SUMMARY
Members of the bacterial genus Bacillus are known as producers of a broad spectrum of antibiotic compounds of proteinaceous nature that possess inhibitory activity against different saprophytic and pathogenic microorganisms. In the current research, a peptide synthesized by Bacillus methylotrophicus strain BM47, previously isolated from a natural thermal spring in Bulgaria, was identified and characterized as a bacteriocin. In vitro antimicrobial screening of the crude bacteriocin substance of B. methylotrophicus BM47 showed activity against the plant pathogenic fungi Fusarium moniliforme, Aspergillus awamori, Penicillium sp., Aspergillus niger and Gram-negative bacterium Pseudomonas aeruginosa. The antimicrobial activity of the crude bacteriocin substance was partially inhibited by the enzymes trypsin, Alcalase®, Savinase®, proteinase K, papain and Esperase®, while catalase was not effective. The crude bacteriocin substance was relatively pH resistant, but sensitive to the action of heat and most organic solvents and detergents tested. To obtain the active protein fractions, crude bacteriocin substance was purified by fast protein liquid chromatography (FPLC) using a strong anion exchange column. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis demonstrated that the purified bacteriocin had molecular mass of 19 578 Da. The amino acid analysis performed by high-performance liquid chromatography (HPLC) revealed that the isolated bacteriocin consisted of 17 types of amino acids, with the highest mol fraction expressed as percent of serine (29.3), valine (10.3), alanine (9.8) and tyrosine (7.1).
Key words: Bacillus methylotrophicus, bacteriocin, antimicrobial activity, amino acid composition
INTRODUCTION
The bacterial genus Bacillus unifies Gram-positive, spore-forming rods that produce a wide spectrum of biologically active substances that have antimicrobial activity against closely-related or non-related microorganisms. This large and varied group of antimicrobial compounds of proteinaceous nature includes different peptide and lipopeptide antibiotics, and bacteriocins. Most bacteriocins synthesized by Bacillus sp. belong to the lantibiotics, a group of peptides that undergo various post-translational modifications (1).
According to the latest classifications Bacillus bacteriocins are divided into three main groups. Class I unifies post-translationally modified peptides. This class can be subdivided into four subclasses. Subclass I.1 comprises type A lantibiotics that exhibit an enlongated structure (subtilin, ericin S and ericin A), subclass I.2 unifies type B globular lantibiotics (mersacidin, sublancin 168 and paenibacillin), subclass I.3 includes two-component lantibiotics (haloduracin and lichenicidin), while subclass I.4 includes cyclic peptides (subtilosin A). Class II bacteriocins comprise small, ribosomally synthesized, non-modified and linear peptides. This class can be subdivided into three subclasses. Subclass II.1 includes pediocin-like peptides produced by Bacillus coagulans, Bacillus circulans and Paenibacillus polymyxa strains (coagulin, SRCAM 37, 602 and 1580). Subclass II.2 includes thuricin-like peptides produced by Bacillus thuringiensis strains (bacthuricin F4, thurincin H, S and 17) and Bacillus cereus strains (cerein MRX1). Subclass II.3 includes other linear peptides produced by Bacillus licheniformis (lichenin) as well as cerein 7A and 7B, and thuricin 439. Class III unifies large proteins with phospholipase activity produced by Bacillus megaterium strains (megacins A-216 and A-19213) (2).
Due to their antimicrobial properties against various saprophytic and pathogenic microorganisms, the bacteriocins synthesized from Bacillus sp. find a wide application in the food industry as biopreservatives (3) and in the medicine as alternatives to conventional antibiotics and even as means for cancer therapy (4). Bacillus bacteriocins are also of interest in the veterinary medicine against animal pathogens and in the agriculture as plant protection and biocontrol agents (5). Most Bacillus bacteriocins are active against Gram-positive bacteria, but a small number has been found to have promising activity against Gram-negative bacteria, yeasts and fungi (6).
Bacillus methylotrophicus strains are known for their excellent plant-growth promoting properties and as producers of biologically active compounds that effectively control plant diseases caused by bacteria and fungi (7). Despite the increasing scientific interest and efforts to characterize some secondary metabolites of B. methylotrophicus strains, presently little is known about the physicochemical properties and chemical composition of the antimicrobial substances they produce. Therefore, the aim of the present research is to isolate, characterize and determine the amino acid composition of a bacteriocin synthesized by B. methylotrophicus BM47, a strain previously isolated from a natural thermal spring in Bulgaria.
MATERIALS AND METHODS
Studied strain
Bacillus methylotrophicus BM47, as reported in our previous works (8, 9), was isolated from a natural thermal spring (water temperature=57 °С) in Haskovo mineral spa, Haskovo region, Southern Bulgaria. The results from comparative 16S rRNA gene sequence-based phylogenetic analysis revealed 99% pairwise homology with the reference strain B. methylotrophicus PY5.
Test microorganisms
Twelve test microorganisms including Gram-positive bacteria (Enterococcus faecalis ATCC 19433 and Staphylococcus aureus ATCC 25923), Gram-negative bacteria (Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027 and Salmonella sp. – our isolate), yeasts (Saccharomyces cerevisiae – our isolate) and fungi (Aspergillus niger, Aspergillus awamori, Aspergillus oryzae, Fusarium moniliforme, Rhizopus sp. and Penicillium sp. – our isolates) from the collection of the Department of Microbiology at the University of Food Technologies, Plovdiv, Bulgaria, were used.
Culture media
Luria-Bertani medium (HiMedia®, Mumbai, India) supplemented with glucose (LBG broth) was used for cultivation of B. methylotrophicus BM47. LBG agar medium (Laboratorios Conda S.A., Madrid, Spain) was used for cultivation of the test bacteria and yeasts, as well as for implementation of agar well diffusion assay. Malt agar (HiMedia®) was used for cultivation/maintenance of the test fungi. The culture media were prepared according to the typical formula (8, 9).
Antimicrobial assay
The antimicrobial activity of B. methylotrophicus BM47 was determined by the conventional agar well diffusion method (8–10). The test bacteria were cultured on LBG agar medium at 37 °C for 24 h, while the yeasts were cultured on LBG agar medium at 30 °C for 24 h. The test fungi were grown on malt agar at 30 °C for 7 days or until sporulation. The inocula of bacteria/yeasts were prepared by homogenization of a small amount of biomass in 5 mL of sterile 0.5% NaCl. The inocula of fungi were prepared by the addition of 5 mL of sterile 0.5% NaCl into the tubes. After vigorous shaking, they were filtered and replaced in other tubes before use. The number of viable cells and fungal spores was determined using a bacterial counting chamber Thoma (Poly-Optik GmbH, Bad Blankenburg, Germany). Their final concentrations were adjusted to 108 CFU/mL for bacterial/yeast cells and 105 CFU/mL for fungal spores, and then inoculated in preliminarily melted and tempered at 45–48 °C LBG agar media. The inoculated media were transferred in quantity of 17 mL in sterile Petri plates (d=90 mm; Gosselin™, Hazebrouck, France) and allowed to solidify. Then six wells (d=6 mm) per plate were cut.
B. methylotrophicus BM47 was cultured in centrifuge tubes (Isolab, Wertheim, Germany) containing 5 mL of LBG broth at 30 °C for 48 h. Cells were removed by centrifugation at 5000×g for 15 min and the cell-free culture supernatant was collected. The supernatant was sterilized through a bacterial filter (dpore=0.2 μm; Sartorius, Goettingen, Germany). Triplicates of 60 μL were pipetted into the agar wells. Antimicrobial activity was determined by measuring the diameter of inhibition zones (mm) around the wells after incubation at 30 °C (for yeasts and fungi) and 37 °C (for bacteria) for 48 h. One unit (U) of antimicrobial activity was defined as equal to 1 mm2 of the zone of inhibition beyond the well diameter (11).
Production of bacteriocin and preparation of crude bacteriocin substance
The strain B. methylotrophicus BM47 was grown in tubes (Isolab), each containing 40 mL of LBG broth at 30 °C for 48 h. Then the tubes were centrifuged at 5000×g for 15 min, the cell-free culture supernatant was sterilized with a bacterial filter (Sartorius) and collected in a sterile bottle. This preparation was designated as crude bacteriocin substance, which was transferred in freeze-drying flasks and lyophilized using a freeze dryer Lyovac GT2 (Leybold-Heraeus, Cologne, Germany) for 48 h. Then the freeze-dried crude bacteriocin substance was collected and stored for further investigations.
Effects of enzymes, temperature, pH, organic solvents and detergents on antimicrobial activity of crude bacteriocin substance
Effect of enzymes
The effect of enzymes on antimicrobial activity was determined according to the method described by Xie et al. (12) with slight modification. Aliquots of crude bacteriocin substance were treated with different enzymes: trypsin (EC 3.4.21.4), Alcalase®, Savinase®, Esperase® (EC 3.4.21.62), proteinase K (EC 3.4.21.64), papain (EC 3.4.22.2) and catalase (EC 1.11.1.6) (all from Sigma-Aldrich, Merck, St. Louis, MO, USA) at final concentration of 1 mg/mL. The samples were incubated for 1 h under the optimal conditions (pH and temperature) for each enzyme. Samples of enzymes plus buffer, enzymes plus LBG broth, LBG broth alone and buffer alone were used as negative controls, while untreated crude bacteriocin substance was used as a positive one. The residual antimicrobial activity against the indicator strain F. moniliforme was determined by the agar well diffusion method described above.
Thermal stability
To determine the thermal stability, aliquots (1 mL) of crude bacteriocin substance were put in micro tubes (Isolab) and sealed. Samples were exposed for 15 min to different temperatures as follows: 45, 60, 75, 90, 100 and 121 °C (autoclaving). Untreated crude bacteriocin substance sample was used as a control (12). The residual antimicrobial activity against the indicator strain F. moniliforme was determined by the agar well diffusion method.
Effect of pH
The effect of pH on antimicrobial activity was determined by adjusting the pH of the crude bacteriocin substance with diluted HCl and NaOH (Sigma-Aldrich, Merck). Samples with different pH values (from pH=3 to 11) were incubated at room temperature for 2 h, then neutralized to pH=6.8 (12). The residual antimicrobial activity against the indicator strain F. moniliforme was determined by the agar well diffusion method.
Effect of organic solvents and detergents
Different organic solvents and detergents at their working concentrations: methanol (50%), isopropanol (50%), acetonitrile (50%), acetone (50%), EDTA (0.1 M), Tween 20 (10%), Tween 60 (10%) and Tween 80 (10%) (all from Sigma-Aldrich, Merck) were added to crude bacteriocin substance samples and incubated at room temperature for 1 h. Crude bacteriocin substance sample with added water was used as a control (12). The residual antimicrobial activity against the indicator strain F. moniliforme was determined by the agar well diffusion method.
Bacteriocin purification by fast protein liquid chromatography
The lyophilized crude bacteriocin substance was dissolved in distilled water to final concentration of 1 g/mL and then dialyzed against 50 mM sodium phosphate buffer at pH=6.8. The separation of protein fractions was performed by strong anion exchange column High Q (Bio-Rad, Hercules, CA, USA) on FPLC system (Bio-Rad) coupled with UV detector, conductometry cell and automatic collector. A gradient elution was applied by using 50 mM sodium phosphate buffer at pH=6.8 and 1 М NaCl buffered in the same buffer. The antimicrobial activity of the obtained protein fractions against the indicator strain F. moniliforme was determined by the conventional agar well diffusion method described above.
The active protein fractions were collected and lyophilized in a freeze dryer Lyovac GT2 (Leybold-Heraeus) for 48 h, and then stored for further investigations.
Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (tricine-SDS-PAGE) was performed following the method described by Schägger (13) on 10 cm×8 cm gels in Mini-PROTEAN Tetra vertical electrophoresis cell (Bio-Rad). The gels were stained using SYPRO™ Ruby Protein Gel Stain (Life Technologies, Thermo Fisher Scientific, Eugene, OR, USA). The molecular mass of the purified bacteriocin was estimated according to ultra-low-range molecular mass calibration kit as markers (Sigma-Aldrich, Merck) consisting of: triosephosphate isomerase from rabbit muscle (26 600 Da), myoglobin from horse heart (17 000 Da), α-lactalbumin from bovine milk (14 200 Da), aprotinin from bovine lung (6500 Da) and oxidized bovine insulin chain B (3496 Da). The image analysis of the gels was performed using TotalLab software (14).
Hydrolysis of the purified bacteriocin into amino acids
A mass of 36 mg of the lyophilised purified bacteriocin was put in a vial and the sample was hydrolysed with 2 mL of 6 M HCl. Then the vial was sealed and heated in a laboratory oven JBD001 (Robotika, Velingrad, Bulgaria) at 105 °C for 24 h. After completion of hydrolysis, the sample was transferred in a crystallizer. The vial was washed twice with 1 mL of distilled water and the sample was dried in a laboratory oven JBD001 (Robotika) at 40–50 °C. After evaporation of the excess water, the residue was treated with 2 mL of 20 mM HCl, vortexed and filtered. The filtered sample was collected and processed to derivatization.
Derivatization of amino acids
The sample (20 μL) was derivatized with AccQ-Fluor reagent kit WAT052880 (Waters, Dublin, Ireland). AccQ-Fluor borate buffer (60 μL) was added by micropipette to the sample and vortexed. Then, 20 μL of AccQ-Fluor reagent were added and the sample was additionally vortexed for 30 s. The sample was heated in a waterbath MLW W3 (Labexchange, Burladingen, Germany) at 55 °C for 10 min before separation of amino acids using HPLC system.
High-performance liquid chromatography separation of amino acids
The AccQ-Fluor amino acid derivates were separated on ELITE LaChrom HPLC system (VWR™ Hitachi, Tokyo, Japan). Sample of 20 μL was injected into an HPLC reversed phase AccQ-Tag™ silica-bonded amino acid column C18, 3.9 mm×150 mm (Waters). The elution of the amino acids was performed by gradient system with mobile phase, eluent A, buffer WAT052890 (Waters) and mobile phase, eluent B, 60% acetonitrile (Sigma-Aldrich, Merck), in a separation gradient with a flow rate of 1.0 mL/min (Table 1). The amino acids were detected using a diode array detector (DAD) at 254 nm with the column condition set at 37 °C for 40 min. The amino acid peaks were acquired using EZChrom Elite™ software (15) and were calculated based on amino acid calibration standard (amino acid standard H, Thermo Fisher Scientific). The proportional molar concentration for each amino acid was calculated based on the concentration of standard amino acids and expressed as percent in the purified bacteriocin (16).
Table 1. Gradient conditions of high-performance liquid chromatography separation of amino acids.
| t/min | Flow rate/(mL/min) | φ(phase A)/% | φ(phase B)/% |
|---|---|---|---|
| 0 | 1.0 | 100 | 0 |
| 0.5 | 1.0 | 98 | 2 |
| 15.0 | 1.0 | 93 | 7 |
| 19.0 | 1.0 | 90 | 10 |
| 32.0 | 1.0 | 67 | 33 |
| 33.0 | 1.0 | 67 | 33 |
| 34.0 | 1.0 | 0 | 100 |
| 37.0 | 1.0 | 0 | 100 |
| 38.0 | 1.0 | 100 | 0 |
| 64.0 | 1.0 | 100 | 0 |
| 65.0 | 1.0 | 0 | 100 |
Statistical analysis
The experiments (antimicrobial activity and effects of enzymes, temperature, pH, organic solvents and detergents on the antimicrobial activity) were performed in triplicates. Data are presented as mean value±standard deviation (S.D.).
RESULTS AND DISCUSSION
In vitro antimicrobial activity of crude bacteriocin substance of B. methylotrophicus BM47
As seen from the results presented in Table 2, the crude bacteriocin substance of B. methylotrophicus BM47 demonstrated mostly antifungal, rather than antibacterial activity. The screening for antimicrobial activity showed that the studied strain had the strongest inhibitory activity against the fungus F. moniliforme. Therefore, F. moniliforme was chosen as an indicator microorganism for further analyses. The antifungal activity against other test fungi (A. niger, A. awamori and Penicillium sp.) was moderate. The inhibitory effect of crude bacteriocin substance of B. methylotrophicus BM47 on the Gram-negative bacterium P. aeruginosa ATCC 9027 also was moderate. Gram-positive bacteria E. faecalis ATCC 19433 and S. aureus ATCC 25923, Gram-negative bacteria E. coli ATCC 8739 and Salmonella sp., yeast S. cerevisiae, as well as fungi A. oryzae and Rhizopus sp. remained unaffected.
Table 2. In vitro antimicrobial activity of crude bacteriocin substance of Bacillus methylotrophicus strain BM47.
| Test microorganism | Activity/U |
|---|---|
| Aspergillus niger | 84.80±0.01 |
| Aspergillus awamori | 172.70±0.05 |
| Aspergillus oryzae | 0 |
| Fusarium moniliforme | 285.70±0.04 |
| Rhizopus sp. | 0 |
| Penicillium sp. | 125.60±0.01 |
| Salmonella sp. | 0 |
| Pseudomonas aeruginosa ATCC 9027 | 198.60±0.02 |
| Enterococcus faecalis ATCC 19433 | 0 |
| Escherichia coli ATCC 8739 | 0 |
| Staphylococcus aureus ATCC 25923 | 0 |
| Saccharomyces cerevisiae | 0 |
Activity values are expressed as units (U)±standard deviation (S.D.) (N=3)
The antifungal potential of B. methylotrophicus BM47 was also studied in our previous works, where the strain demonstrated significant inhibitory effect on some plant pathogenic fungi of agricultural and economic importance such as Aspergillus flavus, Fusarium oxysporum (8) and Botrytis cinerea (9). The results revealed the promising potential of B. methylotrophicus BM47 as a producer of biologically active compounds (bacteriocins) for application in agriculture for plant disease control and prevention.
The antifungal properties of B. methylotrophicus have also been investigated by Ge et al. (17), stating that B. methylotrophicus strain NKG-1 was an effective and environmentally friendly means to increase the crop yields and prevent the outbreaks of plant pathogens such as Botryosphaeria dothidea, Phyllosticta ampelicida, Valsa ceratosperma, B. cinerea, Pyricularia oryzae, Gloeosporium capsici, Fusarium graminearum, Colletotrichum lagenarium, Fulvia fulva, Alternaria alternata, Rhizoctonia cerealis, F. oxysporum and Bipolaris maydis.
Antimicrobial activity of crude bacteriocin substance affected by enzymes, temperature, pH, organic solvents and detergents
The results summarized in Table 3 showed that the enzyme catalase did not affect the antimicrobial activity of crude bacteriocin substance, which means that the antimicrobial action is not due to hydrogen peroxide production. Trypsin, papain and Esperase® partially inhibited the crude bacteriocin substance, resulting in a decrease of the antimicrobial activity to 85.9%. Alcalase®, Savinase® and proteinase K also partially inhibited the crude bacteriocin substance, but the antimicrobial activity decreased to 72.7%, compared to the activity of the untreated crude bacteriocin substance serving as a positive control (100%). The negative controls (enzymes plus buffer, enzymes plus LBG broth, LBG broth alone and buffer alone) did not show antimicrobial activity.
Table 3. Effects of enzymes, temperature, pH, organic solvents and detergents on the antimicrobial activity of crude bacteriocin substance of Bacillus methylotrophicus BM47 against Fusarium moniliforme.
| Treatment | Residual activity/% | |
|---|---|---|
| Enzyme | ||
| Alcalase | 72.70±0.03 | |
| Savinase | 72.70±0.01 | |
| Proteinase K | 72.70±0.04 | |
| Trypsin | 85.90±0.03 | |
| Papain | 85.90±0.02 | |
| Esperase | 85.90±0.01 | |
| Catalase | 100 | |
| Temperature/°C | t/min | |
| 45 | 15 | 42.70±0.04 |
| 60 | 15 | 33.60±0.05 |
| 75 | 15 | 17.80±0.01 |
| 90 | 15 | 11.00±0.02 |
| 100 | 15 | 0 |
| 121 | 15 | 0 |
| pH | ||
| 3 | 42.70±0.05 | |
| 4 | 63.20±0.02 | |
| 5 | 74.70±0.01 | |
| 6 | 87.00±0.03 | |
| 7 | 100 | |
| 8 | 100 | |
| 9 | 63.20±0.04 | |
| 10 | 63.20±0.01 | |
| 11 | 42.70±0.02 | |
| Organic solvent and detergent | ||
| Methanol | 63.20±0.05 | |
| Isopropanol | 0 | |
| Acetonitrile | 0 | |
| Acetone | 42.70±0.03 | |
| EDTA | 0 | |
| Tween 20 | 0 | |
| Tween 60 | 0 | |
| Tween 80 | 0 | |
| Untreated crude bacteriocin substance | 100 | |
Residual activity values are expressed as percentage±standard deviation (S.D.) (N=3)
The crude bacteriocin substance was heat-labile, preserving only 42.7% of its initial activity after 15 min of exposure to 45 °C and a lower activity at higher temperatures. Full loss of antimicrobial activity was observed at 100 °C for 15 min. The crude bacteriocin substance also remained active at all of the tested pH values, in which the residual antimicrobial activity varied between 42.7 and 100%. The crude bacteriocin substance was sensitive to the action of the most organic solvents and detergents, such as isopropanol, acetonitrile, EDTA, Tween 20, Tween 60 and Tween 80, which completely inhibited its antimicrobial activity. The organic solvents methanol and acetone partially inhibited the antimicrobial activity of the crude bacteriocin substance and it decreased to 63.2 and 42.7% respectively (Table 3).
The results of antimicrobial activity and biochemical characterisation of lipopeptides (surfactins, iturins and fengycins) of B. methylotrophicus strain 39b reported by Frikha-Gargouri et al. (18) demonstrated that these compounds were thermostable, maintaining 100% activity against the phytopathogenic strains of Agrobacterium tumefaciens C58 and B6 at 70 °C, and 90% at 100 °C after incubation for 15 min. The effect of pH variation on the activity of these antimicrobial compounds showed that they were pH-stable, which is also important feature for their potential biocontrol application.
Significant antibacterial and antifungal activity against A. niger, A. flavus, Pythium ultiumum, Fusarium solani and Rhizoctonia bataticola, but different physicochemical properties of lipopeptides of B. methylotrophicus strain DCS1 were reported by Jemil et al. (19). In addition to their high pH and heat resistance, these lipopeptides retained their antibacterial activity against the indicator strain Klebsiella pneumoniae after incubation for 1 h with proteolytic enzymes pepsin, trypsin and chymotrypsin at a concentration of 1 mg/mL. The maintenance of antimicrobial activity after treatment with proteolytic enzymes or high temperature resembles the characteristics of cyclic lipopeptides synthesized by Bacillus sp., which contain unusual amino acids (20) and this could be used as a prerequisite for their potential food, pharmaceutical and therapeutic applications.
Bacteriocin purification and molecular mass determination
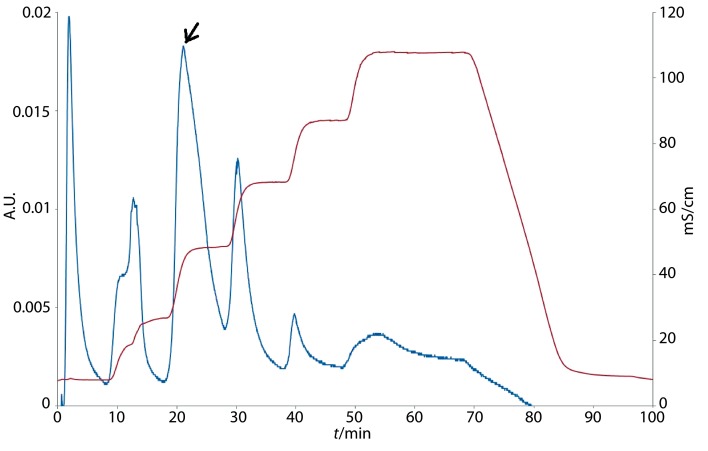
The purification of the crude bacteriocin substance and separation of the protein fractions were performed by fast protein liquid chromatography (FPLC) on a strong anion exchange column High Q (Bio-Rad). The active protein fractions 5 and 6 coincided with the highest inhibitory effect of crude bacteriocin substance on the indicator microorganism F. moniliforme with antimicrobial activity of 148.3 U (fraction 5) and 198.6 U (fraction 6), and corresponded to the bacteriocin elution (Fig. 1).
Fig. 1.
Fast protein liquid chromatogram obtained during the final step of purification of Bacillus methylotrophicus BM47 bacteriocin (the active protein fractions 5 and 6 are indicated by an arrow)
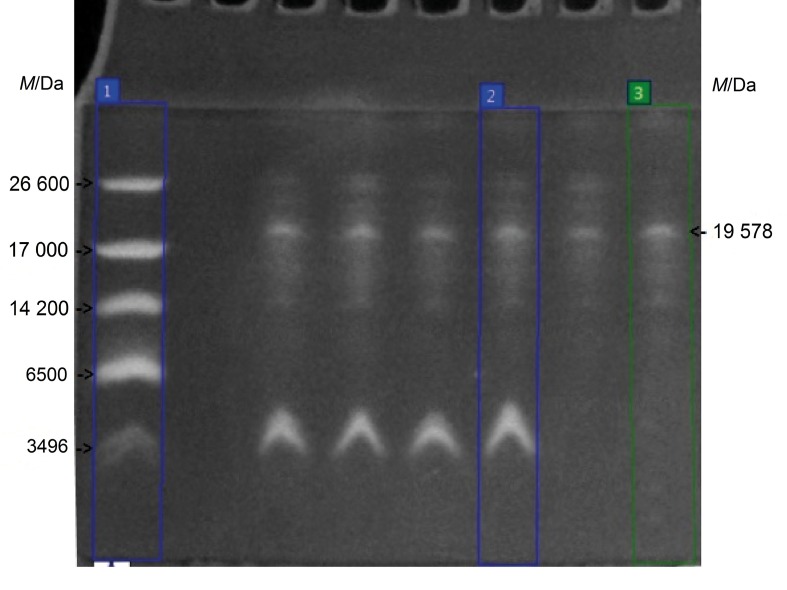
The estimation of the apparent molecular mass of the purified bacteriocin of B. methylotrophicus BM47 (Fig. 2) was performed by tricine-SDS-PAGE, where the low molecular mass protein markers were applied on lane 1, the sample of crude bacteriocin substance (after lyophilization and dialysis) of B. methylotrophicus strain BM47 was applied on lane 2, the sample of combined protein fractions 5 and 6 was applied on lane 3. The analysis of the obtained electrophoregram was carried out using TotalLab software (14). The presence of a single protein band in lane 3 with molecular mass of 19 578 Da was detected and classified as a peptide of intermediate size (10–30 kDa). The protein band of the bacteriocin corresponded to the band of crude bacteriocin substance control after lyophilization and dialysis, shown in lane 2. For the sake of clarity, the calculation of the molecular mass of proteins in the samples was performed towards the low molecular mass protein markers referenced in lane 1.
Fig. 2.
Electropherogram of the bacteriocin of Bacillus methylotrophicus BM47 and controls: lane 1=low molecular mass protein markers, lane 2=crude bacteriocin substance (after lyophilization and dialysis), lane 3=purified bacteriocin (combined protein fractions 5 and 6)
Amino acid composition of the purified bacteriocin
As seen from the results in Table 4, the amino acid analysis performed by high-performance liquid chromatography (HPLC) demonstrated that the bacteriocin produced by B. methylotrophicus strain BM47 consisted of 17 types of amino acids, with the highest mol fraction expressed as percent of serine (29.3), valine (10.3), alanine (9.8) and tyrosine (7.1).
Table 4. Mole fractions of amino acids expressed as percent in the purified bacteriocin of Bacillus methylotrophicus strain BM47.
| Amino acid | X(amino acid)/% |
|---|---|
| Asparagine | 4.4 |
| Serine | 29.3 |
| Glutamine | 2.3 |
| Glycine | 6.0 |
| Histidine | 4.4 |
| Arginine | 0.8 |
| Threonine | 3.7 |
| Alanine | 9.8 |
| Proline | 2.6 |
| Cysteine | 3.1 |
| Tyrosine | 7.1 |
| Valine | 10.3 |
| Methionine | 4.7 |
| Lysine | 6.1 |
| Isoleucine | 3.8 |
| Leucine | 0.3 |
| Phenylalanine | 1.3 |
| Total | 100 |
Presently, the isolation, FPLC/HPLC purification and molecular characterization of antimicrobial peptides synthesized by B. methylotrophicus strains have not yet been sufficiently investigated; therefore, the lack of data about the characteristics of these compounds significantly hampers the interpretation of our results and their comparison to others. The existing reports in the scientific literature consider only the metabolites of lipopeptide nature (18, 19) or the genes encoding the biosynthesis of some antimicrobial compounds (7), but data concerning the amino acid composition of peptides produced by B. methylotrophicus strains are still not available. Therefore, the antimicrobial peptide produced by B. methylotrophicus strain BM47 should be a novel bacteriocin. Although there are some disadvantages (low antibacterial activity and thermal instability), the bacteriocin of B. methylotrophicus BM47 possesses high antifungal activity and all the necessary properties to be attractive and perspective for practical applications in different biotechnological processes.
CONCLUSION
This is the first report describing the isolation, characterization and amino acid composition of an antimicrobial peptide produced by Bacillus methylotrophicus. The bacteriocin isolated from the strain Bacillus methylotrophicus BM47 demonstrated promising antifungal activity and a moderate stability to different environmental conditions, which makes it suitable for application in the agriculture as a perspective agent for biocontrol and plant protection as well as in the food industry as biopreservative against food spoilage microorganisms of fungal origin.
Footnotes
FUNDING: This work was financed by Fund ’Science‘ of the University of Food Technologies, Plovdiv, Bulgaria (Grant 06/16-H).
CONFLICT OF INTEREST: The authors declare that no conflict of interest exists. This article does not contain any studies related to human participants or animals.
REFERENCES
- 1.Bhuvaneswari S, Madhavan S, Panneerselvam A. Optimization of bacteriocin production by Bacillus subtilis BMP01 isolated from Solanum trilobatum L. Int J Curr Microbiol Appl Sci. 2015;4(3):617–26. [Google Scholar]
- 2.Abriouel H, Franz C, Omar N, Galvez A. Diversity and applications of Bacillus bacteriocins [review]. FEMS Microbiol Rev. 2011;35:201–32. 10.1111/j.1574-6976.2010.00244.x [DOI] [PubMed] [Google Scholar]
- 3.Cotter PD, Hill C, Ross R. Bacteriocins: Developing innate immunity for food. Nat Rev Microbiol. 2005;3(10):777–88. 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- 4.Yang SC, Lin CH, Sung CT, Fang JY. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front Microbiol. 2014;5:241. 10.3389/fmicb.2014.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramanian S, Smith DL. Bacteriocins from the rhizosphere microbiome – from an agriculture perspective. Front Plant Sci. 2015;6:909. 10.3389/fpls.2015.00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dev Sharma S, Shovon M, Sarowar Jahan M, Asaduzzaman A, Rahman M, Biswas K, et al. Antibacterial and cytotoxic activity of Bacillus methylotrophicus-SCS2012, isolated from soil. J Microbiol Biotechnol Food Sci. 2013;2(4):2293–307. [Google Scholar]
- 7.Dias L, Caetano T, Pinheiro M, Mendo S. The lanthipeptides of Bacillus methylotrophicus and their association with genomic islands. Syst Appl Microbiol. 2015;38(8):525–33. 10.1016/j.syapm.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 8.Tumbarski Y, Petkov E, Denkova Z. Study on the influence of the cultural conditions and the composition of culture medium on the antimicrobial activity of Bacillus methylotrophicus BM47 against some fungal phytopathogens. J Glob Biosci. 2015;4(8):2990–6. [Google Scholar]
- 9.Tumbarski Y. Antimicrobial activity of Bacillus methylotrophicus BM47 against Botrytis cinerea in different conditions of cultivation. Bulg J Vet Med. 2017;20 Suppl.1:89–94. [Google Scholar]
- 10.Ivanov I, Petkova N, Tumbarski J, Dincheva I, Badjakov I, Denev P, et al. GC-MS characterization of n-hexane soluble fraction from dandelion (Taraxacum officinale Weber ex F.H. Wigg.) aerial parts and its antioxidant and antimicrobial properties. Z Naturforsch. 2018;73(1–2):41–7. 10.1515/znc-2017-0107 [DOI] [PubMed] [Google Scholar]
- 11.Delgado A, Brito D, Fevereiro P, Tenreiro R, Peres C. Bioactivity quantification of crude bacteriocin solutions. J Microbiol Methods. 2005;62(1):121–4. 10.1016/j.mimet.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Xie J, Zhang R, Shang C, Guo Y. Isolation and characterization of a bacteriocin produced by an isolated Bacillus subtilis LFB112 that exhibits antimicrobial activity against domestic animal pathogens. Afr J Biotechnol. 2009;8(20):5611–9. [Google Scholar]
- 13.Schägger H. Tricine–SDS-PAGE. Nat Protoc. 2006;1(1):16–22. 10.1038/nprot.2006.4 [DOI] [PubMed] [Google Scholar]
- 14.TotalLab Software. CLIQS, v. 1.1, Newcastle upon Tyne, UK; 2015. Available from: https://www.totallab.com/home/cliqs.
- 15.EZChrom Elite™ Software, Agilent Technologies Inc., v. 3.2.1 (Build 31b), Santa Clara, California, USA; 2007. Available from: https://www.agilent.com.
- 16.Dhillon MK, Kumar S, Gujar G. A common HPLC-PDA method for amino acid analysis in insects and plants. Indian J Exp Biol. 2014;52(1):73–9. [PubMed] [Google Scholar]
- 17.Ge B, Liu B, Nwet T, Zhao W, Shi L, Zhang K. Bacillus methylotrophicus strain NKG-1, isolated from Changbai Mountain, China, has potential applications as a biofertilizer or biocontrol agent. PLoS One. 2016;11(11):e0166079. 10.1371/journal.pone.0166079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frikha-Gargouri O, Ben Abdallah D, Ghorbel I, Charfeddine I, Jlaiel L, Ali Triki M, et al. Lipopeptides from a novel Bacillus methylotrophicus 39b strain suppress Agrobacterium crown gall tumours on tomato plants. Pest Manag Sci. 2017;73:568–74. 10.1002/ps.4331 [DOI] [PubMed] [Google Scholar]
- 19.Jemil N, Ayed H, Manresa A, Nasri M, Hmidet N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. 2017;17(1):144. 10.1186/s12866-017-1050-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56(4):845–57. 10.1111/j.1365-2958.2005.04587.x [DOI] [PubMed] [Google Scholar]