Abstract
Background:
Early palliative care can reduce end-of-life acute-care use, but findings are mainly limited to cancer populations receiving hospital interventions. Few studies describe how early versus late palliative care affects end-of-life service utilization.
Aim:
To investigate the association between early versus late palliative care (hospital/community-based) and acute-care use and other publicly funded services in the 2 weeks before death.
Design:
Retrospective population-based cohort study using linked administrative healthcare data.
Setting/participants:
Decedents (cancer, frailty, and organ failure) between 1 April 2010 and 31 December 2012 in Ontario, Canada. Initiation time before death (days): early (⩾60) and late (⩾15 and <60). ‘Acute-care settings’ included acute-hospital admissions with (‘palliative-acute-care’) and without palliative involvement (‘non-palliative-acute-care’).
Results:
We identified 230,921 decedents. Of them, 27% were early palliative care recipients and 13% were late; 45% of early recipients had a community-based initiation and 74% of late recipients had a hospital-based initiation. Compared to late recipients, fewer early recipients used palliative-acute care (42% vs 65%) with less days (mean days: 9.6 vs 12.0). Late recipients were more likely to use acute-care settings; this was further modified by disease: comparing late to early recipients, cancer decedents were nearly two times more likely to spend >1 week in acute-care settings (odds ratio = 1.84, 95% confidence interval: 1.83–1.85), frailty decedents were three times more likely (odds ratio = 3.04, 95% confidence interval: 3.01–3.07), and organ failure decedents were four times more likely (odds ratio = 4.04, 95% confidence interval: 4.02–4.06).
Conclusion:
Early palliative care was associated with improved end-of-life outcomes. Late initiations were associated with greater acute-care use, with the largest influence on organ failure and frailty decedents, suggesting potential opportunities for improvement.
Keywords: Palliative care, hospitalization, health services, end-of-life care, cohort studies, administrative claims, healthcare, Canada
What is already known about the topic?
Early palliative care has been shown to reduce acute care service use at the end of life, but findings are mostly limited to cancer patients receiving hospital-based palliative care interventions.
Recent studies show that both hospital- and community-based palliative care are associated with improved end-of-life outcomes, but never investigated the association between early versus late palliative care and end-of-life service utilization.
What this paper adds?
Includes palliative care services initiated in both hospital and community-based settings.
Early initiation of palliative care was associated with reduced acute-hospital use in the last 2 weeks of life.
Compared to cancer decedents, late palliative care had a notably greater association with increased acute care use in organ failure and frailty decedents.
Implications for practice, theory or policy
Clear disparities exist in palliative care timing for non-cancer decedents, suggesting that these populations may reap greater benefits if identified earlier. Addressing this gap would ultimately help reduce costly end-of-life acute-care service use.
Future research should examine effective interventions that would allow for earlier identification of patients (including cancer and non-cancer) who may benefit from timely palliative care. Differences in underlying characteristics of early and late palliative care recipients should also be further investigated.
Introduction
End-of-life discussions and interventions to control advanced symptoms often occur only during the last few weeks of life. This late initiation is often also associated with care that is primarily delivered in hospital settings—the default place of care when community-based care (i.e. in patient’s homes) is not established early and adequately. Palliative care that is earlier on in the course of one’s disease—and even concurrently with active treatments—can drastically improve symptom control, reduce distress experienced from standard therapies,1–3 and can fulfill the wishes of many patients who prefer home-based care.4–7 Consequently, policymakers have made a push toward supporting more patients at home during end-of-life—a widely used administrative indicator of end-of-life quality that also strives to reduce acute-care service use.8–11
Past randomized controlled trials have illustrated that early palliative care is associated with better end-of-life outcomes.12–14 For instance, a landmark study by Temel et al.13 demonstrated that early palliative care delivered concurrently with standard oncologic care was associated with improved quality of life, reduced depressive symptoms, longer survival rates, and less aggressive care at the end of life. Although informative, results from these trials were limited to cancer patients who received hospital-based palliative care interventions. Recent research shows that community-based palliative care may also lead to improvements—such as reduced acute-hospital use and hospital deaths—but never investigated the impact of early versus late palliative care on end-of-life service use and mainly focused on small populations (mostly cancer) receiving care from a particular setting.15–24 Furthermore, a large abundance of existing palliative care research uses late-life acute hospitalizations as an outcome to indicate poor quality care. However, not all hospitalizations are considered inappropriate as some involve a palliative care approach; despite this, most research does not differentiate between those who did and did not receive palliative care in acute settings.
To address these knowledge gaps, we conducted a population-based retrospective cohort study of cancer and non-cancer Ontario decedents to investigate the association between early versus late palliative care and acute-hospital use in the last 2 weeks of life. Our study specifically provides information on acute-hospital use with and without palliative care involvement during the hospitalization. We also report on all other end-of-life services used in a publicly funded healthcare system and assess disease-specific trends (frailty, organ failure, cancer). Our study advances prior work by investigating the association between palliative care (both hospital- and community-based) timing and end-of-life service use, which can inform other countries with similar or different healthcare systems.
Methods
Study design and data sources
We conducted a retrospective cohort study of Ontario decedents aged 18 years or older, capturing all deaths from 1 April 2010 to 31 December 2012. To identify all services used across several health sectors in the last 2 weeks of life (defined as: 1–14 days before death + date of death (day 0)), patient data were linked using multiple administrative databases held at ICES25, including the Vital Statistics Database (Office of the Registrar General—Deaths), which captured place, cause, and date of death; Registered Persons Database, which captured all demographic information including sex, age, and postal code; Ontario Health Insurance Plan Claims Database, which captured all claims data for physician services in both inpatient and outpatient settings; Home Care Database, capturing publicly funded home care services; Discharge Abstract Database, capturing all acute-care use, including acute care with and without palliative care (identified using a previously derived comprehensive list of palliative care billing codes);26,27 National Ambulatory Care Reporting System, which captured all emergency department visits; Continuing Care Reporting System, capturing care provided in long-term care and complex continuing care (i.e. equivalent to subacute care settings); and Statistics Canada Census data, which captured income quintile and rurality via postal codes.28
Five distinct categories exist for causes of death: terminal illness (e.g. cancer), organ failure (e.g. chronic heart failure), frailty (e.g. Alzheimer’s disease), sudden death (e.g. accident), and other;28–30 these cohorts have been previously used in Canada.31,32 In this study, we refer to these categories as ‘disease cohorts’. Decedents were assigned to a disease cohort based on the underlying cause of death code (10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10)-CA diagnosis code) found in the vital statistics records, as defined previously.31 For clarity, we replaced the label ‘terminal illness’ with ‘cancer’ since the majority of individuals in this disease cohort had a cancer-related death. Decedents in the ‘sudden death’ and ‘other’ cohorts were excluded in our analyses due to their small numbers and because of the diminished potential role of palliative care in many cases.
Exposure
The main exposure was time from first palliative care initiation to death from hospital or community, defined as the first instance of any palliative care service captured in the last year of life. We used a previously derived comprehensive list of palliative care billing codes to identify each individual’s date of palliative care initiation prior to death.27 The timing of the exposure was calculated by finding the difference (in days) between an individual’s date of death and date of palliative care initiation. We categorized decedents into the following recipient groups, according to initiation time before death: early (⩾60 days), late (⩾15 to <60 days), very late (⩾0 to ⩽14 days), and never (no initiation). Cut-offs for these categories were chosen based on expert opinion consensus and previous literature proposing similar timeframes for defining palliative care receipt.33,34 Note that we mainly focus on comparing early versus late recipients and exclude ‘very late’ recipients from much of our analyses; this was done to avoid confounding issues due to overlap with the outcome period (i.e. it would be unclear if palliative care was initiated prior to or after use of acute care within the last 2 weeks of life).
Outcomes
The primary outcome was use of acute-care and community services during the last 2 weeks of life. We classified these services by care settings. Acute-care settings were composed of (1) ‘palliative-acute care’, defined as an acute-hospital admission that had palliative involvement, and (2) ‘non-palliative-acute care’, defined as an acute-hospital admission without any palliative involvement.27 Other outcomes we examined include subacute care, emergency department, and community-based care (home care, home-based physician visits, and outpatient physician encounters). Within acute-care admissions, all days prior to discharge were counted as a palliative care day (i.e. deemed palliative-acute care) for the entire duration of stay when a decedent had a pre-admitting condition listed as palliative care or the most responsible diagnosis for the hospital stay was also palliative, the main service provider was palliative, or palliative care was consulted for the largest portion of their hospital stay. For all remaining palliative-acute-care encounters, only a single day of the hospitalization was counted as a palliative care day (e.g. individuals initially admitted as acute-care patients but later received a palliative diagnosis at some point during their hospital stay). This approach indirectly captures designated palliative care unit beds in acute hospitals and also palliative care services provided when another admitting service was the main provider service.
Statistical analysis
Descriptive statistics were used to compare cohort characteristics between early versus late palliative care recipients. Characteristics include sex, age, income quintile, rurality, chronic diseases, number of comorbidities, place of death, mean and median time to first palliative care initiation before death, and palliative care initiation sector. Multivariable logistic regression analyses were used to predict in the 2 weeks before death: the likelihood of using an acute-care setting and the likelihood of spending >1 week in acute-care settings. We adjusted for the following covariates in the models: sex, age, income quintile, rurality, and number of comorbidities. Ethics approval for this study was received from the Ottawa Hospital Research Institute Ethics Board in Ottawa, Canada. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA).
Results
We identified 230,921 decedents during the study period, who spent an average of 5.1 days in acute-care settings (of whom 60% had at least one service day in the last 2 weeks of life). Overall, 33% of decedents died from cancer, 31% from organ failure, 29% from frailty, 3% had a sudden death, and 5% from other causes. Almost half (46%) of decedents never received palliative care, and the remaining decedents were split by early palliative care (27%) and late or very late recipients (27%; Table 1). The majority of early and late recipients died from cancer (67% and 53%, respectively), while a large portion of very late recipients died from organ failure (40%). Notably, more than half of cancer decedents were early recipients (56%). Overall, 61% of the study population experienced a hospital-based death, and more late recipients (73%) died in hospital compared to early recipients (60%) (Table 2).
Table 1.
Cohort characteristics.
| Characteristic | Early |
Late |
Very Late |
Never |
Overall |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |
| Overall | 62,728 | 27 | 29,710 | 13 | 31,549 | 14 | 106,934 | 46 | 230,921 | 100 |
| Disease cohort | ||||||||||
| Frailty | 7893 | 12 | 5231 | 8 | 8202 | 12 | 45,467 | 68 | 66,793 | 29 |
| Organ failure | 11,103 | 16 | 7480 | 11 | 12,511 | 18 | 39,596 | 56 | 70,690 | 31 |
| Cancer | 42,255 | 56 | 15,672 | 21 | 8422 | 11 | 8841 | 12 | 75,190 | 33 |
| Sudden death | 166 | 2 | 162 | 2 | 344 | 5 | 6781 | 91 | 7453 | 3 |
| Other | 1311 | 12 | 1165 | 11 | 2070 | 19 | 6249 | 58 | 10,795 | 5 |
| Sex | ||||||||||
| Female | 32,081 | 27 | 14,940 | 13 | 16,752 | 14 | 54,850 | 46 | 118,623 | 51 |
| Male | 30,647 | 27 | 14,770 | 13 | 14,797 | 13 | 52,084 | 46 | 112,298 | 49 |
| Age (years) | ||||||||||
| 18–44 | 1493 | 21 | 391 | 6 | 347 | 5 | 4788 | 68 | 7019 | 3 |
| 45–64 | 13,057 | 35 | 4409 | 12 | 3455 | 9 | 16,234 | 44 | 37,155 | 16 |
| 65–84 | 32,041 | 31 | 14,692 | 14 | 14,302 | 14 | 43,276 | 41 | 104,311 | 45 |
| 85 + | 16,137 | 20 | 10,218 | 12 | 13,445 | 16 | 42,636 | 52 | 82,436 | 36 |
| Income a | ||||||||||
| Lowest | 13,362 | 25 | 6513 | 12 | 7347 | 14 | 25,540 | 48 | 52,762 | 23 |
| Low | 13,116 | 27 | 6311 | 13 | 6669 | 14 | 22,099 | 46 | 48,195 | 21 |
| Middle | 11,983 | 27 | 5818 | 13 | 5962 | 13 | 20,720 | 47 | 44,483 | 19 |
| High | 12,141 | 28 | 5663 | 13 | 5919 | 14 | 19,689 | 45 | 43,412 | 19 |
| Highest | 11,858 | 29 | 5267 | 13 | 5465 | 13 | 18,081 | 44 | 40,671 | 18 |
| Ruralitya | ||||||||||
| Urban | 53,978 | 27 | 25,780 | 13 | 27,221 | 14 | 89,977 | 46 | 196,956 | 85 |
| Rural | 8689 | 26 | 3897 | 12 | 4288 | 13 | 16,745 | 50 | 33,619 | 15 |
| Chronic diseases | ||||||||||
| Hypertension | 45,150 | 26 | 22,538 | 13 | 25,051 | 14 | 80,611 | 47 | 173,350 | 75 |
| Osteoarthritis | 31,523 | 28 | 14,658 | 13 | 15,872 | 14 | 52,080 | 46 | 114,133 | 49 |
| Cancer | 47,786 | 47 | 18,516 | 18 | 12,441 | 12 | 23,296 | 23 | 102,039 | 44 |
| Diabetes | 21,735 | 26 | 10,398 | 13 | 11,380 | 14 | 38,871 | 47 | 82,384 | 36 |
| Congestive heart failure | 18,305 | 23 | 9415 | 12 | 12,273 | 16 | 38,882 | 49 | 78,875 | 34 |
| Coronary heart disease | 18,422 | 24 | 9088 | 12 | 11,127 | 15 | 37,309 | 49 | 75,946 | 33 |
| Dementia | 12,132 | 18 | 7159 | 11 | 10,099 | 15 | 36,970 | 56 | 66,360 | 29 |
| COPD | 15,866 | 27 | 7373 | 13 | 9005 | 15 | 26,393 | 45 | 58,637 | 25 |
| Renal disease | 14,703 | 27 | 7399 | 13 | 9245 | 17 | 23,986 | 43 | 55,333 | 24 |
| No. of comorbidities | ||||||||||
| 0 | 131 | 2 | 163 | 3 | 298 | 5 | 4855 | 89 | 5447 | 2 |
| 1–2 | 12,297 | 29 | 5346 | 12 | 4722 | 11 | 20,777 | 48 | 43,142 | 19 |
| 3–5 | 31,082 | 28 | 15,284 | 14 | 15,579 | 14 | 48,664 | 44 | 110,609 | 48 |
| 6+ | 19,218 | 27 | 8917 | 12 | 10,950 | 15 | 32,638 | 46 | 71,723 | 31 |
Does not equal 100%: a very small number of records are missing this information.
Table 2.
Palliative care delivery and place of death.
| Characteristic | Early |
Late |
Very late |
Never |
Overall |
|---|---|---|---|---|---|
| N = 62,728 | N = 29,710 | N = 31,549 | N = 106,934 | N = 230,921 | |
| Place of palliative care initiation (%) | |||||
| Hospital | 54 | 74 | 83 | N/A | 35 |
| Long-term care | 0.2 | 0.3 | 0.8 | N/A | 0.2 |
| Community | 45 | 26 | 16 | N/A | 18 |
| Initiation time before death (days) | |||||
| Mean, median (IQR) | 210, 201 (116, 307) | 32, 30 (21, 42) | 6, 6 (3, 9) | N/A | 114, 59 (13, 200) |
| Place of death (%) | |||||
| Hospital | 60 | 73 | 83 | 52 | 61 |
| Long-term care | 11 | 10 | 11 | 28 | 19 |
| Community | 29 | 17 | 6 | 21 | 20 |
Palliative care initiations
Early recipients initiated palliative care at a mean time of 210 days prior to death, compared with a mean of 32 days for late recipients (Table 2). Overall, 45% of early recipients initiated in a community-based setting, which was almost two times greater than the proportion of late recipients (26%). Late recipients had considerably more hospital-based initiations (74%) when compared to early recipients (54%). Disease-specific differences show that organ failure and frailty decedents had the most hospital-based initiations (82% and 73%, respectively), while cancer decedents had the most community-based initiations (44%).
Place of care utilization trends (among service users)
About 63% of early recipients used an acute-care setting at least once in the last 2 weeks of life (spent 9.2 mean days), compared to 80% of late recipients (spent 11.7 mean days; Table 3). Early and late recipients had a similar proportion of non-palliative-acute-care users (26% and 23%, respectively), with similar days of service use (6.4 and 6.8 mean days, respectively). Compared to late recipients, fewer early recipients used palliative-acute care (65% vs 42%) and spent less service days (9.6 vs 12 mean days) in the last 2 weeks of life. In addition, early recipients made more use of community-based care, having almost double the percentage of individuals receiving home-based physician visits compared to late recipients (28% vs 17%).
Table 3.
Place of care utilization by palliative care initiation time before death (among users).
| Place of care | Palliative care initiation time |
||
|---|---|---|---|
| Early |
Late |
Never |
|
| (N = 62,728) | (N = 29,728) | (N = 106,934) | |
| Care in hospitals/institutions | |||
| Acute-care settings: mean days (% users) | 9.2 (62.5) | 11.7 (79.8) | 7.3 (44.6) |
| Non-palliative-acute care: mean days (% users) | 6.4 (26.4) | 6.8 (22.8) | 7.3 (44.6) |
| Palliative-acute care: mean days (% users) | 9.6 (42.3) | 12 (65.2) | 0 (0) |
| Emergency department: mean days (% users) | 1.8 (32.5) | 1.6 (25.3) | 1.6 (44.6) |
| Subacute care: mean days (% users) | 11.3 (16.9) | 9.6 (13.6) | 12.2 (3.6) |
| Long-term care: mean days (% users) | 13.3 (11.6) | 12.8 (9.9) | 14.1 (32) |
| Care in the community | |||
| Home care: mean days (% users) | 7.4 (55.4) | 5.9 (42.5) | 4.9 (19.2) |
| Home-based physician visit: mean days (% users) | 2.4 (28) | 2.1 (16.6) | 1.1 (7.5) |
| Outpatient physician encounter: mean days (% users) | 2.9 (69.9) | 2.8 (69.5) | 1.6 (43.5) |
Multivariable analyses
When examining the odds of using acute-care settings, late recipients from each disease cohort have a higher odds ratio (OR; cancer: OR = 2.31, 95% confidence interval (CI): 2.30–2.32, frailty: OR = 3.05, 95% CI: 3.03–3.07, organ failure: OR = 3.25, 95% CI: 3.23–3.27) compared to early recipients, controlling for covariates (Table 4). Similarly, when examining the odds of spending >1 week in acute-care settings during the last 2 weeks of life, late recipients have a higher OR (cancer: OR = 1.84, 95% CI: 1.83–1.85, frailty: OR = 3.04, 95% CI: 3.01–3.07, and organ failure: OR = 4.04, 95% CI: 4.02–4.06) compared to early recipients. An increasing number of comorbidities was also associated with increased odds of using acute-care settings and increased odds of spending >1 week in acute-care settings—especially for those with frailty.
Table 4.
Multivariate logistic regression: (1) odds of ever using acute-care settings in the last 2 weeks of life, and (2) odds of spending >1 week in acute-care settings in the last 2 weeks of life.
| Exposure | Odds of ever using acute-care settings in the last 2 weeks of life |
Odds of spending >1 week in acute-care settings in the last 2 weeks of life |
||||
|---|---|---|---|---|---|---|
| Cancer | Frailty | Organ failure | Cancer | Frailty | Organ failure | |
| Palliative care initiation time | ||||||
| Early | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Late | 2.31 (2.3, 2.32) | 3.05 (3.03, 3.07) | 3.25 (3.23, 3.27) | 1.84 (1.83, 1.85) | 3.04 (3.01, 3.07) | 4.04 (4.02, 4.06) |
| Never | 0.96 (0.95, 0.97) | 0.52 (0.51, 0.53) | 0.61 (0.6, 0.62) | 0.77 (0.75, 0.79) | 0.41 (0.39, 0.43) | 0.48 (0.47, 0.49) |
| Sex | ||||||
| Male | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Female | 0.8 (0.79, 0.81) | 0.66 (0.65, 0.67) | 0.76 (0.75, 0.77) | 1.06 (1.05, 1.07) | 0.87 (0.86, 0.88) | 0.87 (0.86, 0.88) |
| Age (years) | ||||||
| 18–44 | 1.88 (1.85, 1.91) | 1.57 (1.51, 1.63) | 1.41 (1.38, 1.44) | 1.29 (1.26, 1.32) | 1.52 (1.43, 1.61) | 1.06 (1.02, 1.1) |
| 45–64 | 1.4 (1.39, 1.41) | 1.06 (1.04, 1.08) | 1.16 (1.15, 1.17) | 1 (0.99, 1.01) | 0.88 (0.85, 0.91) | 1.09 (1.07, 1.11) |
| 65–84 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 85+ | 0.6 (0.59, 0.61) | 0.61 (0.6, 0.62) | 0.52 (0.51, 0.53) | 0.95 (0.94, 0.96) | 0.84 (0.82, 0.86) | 0.82 (0.81, 0.83) |
| Income | ||||||
| Lowest | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Low | 0.96 (0.95, 0.97) | 1.04 (1.03, 1.05) | 1.07 (1.06, 1.08) | 1 (0.98, 1.02) | 0.97 (0.95, 0.99) | 0.95 (0.93, 0.97) |
| Middle | 0.9 (0.89, 0.91) | 0.97 (0.96, 0.98) | 1 (0.99, 1.01) | 0.98 (0.96, 1) | 0.95 (0.93, 0.97) | 0.93 (0.91, 0.95) |
| High | 0.9 (0.89, 0.91) | 0.95 (0.94, 0.96) | 1.05 (1.04, 1.06) | 0.94 (0.92, 0.96) | 0.99 (0.97, 1.01) | 0.96 (0.94, 0.98) |
| Highest | 0.82 (0.81, 0.83) | 0.94 (0.93, 0.95) | 1.02 (1.01, 1.03) | 0.95 (0.93, 0.97) | 0.92 (0.9, 0.94) | 0.88 (0.86, 0.9) |
| Rurality | ||||||
| Urban | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Rural | 1.11 (1.1, 1.12) | 0.99 (0.98, 1) | 0.95 (0.94, 0.96) | 1 (0.99, 1.01) | 0.92 (0.9, 0.94) | 0.91 (0.89, 0.93) |
| No. of comorbidities | ||||||
| 0 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 1 | 3.45 (3.38, 3.52) | 2.23 (2.18, 2.28) | 1.76 (1.72, 1.8) | 1.18 (1.06, 1.3) | 1.54 (1.44, 1.64) | 1.13 (1.07, 1.19) |
| 2 | 4.14 (4.07, 4.21) | 3.07 (3.02, 3.12) | 2.44 (2.41, 2.47) | 1.37 (1.25, 1.49) | 1.58 (1.49, 1.67) | 1.18 (1.13, 1.23) |
| 3 | 4.77 (4.7, 4.84) | 4.48 (4.44, 4.52) | 2.97 (2.94, 3) | 1.45 (1.33, 1.57) | 1.85 (1.76, 1.94) | 1.39 (1.34, 1.44) |
| 4 | 5.01 (4.94, 5.08) | 5.86 (5.82, 5.9) | 3.51 (3.48, 3.54) | 1.66 (1.54, 1.78) | 1.99 (1.9, 2.08) | 1.58 (1.53, 1.63) |
| 5 | 5.52 (5.45, 5.59) | 7.76 (7.72, 7.8) | 4.36 (4.33, 4.39) | 1.57 (1.45, 1.69) | 2.23 (2.14, 2.32) | 1.67 (1.62, 1.72) |
| 6 | 6.38 (6.31, 6.45) | 12.03 (11.99, 12.07) | 5.51 (5.48, 5.54) | 1.74 (1.62, 1.86) | 2.53 (2.44, 2.62) | 1.9 (1.85, 1.95) |
| Ref: Never Used Acute-Care Settings | Ref: Spent <1 Week in Acute-Care Settings | |||||
Place of care utilization trajectories
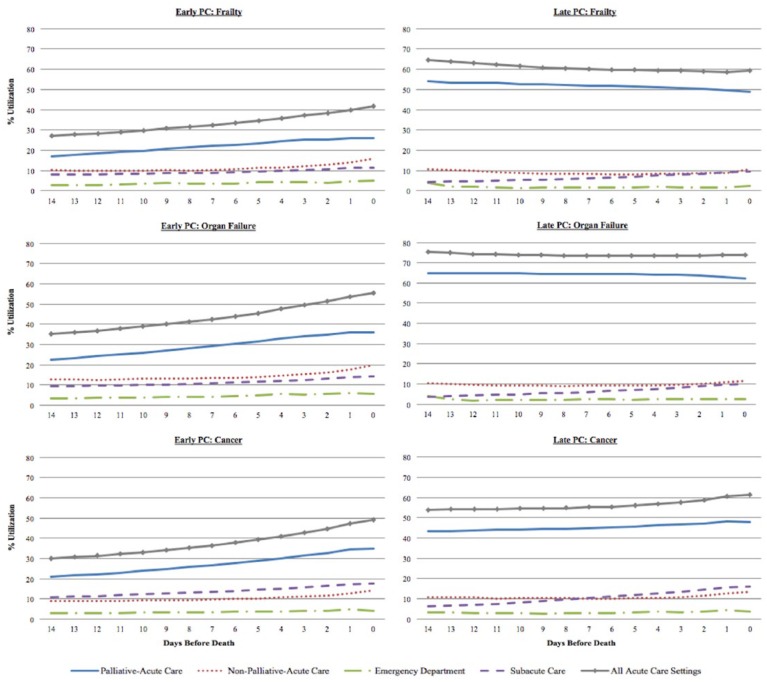
We examined the percentage of patients using hospital-based care on each day within the last 2 weeks of life by disease cohort and by early versus late initiation times (Figure 1). Late palliative care recipients used a consistently high proportion of acute-care services (palliative + nonpalliative) across the entire duration of the last 2 weeks of life (consistently over 50%). Although early recipients experienced notable increases in acute-care service use as death got closer, the proportion was always well below that of late recipients. These differing trends of acute-care service utilization in early versus late recipients were consistent across all three disease cohorts. Palliative-acute-care accounted for most of the acute-care service use for both early and late recipients.
Figure 1.
Disease-specific utilization trends of hospital-based care by palliative care initiation time.
Discussion
To our knowledge, this is the first study of its kind that uses population-based data from a universal healthcare system to study the association between early versus late palliative care and a rich set of services used at the end-of-life by both cancer and non-cancer patients. Our study findings show that early palliative care (as opposed to late palliative care) was associated with improved outcomes near the end of life. In the 2 weeks before death, early recipients had lower odds of using acute care and lower odds of spending >1 week in acute care compared to late recipients. Moreover, we found that early recipients made greater use of community-based services. Many early initiations occurred in a community-based setting, while late initiations occurred mainly in hospital. Early recipients had twice as many community-based deaths and 13% less hospital-based deaths; early recipients were largely receiving out-of-hospital care, such as within the home, while most late recipients remained hospitalized throughout the last 2 weeks of life.
Reducing end-of-life acute-care service use is an indicator of higher quality of care35 while lowering healthcare costs.36 Past research has also shown that early palliative care (defined variably, ranging from 1 to 6 months before death) is associated with reduced end-of-life acute-care service use. Seow and colleagues previously conducted a population-based analysis of Ontario decedents, showing that early home-based palliative care reduced the risk of needing acute care in the last 2 weeks of life.19 Several US cancer studies also highlight the benefits of early palliative care which include less aggressiveness at the end of life,13 fewer hospital admissions, and reduced hospital-based deaths.37,38 Similarly in Western Australia, earlier community-based palliative care was found to reduce acute-hospital stays,22 emergency department use,18 and unplanned hospitalizations.23 Moreover, a Singapore study found that earlier referrals to hospital-based palliative care was associated with a higher likelihood of dying out of hospital.39 Several European studies also reach similar conclusions.15,40
Frailty and organ failure decedents received a late initiation of palliative care more often than cancer decedents, which was also associated with poorer outcomes in the last 2 weeks of life. We found that frailty and organ failure decedents were three times and four times more likely, respectively, to spend a greater duration of time in acute-care hospitals (compared to their early counterparts). A late initiation similarly influenced cancer decedents, but the association was not as pronounced. Several factors may explain these findings. First, non-cancer patients tend to receive lower quality end-of-life care than cancer patients.41 Also, the setting of end-of-life care—which is known to be a key driver of disease-specific disparities41—may play a role; our data show that late palliative care provided to organ failure and frailty decedents was initiated mostly in hospital, which may not necessarily be the most appropriate care setting. Non-cancer populations also experience greater incongruence between their care preferences and what happens in reality. Differences in trajectory of functional decline and its predictability may also explain our findings; for instance, patients with organ failure experience an end-of-life trajectory marked by acute exacerbations, warranting a greater need for acute-care services.28,42 Therefore, earlier identification and increased understanding of patient needs may help improve palliative care provision; accomplishing this requires extensive knowledge of the trajectories of functional decline, existing comorbidities, and the social and environmental circumstances under which care is provided.
Strengths and limitations
Past studies examine recipients of hospital-based palliative care or community-based services, but not both together. A major strength of this study is the inclusion of a population-based sample from Ontario, Canada, where patients rely on a universal health system in which they are provided with concurrent access to hospital and community palliative care services without needing to forego curative treatment. Unlike the United States, where patients are required to forego curative care to be eligible for the Medicare Hospice Benefit at the end of life, we are able to observe palliative care provision in the entire population. Thus, our data are largely generalizable to other high-income countries with similar publicly funded healthcare (i.e. United Kingdom and Australia). Another strength of our study is that we include cancer and non-cancer decedents and a large set of health sectors to observe various services used at the end of life. We also capture officially and unofficially designated palliative beds in acute-care hospitals, allowing us to distinguish palliative- from non-palliative-acute-care use; this information lets us gauge which end-of-life hospitalizations were appropriate or inappropriate and serves as a useful comparator for other countries whose systems may or may not allow for such distinctions to be made.
Our study has several limitations. First, our study does not address the quality of care being delivered, nor do we describe the intensity of palliative care services provided in acute hospitals. Second, residential (i.e. free-standing) hospice facilities were not included as a place of care setting due to the lack of a central hospice database. About 1%–3% of individuals die in hospice annually, though most who do so use services such as home care or palliative-acute care—which is included in our study—before being admitted to a hospice. Moreover, our study only includes home care services that are publicly funded. In addition, relying mainly on physician billing codes might undercount palliative care provision in long-term care (where a large portion of frailty patients reside) as many individuals receive palliative care services from nurses or personal support workers in this setting, which are not billed under publicly funded home care—the latter of which we did capture. Our findings may also be susceptible to indication bias; patients initiating late palliative care are often close to death with more unstable conditions and thus more likely to receive care in acute settings at the end of life. Early palliative care recipients may also have distinct underlying characteristics than late recipients, such as differences in care preferences and disease symptoms (which we could not measure). Moreover, several important factors may play a role in explaining our finding of more aggressive end-of-life care among organ failure and frailty patients. These factors include lack of access to early palliative care43 and challenges in early identification for a palliative care approach among non-cancer patients.44,45 Healthcare providers also seem to experience difficulty in determining the end-of-life stage for non-cancer patients46 and feel ill-prepared in making end-of-life prognostications due to perceived unpredictability of the disease course.47,48 For example, it may be challenging to know when to initiate palliative care for patients with heart failure, who get hospitalized for exacerbations, but later discharged having regained some of their prior physical function.
Conclusion
In conclusion, this study demonstrates that early palliative care is associated with reduced acute-hospital use (with or without palliative involvement) in the last 2 weeks of life. Clear disparities exist in palliative care timing, with organ failure and frailty decedents receiving late palliative care more often than early. These findings suggest that non-cancer populations might reap greater benefits if identified earlier for palliative care, which may also help reduce costly end-of-life acute-care service use. Future research should examine effective interventions that would allow for earlier identification of patients (including cancer and non-cancer) who may benefit from timely palliative care. Differences in the underlying characteristics of early and late palliative care recipients should also be further investigated.
Acknowledgments
All authors made significant contributions to the design and conduct of the study. D.Q. planned and executed the data collection, statistical analyses, and writing of the draft manuscript. All authors contributed to the interpretation of the results, as well as preparation and approval of the final manuscript.
The datasets used in this article were linked using unique encoded identifiers and analyzed at ICES. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI.
Footnotes
Data Sharing: The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval and Informed consent: Ethical approval for this study was received from the Ottawa Hospital Research Institute Ethics Board in Ottawa, Canada. This study used health administrative data, and thus, no consent was required. To ensure the protection of patients’ identity, cells that contain six or fewer observations were not reported.
Funding: ICES is an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). As a prescribed entity under Ontario’s privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. The results, conclusions, opinions, and statements expressed in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or MOHLTC is intended or should be inferred.
ORCID iD: Hsien-Yeang Seow  https://orcid.org/0000-0001-6701-1714
https://orcid.org/0000-0001-6701-1714
References
- 1. Keating NL, BethLandrum M, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles. J Clin Oncol 2010; 28(28): 4364–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010; 28(7): 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008; 300(14): 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell C, Somogyi-Zalud E, Masaki KH. Methodological review: measured and reported congruence between preferred and actual place of death. Palliat Med 2009; 23(6): 482–490. [DOI] [PubMed] [Google Scholar]
- 5. Billingham MJ, Billingham SJ. Congruence between preferred and actual place of death according to the presence of malignant or non-malignant disease: a systematic review and meta-analysis. BMJ Support Palliat Care 2013; 3(2): 144–154. [DOI] [PubMed] [Google Scholar]
- 6. Gomes B, Calanzani N, Gysels M, et al. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care 2013; 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gomes B, Higginson IJ, Calanzani N, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012; 23(8): 2006–2015. [DOI] [PubMed] [Google Scholar]
- 8. Sato K, Miyashita M, Morita T, et al. Reliability assessment and findings of a newly developed quality measurement instrument: quality indicators of end-of-life cancer care from medical chart review at a Japanese regional cancer center. J Palliat Med 2008; 11(5): 729–737. [DOI] [PubMed] [Google Scholar]
- 9. Barbera L, Paszat L, Chartier C. Indicators of poor quality end-of-life cancer care in Ontario. J Palliat Care 2006; 22(1): 12–17. [PubMed] [Google Scholar]
- 10. Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004; 22(2): 315–321. [DOI] [PubMed] [Google Scholar]
- 11. Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 2005; 17(6): 505–509. [DOI] [PubMed] [Google Scholar]
- 12. Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015; 33(13): 1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010; 363(8): 733–742. [DOI] [PubMed] [Google Scholar]
- 14. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014; 383(9930): 1721–1730. [DOI] [PubMed] [Google Scholar]
- 15. Costantini M, Higginson IJ, Boni L, et al. Effect of a palliative home care team on hospital admissions among patients with advanced cancer. Palliat Med 2003; 17(4): 315–321. [DOI] [PubMed] [Google Scholar]
- 16. Ferroni E, Avossa F, Figoli F, et al. Intensity of integrated primary and specialist home-based palliative care for chronic diseases in Northeast Italy and its impact on end-of-life hospital access. J Palliat Med 2016; 19(12): 1260–1266. [DOI] [PubMed] [Google Scholar]
- 17. Iupati S, Ensor BR. Do community hospice programmes reduce hospitalisation rate in patients with advanced chronic obstructive pulmonary disease. Intern Med J 2016; 46(3): 295–300. [DOI] [PubMed] [Google Scholar]
- 18. McNamara BA, Rosenwax LK, Murray K, et al. Early admission to community-based palliative care reduces use of emergency departments in the ninety days before death. J Palliat Med 2013; 16(7): 774–779. [DOI] [PubMed] [Google Scholar]
- 19. Seow H, Barbera L, Howell D, et al. Using more end-of-life homecare services is associated with using fewer acute care services: a population-based cohort study. Med Care 2010; 48(2): 118–124. [DOI] [PubMed] [Google Scholar]
- 20. Seow H, Brazil K, Sussman J, et al. Impact of community based, specialist palliative care teams on hospitalisations and emergency department visits late in life and hospital deaths: a pooled analysis. BMJ 2014; 348: g3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seow H, Dhaliwal G, Fassbender K, et al. The effect of community-based specialist palliative care teams on place of care. J Palliat Med 2016; 19(1): 16–21. [DOI] [PubMed] [Google Scholar]
- 22. Spilsbury K, Rosenwax L, Arendts G, et al. The impact of community-based palliative care on acute hospital use in the last year of life is modified by time to death, age and underlying cause of death. PLoS ONE 2017; 12(9): e0185275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright CM, Youens D, Moorin RE. Earlier initiation of community-based palliative care is associated with fewer unplanned hospitalizations and emergency department presentations in the final months of life: a population-based study among cancer decedents. J Pain Symptom Manage 2018; 55(3): 745.e8–754.e8. [DOI] [PubMed] [Google Scholar]
- 24. Youens D, Moorin R. The impact of community-based palliative care on utilization and cost of acute care hospital services in the last year of life. J Palliat Med 2017; 20(7): 736–744. [DOI] [PubMed] [Google Scholar]
- 25. Qureshi D, Tanuseputro P, Perez R, et al. Place of care trajectories in the last two weeks of life: a population-based cohort study of Ontario decedents. J Palliat Med 2018; 21: 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanuseputro P. Delivering care to those in need: improving palliative care using linked data. London: SAGE, 2017. [DOI] [PubMed] [Google Scholar]
- 27. Tanuseputro P, Budhwani S, Bai YQ, et al. Palliative care delivery across health sectors: a population-level observational study. Palliat Med 2017; 31(3): 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA 2003; 289(18): 2387–2392. [DOI] [PubMed] [Google Scholar]
- 29. Gill TM, Gahbauer EA, Han L, et al. Trajectories of disability in the last year of life. N Engl J Med 2010; 362(13): 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray SA, Kendall M, Boyd K, et al. Illness trajectories and palliative care. BMJ 2005; 330(7498): 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Canadian Institute for Health Information (CIHI). Health care use at the end of life in Atlantic Canada. Ottawa, ON, Canada: CIHI, 2011. [Google Scholar]
- 32. Fassbender K, Fainsinger RL, Carson M, et al. Cost trajectories at the end of life: the Canadian experience. J Pain Symptom Manage 2009; 38(1): 75–80. [DOI] [PubMed] [Google Scholar]
- 33. Hui D, Kim YJ, Park JC, et al. Integration of oncology and palliative care: a systematic review. Oncologist 2015; 20(1): 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis MP, Temel JS, Balboni T, et al. A review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015; 4(3): 99–121. [DOI] [PubMed] [Google Scholar]
- 35. Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003; 21(6): 1133–1138. [DOI] [PubMed] [Google Scholar]
- 36. Hollander MJ, Chappell N. Synthesis report: final report of the national evaluation of the cost-effectiveness of home care (A report prepared for the Health Transition Fund, Health Canada). Victoria, BC: National Evaluation of the Cost-Effectiveness of Home Care, 2002. [Google Scholar]
- 37. Hui D, Kim SH, Roquemore J, et al. Impact of timing and setting of palliative care referral on quality of end-of-life care in cancer patients. Cancer 2014; 120(11): 1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Romano AM, Gade KE, Nielsen G, et al. Early palliative care reduces end-of-life intensive care unit (ICU) use but not ICU course in patients with advanced cancer. Oncologist 2017; 22(3): 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poulose JV, Do YK, Neo PS. Association between referral-to-death interval and location of death of patients referred to a hospital-based specialist palliative care service. J Pain Symptom Manage 2013; 46(2): 173–181. [DOI] [PubMed] [Google Scholar]
- 40. Higginson IJ, McCrone P, Hart SR, et al. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009; 38(6): 816–826. [DOI] [PubMed] [Google Scholar]
- 41. Wachterman MW, Pilver C, Smith D, et al. Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med 2016; 176(8): 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc 2002; 50(6): 1108–1112. [DOI] [PubMed] [Google Scholar]
- 43. Seow H, O’Leary E, Perez R, et al. Access to palliative care by disease trajectory: a population-based cohort of Ontario decedents. BMJ Open 2018; 8(4): e021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huijberts S, Buurman BM, deRooij SE. End-of-life care during and after an acute hospitalization in older patients with cancer, end-stage organ failure, or frailty: a sub-analysis of a prospective cohort study. Palliat Med 2016; 30(1): 75–82. [DOI] [PubMed] [Google Scholar]
- 45. Tapsfield J, Hall C, Lunan C, et al. Many people in Scotland now benefit from anticipatory care before they die: an after death analysis and interviews with general practitioners. BMJ Support Palliat Care. Epub ahead of print 13 April 2016. DOI: 10.1136/bmjspcare-2015-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buurman BM, vanMunster BC, Korevaar JC, et al. Prognostication in acutely admitted older patients by nurses and physicians. J Gen Intern Med 2008; 23(11): 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Christakis NA, Smith JL, Parkes CM, et al. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study Commentary: why do doctors overestimate? Commentary: prognoses should be based on proved indices not intuition. BMJ 2000; 320: 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med 1998; 158(21): 2389–2395. [DOI] [PubMed] [Google Scholar]