus) could be reproduced between analysis with SPM8 and SPM12 (De Bondt et al., 2016). Finally, this study was cross-sectional, so directionality can only be inferred. Further investigations should be designed to confirm the causal relationships among brain structure, personality and social well-being.
In summary, the present study provides the further evidence for a neuroanatomical marker for social well-being by revealing that rGMD in the left OFC was significantly associated with social well-being. Furthermore, even when controlling for SSS, the association remained significant, indicating specificity to social well-being. Moreover, our study provides possible mediational mechanisms (i.e. neuroticism and dispositional forgiveness) that explain the link between rGMD in the OFC and social well-being. In addition, our study invites further research to explore how to develop neural interventions (non-invasive brain stimulation to the left OFC) related to neuroticism and forgiveness to promote social well-being.
Funding
This work was supported by the National Natural Science Foundation of China (31800942; 31700945), Young Talent Fund of University Association for Science and Technology in Shaanxi, China (20180206), and Fundamental Research Funds for the Central Universities (GK201903106).
Conflict of interest. None declared.
References
- Abu-Akel A., Shamay-Tsoory S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia, 49, 2971–2984. [DOI] [PubMed] [Google Scholar]
- Arnone D., Mckie S., Elliott R., et al. (2013). State-dependent changes in hippocampal grey matter in depression. Molecular Psychiatry, 18, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38, 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. (2005). Unified segmentation. NeuroImage, 26, 839–851. [DOI] [PubMed] [Google Scholar]
- Blakemore S. (2008). The social brain in adolescence. Nature Reviews. Neuroscience, 9, 267–277. [DOI] [PubMed] [Google Scholar]
- Brown R.P. (2003). Measuring individual differences in the tendency to forgive: Construct validity and links with depression. Personality and Social Psychology Bulletin, 29, 759–71. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z., Pisoni A., Papagno C. (2011). Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience, 183, 64–70. [DOI] [PubMed] [Google Scholar]
- Chung H.K., Tymula A., Glimcher P. (2017). The reduction of ventrolateral prefrontal cortex gray matter volume correlates with loss of economic rationality in aging. The Journal of Neuroscience, 37, 12068–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P.T. Jr., McCrae R.R. (1992). Normal personality assessment in clinical practice: the NEO personality inventory. Psychological Assessment, 4, 5–13. [Google Scholar]
- De Bondt T., Pullens P., Van Hecke W., Jacquemyn Y., Parizel P.M. (2016). Reproducibility of hormone-driven regional grey matter volume changes in women using SPM8 and SPM12. Brain Structure & Function, 221, 4631–4641. [DOI] [PubMed] [Google Scholar]
- Deckersbach T., Miller K.K., Klibanski A., et al. (2006). Regional cerebral brain metabolism correlates of neuroticism and extraversion. Depression and Anxiety, 23, 133–138. [DOI] [PubMed] [Google Scholar]
- Deng Z., Wei D., Xue S., Du X., Hitchman G., Qiu J. (2014). Regional gray matter density associated with emotional conflict resolution: evidence from voxel-based morphometry. Neuroscience, 275, 500–507. [DOI] [PubMed] [Google Scholar]
- DeYoung C.G., Hirsh J.B., Shane M.S., Papademetris X., Rajeevan N., Gray J.R. (2010). Testing predictions from personality neuroscience: brain structure and the Big Five. Psychological Science, 21, 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eres R., Decety J., Louis W.R., Molenberghs P. (2015). Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. NeuroImage, 117, 305–310. [DOI] [PubMed] [Google Scholar]
- Evans T.M., Kochalka J., Ngoon T.J., et al. (2015). Brain structural integrity and intrinsic functional connectivity forecast 6 year longitudinal growth in children’s numerical abilities. The Journal of Neuroscience, 35, 11743–11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow T.F., Zheng Y., Wilkinson I.D., et al. (2001). Investigating the functional anatomy of empathy and forgiveness. Neuroreport, 12, 2433–2438. [DOI] [PubMed] [Google Scholar]
- Fregni F., Boggio P.S., Nitsche M., et al. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research, 166, 23–30. [DOI] [PubMed] [Google Scholar]
- Fujiwara J., Tobler P.N., Taira M., Iijima T., Tsutsui K.I. (2008). Personality-dependent dissociation of absolute and relative loss processing in orbitofrontal cortex. The European Journal of Neuroscience, 27, 1547–1552. [DOI] [PubMed] [Google Scholar]
- Gallagher M.W., Lopez S.J., Preacher K.J. (2009). The hierarchical structure of well-being. Journal of Personality, 77, 1025–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S., Kanai R., Bahrami B., Rees G., Saygin A.P. (2013). Neuroanatomical correlates of biological motion detection. Neuropsychologia, 51, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S., Tymula A., Cooper N., Kable J.W., Glimcher P.W., Levy I. (2014). Neuroanatomy predicts individual risk attitudes. The Journal of Neuroscience, 34, 12394–12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golkar A., Lonsdorf T.B., Olsson A., et al. (2012). Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One, 7, e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N.A., Fristen K.J., Frackowiak R.S.J. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage, 14, 21–36. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Phan K.L., Liberzon I., Worsley K.J., Nichols T.E. (2004). Nonstationary cluster-size inference with random field and permutation methods. NeuroImage, 22, 676–687. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Abe N., Ueno A., et al. (2010). Neural correlates of forgiveness for moral transgressions involving deception. Brain Research, 1332, 90–99. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, New York: Guilford Press. [Google Scholar]
- Heberlein A.S., Padon A.A., Gillihan S.J., Farah M.J., Fellows L.K. (2008). Ventromedial frontal lobe plays a critical role in facial emotion recognition. Journal of Cognitive Neuroscience, 20, 721–733. [DOI] [PubMed] [Google Scholar]
- Hill P.L., Turiano N.A., Mroczek D.K., Roberts B.W. (2012). Examining concurrent and longitudinal relations between personality traits and social well-being in adulthood. Social Psychological and Personality Science, 3, 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A.J., Dopko R.L., Passmore H.-A., Buro K. (2011). Nature connectedness: associations with well-being and mindfulness. Personality and Individual Differences, 51, 166–171. [Google Scholar]
- Huang S., Hou J., Sun L., Dou D., Liu X., Zhang H. (2017). The effects of objective and subjective socioeconomic status on subjective well-being among rural-to-urban migrants in China: the moderating role of subjective social mobility. Frontiers in Psychology, 8, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J., Balota D.A., Head D. (2011). Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of Aging, 32, 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Bahrami B., Duchaine B., Janik A., Banissy M.J., Rees G. (2012). Brain structure links loneliness to social perception. Current Biology, 22, 1975–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Rees G. (2011). The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews. Neuroscience, 12, 231–242. [DOI] [PubMed] [Google Scholar]
- Kanske P., Heissler J., Schönfelder S., Bongers A., Wessa M. (2010). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex, 21, 1379–1388. [DOI] [PubMed] [Google Scholar]
- Keyes C.L.M. (1998). Social well-being. Social Psychology Quarterly, 61, 121–140. [Google Scholar]
- Kitayama S., Yanagisawa K., Ito A., Ueda R., Uchida Y., Abe N. (2017). Reduced orbitofrontal cortical volume is associated with interdependent self-construal. Proceedings of the National Academy of Sciences of the United States of America, 114, 7969–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Chen Z., Xue S., Wang X., Liu J. (2015a). Mother’s but not father’s education predicts general fluid intelligence in emerging adulthood: behavioral and neuroanatomical evidence. Human Brain Mapping, 36, 4582–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Ding K., Yang Z., et al. (2014). Examining gray matter structures associated with individual differences in global life satisfaction in a large sample of young adults. Social Cognitive and Affective Neuroscience, 10, 952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., He Q., Liu X., Chen X., Wang X., Zhao J. (2018a). Amplitude of low-frequency fluctuations during resting state differentially predicts authentic and hubristic pride. Journal of Personality, 86, 213–219. [DOI] [PubMed] [Google Scholar]
- Kong F., Hu S., Wang X., Song Y., Liu J. (2015b). Neural correlates of the happy life: the amplitude of spontaneous low frequency fluctuations predicts subjective well-being. NeuroImage, 107, 136–145. [DOI] [PubMed] [Google Scholar]
- Kong F., Hu S., Xue S., Song Y., Liu J. (2015c). Extraversion mediates the relationship between structural variations in the dorsolateral prefrontal cortex and social well-being. NeuroImage, 105, 269–275. [DOI] [PubMed] [Google Scholar]
- Kong F., Ma X., You X., Xiang Y. (2018). The resilient brain: psychological resilience mediates the effect of amplitude of low-frequency fluctuations in orbitofrontal cortex on subjective well-being in young healthy adults. Social Cognitive and Affective Neuroscience, 13, 755–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Wang X., Song Y., Liu J. (2016). Brain regions involved in dispositional mindfulness during resting state and their relation with well-being. Social Neuroscience, 11, 331–343. [DOI] [PubMed] [Google Scholar]
- Kraus C., Ganger S., Losak J., et al. (2014). Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. NeuroImage, 84, 236–244. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72, 341–372. [DOI] [PubMed] [Google Scholar]
- Krueger F., Barbey A.K., McCabe K., et al. (2009). The neural bases of key competencies of emotional intelligence. Proceedings of the National Academy of Sciences of the United States of America, 106, 22486–22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers S., Westerhof G.J., Bohlmeijer E.T., Klooster P.M., Keyes C.L. (2011). Evaluating the psychometric properties of the Mental Health Continuum-Short Form (MHC-SF). Journal of Clinical Psychology, 67, 99–110. [DOI] [PubMed] [Google Scholar]
- Lamers S.M., Westerhof G.J., Kovács V., Bohlmeijer E.T. (2012). Differential relationships in the association of the Big Five personality traits with positive mental health and psychopathology. Journal of Research in Personality, 46, 517–524. [Google Scholar]
- Lawler-Row K.A., Piferi R.L. (2006). The forgiving personality: describing a life well lived? Personality and Individual Differences, 41, 1009–1020. [Google Scholar]
- Lee D. (2008). Game theory and neural basis of social decision making. Nature Neuroscience, 11, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G., Panizzon M.S., Eyler L., et al. (2014). Heritable influences on amygdala and orbitofrontal cortex contribute to genetic variation in core dimensions of personality. NeuroImage, 103, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.A., Rezaie R., Brown R., Roberts N., Dunbar R.I. (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage, 57, 1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Dong M., Ren A., Ren J., Zhang J., Huang L. (2016). Structural attributes of the temporal lobe predict face recognition ability in youth. Neuropsychologia, 84, 1–6. [DOI] [PubMed] [Google Scholar]
- Li M., Yang D., Ding C., Kong F. (2015). Validation of the social well-being scale in a Chinese sample and invariance across gender. Social Indicators Research, 121, 607–618. [Google Scholar]
- Liu Y., Lin W., Liu C., et al. (2016). Memory consolidation reconfigures neural pathways involved in the suppression of emotional memories. Nature Communications, 7, 13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead R.A., Parsey R.V., Oquendo M.A., Mann J.J. (2004). Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biological Psychiatry, 55, 1154–1162. [DOI] [PubMed] [Google Scholar]
- Madsen M.K., Mc Mahon B., Andersen S.B., Siebner H.R., Knudsen G.M., Fisher P.M. (2015). Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Social Cognitive and Affective Neuroscience, 11, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A.K., Hu Z.G., Zhang J.X., Xiao Z., Lee T.M. (2009). Sex-related differences in neural activity during emotion regulation. Neuropsychologia, 47, 2900–2908. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Mobbs D., Hagan C.C., Azim E., Menon V., Reiss A.L. (2005). Personality predicts activity in reward and emotional regions associated with humor. Proceedings of the National Academy of Sciences of the United States of America, 102, 16502–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. (2002). Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., et al. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Omar R., Henley S.M., Bartlett J.W., et al. (2011). The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration. NeuroImage, 56, 1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. (2005). Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences, 9, 60–68. [DOI] [PubMed] [Google Scholar]
- Qin S., Cho S., Chen T., Rosenberg-Lee M., Geary D.C., Menon V. (2014). Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nature Neuroscience, 17, 1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S.L., Milad M.R., Orr S.P., Quinn B.T., Fischl B., Pitman R.K. (2005). Orbitofrontal thickness, retention of fear extinction, and extraversion. Neuroreport, 16, 1909–1912. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., King-Casas B., Sanfey A.G. (2008). The neurobiology of social decision-making. Current Opinion in Neurobiology, 18, 159–165. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., Sanfey A.G. (2011). The neuroscience of social decision-making. Annual Review of Psychology, 62, 23–48. [DOI] [PubMed] [Google Scholar]
- Riva P., Romero Lauro L.J., Vergallito A., DeWall C.N., Bushman B.J. (2015). Electrified emotions: modulatory effects of transcranial direct stimulation on negative emotional reactions to social exclusion. Social Neuroscience, 10, 46–54. [DOI] [PubMed] [Google Scholar]
- Sabbagh M.A. (2004). Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain and Cognition, 55, 209–219. [DOI] [PubMed] [Google Scholar]
- Sampaio A., Soares J.M., Coutinho J., Sousa N., Gonçalves Ó.F. (2014). The Big Five default brain: functional evidence. Brain Structure & Function, 219, 1913–1922. [DOI] [PubMed] [Google Scholar]
- Sanfey A.G. (2007). Social decision-making: insights from game theory and neuroscience. Science, 318, 598–602. [DOI] [PubMed] [Google Scholar]
- Schmidt L., Tusche A., Manoharan N., Hutcherson C., Hare T., Plassmann H. (2018). Neuroanatomy of the vmPFC and dlPFC predicts individual differences in cognitive regulation during dietary self-control across regulation strategies. Journal of Neuroscience, 3402–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaro R., Nitsche M.A., Colzato L.S. (2016). The stimulated social brain: effects of transcranial direct current stimulation on social cognition. Annals of the New York Academy of Sciences, 1369, 218–239. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Harari H., Aharon-Peretz J., Levkovitz Y. (2010). The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex, 46, 668–677. [DOI] [PubMed] [Google Scholar]
- Skuse D.H., Gallagher L. (2009). Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Sciences, 13, 27–35. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. (1999). In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience, 2, 859–861. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. (2001). Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. The Journal of Neuroscience, 21, 8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Swigart A.G., Tenison C., et al. (2013). Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proceedings of the National Academy of Sciences of the United States of America, 110, 8230–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Nouchi R., et al. (2014). Regional gray matter density is associated with achievement motivation: evidence from voxel-based morphometry. Brain Structure & Function, 219, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Taki Y., Sassa Y., et al. (2011). Regional gray matter density associated with emotional intelligence: evidence from voxel-based morphometry. Human Brain Mapping, 32, 1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal S., Scholte H.S., Lamme V.A., Fahrenfort J.J., Ridderinkhof K.R. (2011). Pre-SMA graymatter density predicts individual differences in action selection in the face of conscious and unconscious response conflict. Journal of Cognitive Neuroscience, 23, 382–390. [DOI] [PubMed] [Google Scholar]
- Van Lente E., Barry M.M., Molcho M., et al. (2012). Measuring population mental health and social well-being. International Journal of Public Health, 57, 421–430. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Yücel M., Dennison M., Simmons J., Allen N.B. (2014). Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Social Cognitive and Affective Neuroscience, 9, 1845–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas I.V., Possin K.L., Miller B.L. (2007). Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Annals of the New York Academy of Sciences, 1121, 528–545. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhou M., Chen T., et al. (2017). Examining gray matter structure associated with academic performance in a large sample of Chinese high school students. Scientific Reports, 7, 893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang L., Kong X., Hong Y., Cheon B., Liu J. (2016). Pathway to neural resilience: self-esteem buffers against deleterious effects of poverty on the hippocampus. Human Brain Mapping, 37, 3757–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn B.L., Papademetris X., Reis D.L., Rajeevan N., Bloise S.M., Gray J.R. (2009). Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Social Cognitive and Affective Neuroscience, 4, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C.I., Williams D., Feczko E., et al. (2006). Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex, 16, 1809–1819. [DOI] [PubMed] [Google Scholar]
- Zhang W., Chen Q., McCubbin H., McCubbin L., Foley S. (2011). Predictors of mental and physical health: individual and neighborhood levels of education, social well-being, and ethnicity. Health & Place, 17, 238–247. [DOI] [PubMed] [Google Scholar]
- Zhu H. (2015). Social support and affect balance mediate the association between forgiveness and life satisfaction. Social Indicators Research, 124, 671–681. [Google Scholar]


