Abstract
Background:
The synthetic solvent tetrachloroethylene (PCE), commonly used in dry cleaning operations, is a human neurotoxicant and carcinogen. However, its effect on reproduction is poorly understood, as prior studies have been limited to small occupational cohorts. We examined the association between PCE exposure from contamination of the public drinking water supply and time-to-pregnancy (TTP) in a cohort of mothers from Cape Cod, Massachusetts.
Methods:
The Cape Cod Family Health Study is a retrospective cohort study designed to examine the reproductive and developmental health effects of exposure to PCE-contaminated drinking water. Our analysis included 1565 women who reported 3826 planned pregnancies from 1949 to 1990. Women completed self-administered questionnaires that ascertained TTP for each of her pregnancies, regardless of the outcome, as well as residential history and demographic information. We utilized EPANET water distribution system modeling software and a leaching and transport model to assess PCE exposure for each pregnancy. We used log-binomial regression models to estimate relative risks (RR) and 95% confidence intervals (CI), adjusting for potential confounders. We performed a probabilistic bias analysis to examine the effect of outcome misclassification on our results.
Results:
Any cumulative PCE exposure before pregnancy was associated with a 15% reduction in risk of TTP > 12 months (RR = 0.85, 95% CI: 0.70, 1.03). However, women with the highest average monthly PCE exposure around the time of the pregnancy attempt (≥ 2.5g) had increased risk of TTP > 12 months (RR = 1.36, 95% CI: 1.06, 1.76).
Conclusions:
We found little evidence for long-term, cumulative adverse effects of PCE exposure on TTP, but high levels of PCE exposure around the time of the pregnancy attempt were associated with longer TTP. These associations may be underestimated due to the exclusion of unsuccessful pregnancy attempts from our study population, and may be biased by outcome and exposure misclassification given the long-term recall of TTP and use of a leaching and transport model to estimate PCE exposure.
Keywords: Infertility, Organic solvents, Retrospective cohort, Tetrachloroethylene, Time to pregnancy
1. Introduction
Tetrachloroethylene (perchloroethylene; PCE) is a synthetic, volatile organic solvent used in dry cleaning solutions, textile processing, and metal degreasing operations (Environmental Protection Agency, 2012). The primary routes of exposure in the general population are vapor inhalation from conventionally dry-cleaned fabrics and contaminated soil, and ingestion of contaminated water (Environmental Protection Agency, 2012; Sherlach et al., 2011). PCE distributes widely throughout the body, with the highest concentration in adipose tissue due to its lipophilic nature, and has a half-life of approximately 96 days (Environmental Protection Agency, 2012). PCE is a well-recognized animal and human neurotoxicant (Environmental Protection Agency, 2012) and carcinogen (Agency for Toxic Substances and Disease Registry, 1997; International Agency for Research on Cancer, 2014); however, its effects on reproduction are poorly understood. In rodent studies, PCE and the related solvent trichloroethylene (TCE) have adversely affected fertilization (Berger and Horner, 2003; Wu and Berger, 2007; Xu et al., 2004) and semen quality (Beliles et al., 1980). Epidemiologic studies conducted almost exclusively among dry cleaning workers have found evidence that dry cleaning work or suspected occupational PCE exposure is associated with increased risk of poor semen quality (Chia et al., 1996; Eskenazi et al., 1991b; Tielemans et al., 1999), infertility (Eskenazi et al., 1991a; Rachootin and Olsen, 1983), and longer time-to-pregnancy (TTP) (Sallmen et al., 1998, 1995).
From 1968 through 1980, public water departments throughout the New England region of the United States installed vinyl-lined asbestos-cement (VL/AC) water pipes on an as-needed basis to solve taste and odor problems. The liner was painted onto the inner surface of the pipes in a slurry of vinyl toluene resin (Piccotex™) and PCE. The manufacturer recommended that 48 h lapse to allow the liner to dry before shipping the pipes for distribution; it was assumed that most of the PCE would evaporate by the time of shipment (Demond, 1982). However, in 1980, government officials discovered that high levels of PCE persisted in the liner and had been slowly leaking into the public drinking water supply for over a decade. Approximately 660 miles of VL/AC pipes were installed in Massachusetts, with a large proportion in the Cape Cod region (Larson et al., 1983). PCE levels on Cape Cod ranged from 1,600–7750 μg/L in low-flow areas and 1.5–80 μg/L in medium- and high-flow areas (Demond, 1982). Current United States Environmental Protection Agency (US EPA) drinking water regulations set the maximum PCE contaminant level at 5 μg/L (U.S. Environmental Protection Agency, 2009). Because replacing the VL/AC pipes was prohibitively expensive, officials began a program of regular flushing and bleeding in 1980 to remediate the problem. This exposure scenario constitutes a type of natural experiment because the irregular installation pattern of VL/AC pipes resulted in neighboring households with vastly different contaminant levels (Aschengrau et al., 2016). PCE exposure levels were, in some instances, substantially higher than those typically seen in non-occupational populations. Levels of other drinking water contaminants were low (Swartz et al., 2003).
In the present analysis, we examined the association between PCE exposure through contaminated drinking water and TTP in a cohort of women from Cape Cod who had successfully conceived.
2. Materials and methods
2.1. Study design and selection of study population
The Cape Cod Family Health Study is a retrospective cohort designed to examine the association between exposure to PCE-contaminated drinking water and reproductive and developmental health. Study methods have been described in detail elsewhere (Aschengrau et al., 2008). Briefly, in 2002, we identified women who gave birth from 1969 to 1983 while residing in a Cape Cod town with documented VL/AC distribution pipes. We visually inspected water pipe distribution maps in the vicinity of the maternal address at birth to identify exposed and unexposed births. We classified 1492 women as exposed because they either lived adjacent to a VL/AC pipe or the only possible water flow to the residence was through a VL/AC pipe. We selected a comparison group of 1704 unexposed women so that their offspring were frequency-matched to those of the exposed women based on the birth month and year. Initial exposure status was considered preliminary; we later conducted a more detailed exposure assessment, described below (see “PCE exposure assessment”).
We successfully traced 91.2% of exposed and 92.0% of unexposed women. We mailed introductory letters and self-administered questionnaires to all traced participants from 2002 to 2003. Self-administered questionnaires collected information on demographic characteristics, residential history, water consumption and bathing habits, medical history, and reproductive history, including detailed information about each of their pregnancies (live births, still births, miscarriages, induced abortions, and ectopic pregnancies).
Women initially classified as exposed and unexposed had similar response rates (64.3% and 63.8%). Non-participants were younger (mean age 26.0 vs. 27.5 years) and less educated (11.3% vs. 3.6% did not graduate from high school), but were similar with respect to race (96.2% white in both groups) and year of pregnancy (54.7% vs. 56.0% births between 1979 and 1983); these differences were observed for both exposed and unexposed women (Aschengrau et al., 2008, 2009a).
All participants provided informed consent. The institutional review boards of Boston University Medical Center and the Massachusetts Department of Public Health approved the study protocol.
2.2. Geocoding of residential addresses
On self-administered questionnaires, women reported detailed information on each of their Cape Cod residences from 1969 through 1990, including exact street address, nearest cross street, calendar years of occupancy, and drinking water source. Throughout the study period, 29.5% of women did not move, whereas 31.7% of women moved once and 38.8% of women moved more than once. The 5324 reported addresses were incorporated into a geographic information system (GIS) using ArcGIS 8.1. Geocoding to a latitude and longitude was conducted without knowledge of exposure or pregnancy history. The majority of addresses were successfully geocoded to a parcel of land (87.6%) or the nearest reported cross street or the middle of the street in instances where street number was missing (9.6%). We were unable to geocode the remaining 2.7% of addresses, and pregnancies associated with these addresses were excluded from the analysis.
2.3. PCE exposure assessment
We refined our PCE exposure assessment using a leaching and transport model developed by Webler and Brown for our prior epidemiologic studies (Aschengrau et al., 2003; Webler and Brown, 1993). This model estimates the amount of PCE entering the drinking water using the initial PCE loading in the pipe liner (estimated using the pipe dimensions), the age of the pipe, and the leaching rate of PCE from the pipe into the water (Demond, 1982). We incorporated the Webler and Brown algorithm into EPANET water distribution system modeling software to account for water flow and direction, which are functions of the water system geometry and the number of water users. This software was developed by the US EPA and simulates the instantaneous flow of water throughout the entire public water distribution system in a town (Rossman, 1994). It has been used to assess exposure to drinking water contaminants in several epidemiologic studies (Aral et al., 1996; Gallagher et al., 1998; Maslia et al., 1996; Reif et al., 2003).
We created a GIS schematic, which represented the pipe configuration present around 1980, a year that is likely to be representative of the water distribution system during the study period. We included water source locations, nodes (points of water consumption), and pipe characteristics obtained from local water companies and the Massachusetts Department of Environmental Protection. EPANET software was used to model the flow of water through thousands of pipe segments in order to estimate the quantity, or mass, of PCE delivered to each participant’s residence. We assigned each geocoded residence to the closest node on the water distribution system. We assumed that each residence drew the same quantity of water and that the water sources did not change over the study period. We calculated annual PCE exposure for each woman for every year from 1949 through 1990. Annual exposure was zero before 1969 and during years when women lived in residences with private wells or in a town without documented VL/AC pipes.
2.4. Assessment of time to pregnancy
On self-administered questionnaires, women reported how long it took for them to conceive each of their planned pregnancies. Response choices were < 3 months, 3–6 months, 7–12 months, or > 12 months.
2.5. Covariate assessment
We collected covariate information from self-administered questionnaires, including demographics (parental age at pregnancy, maternal education and paternal occupation at time of questionnaire, maternal race/ethnicity), medical history (pre-pregnancy history of pelvic inflammatory disease, uterine leiomyomata, ovarian cysts, endometriosis, polycystic ovarian syndrome, gonorrhea or chlamydia), pregnancy information (year of pregnancy, pregnancy outcome, gravidity, parity, first trimester smoking and alcohol use, caffeine intake and marijuana use during pregnancy, oral contraceptive use in 3 months before conception), and menstrual cycle characteristics at time of questionnaire (cycle regularity, cycle length, pre-pregnancy history of amenorrhea).
2.6. Exclusions
Overall, study participants reported 6519 pregnancies from 1949 to 1990. The earliest exposure occurred in 1963; the amount and timing of exposure depended on location of residence, time residing at a particular address, and the timing of pipe installation on each street. We excluded pregnancies with missing pregnancy end dates or pregnancy end dates after 1990 (n = 246). We also excluded pregnancies with incalculable PCE exposure, either because of missing residential move-in or move-out years (n = 249) or insufficient information to geocode addresses (n = 131), and unplanned pregnancies (n = 2067). The proportion of pregnancies that were unplanned did not differ substantially between exposed and unexposed women (33.1% vs. 36.1%). The final analytic sample included 3826 pregnancies, including both the birth(s) that determined eligibility for the cohort and all other planned pregnancies, to 1565 women. We did not collect information on pregnancy attempts that did not result in a clinically-recognized pregnancy.
2.7. Statistical analysis
We calculated two exposure metrics for each pregnancy: cumulative exposure before the pregnancy attempt (hereafter “cumulative PCE exposure”) and average monthly exposure during the year of the pregnancy attempt (hereafter “average monthly PCE exposure”). Cumulative PCE exposure was calculated by summing the annual mass of PCE between the pipe installation or move-in year, whichever came later, and the year the woman began attempting pregnancy. We calculated average monthly PCE exposure by dividing the annual PCE mass for the year the woman began attempting pregnancy by 12. We identified the month and year each woman began attempting each of her pregnancies by subtracting her reported TTP from the date of her last menstrual period (i.e., the beginning of her pregnancy, estimated using self-reported pregnancy end date and length of gestation). Because TTP was reported in categories, for exposure measurement purposes, we assigned each woman a continuous TTP value based on the approximate midpoint of the category: 1, 4, 9, and 14 months for women who reported TTP categories of < 3, 3–6, 7–12, and > 12 months, respectively. We selected these values erring on the lower side of the midpoint based on the distribution of TTP in a general population of pregnancy planners (Radin et al., 2015). We conducted a sensitivity analysis assigning women continuous TTP values of 2, 5, 10, and 18, respectively, out of concern that selecting shorter TTP values (i.e., later initiation of pregnancy attempt relative to LMP date) may overestimate cumulative exposure.
We examined the two exposures as binary (any vs. no exposure) and categorical variables. For cumulative PCE exposure, categories were based on orders of magnitude (no exposure (reference), > 0- < 10, 10- < 100, and ≥ 100 g); for average monthly PCE exposure, categories were defined based on the distribution in the data (no exposure (reference), > 0- < 1, 1- < 2.5, and ≥ 2.5 g). The outcome variable was analyzed in its original form (< 3, 3–6, 7–12, and > 12 months) and as two binary variables: TTP > 12 vs. ≤ 12 months and TTP > 6 vs. ≤ 6 months. We used log-binomial regression models to estimate risk ratios (RR) and 95% confidence intervals (CI) (Rothman et al., 2008). We used generalized estimating equations to account for correlation of pregnancies contributed by the same woman. We first examined the crude association between each PCE exposure and TTP > 12 months, TTP > 6 months, and TTP categories 3–6, 7–12, and > 12 months (vs. < 3 months). We examined the extent to which any predictors of TTP were confounders by including in final models any variable that changed the coefficient on PCE by > 5% in bivariate analyses. These included maternal age (< 25, 25–29, 30–34, ≥ 35 years), paternal age (< 25, 25–29, 30–34, ≥ 35 years), year of pregnancy (pre-1975, 1975–1980, 1981–1990), maternal education (< 12, 12, 13–15, ≥ 16 years), paternal occupation (blue collar, white collar, other), first trimester cigarette smoking (any, none), first trimester alcohol intake (any, none), and pregnancy number (1, 2, 3, 4, 5, ≥ 6). To allow for the possibility of non-linear associations between PCE exposure and fertility, we fit restricted cubic splines (Durrleman and Simon, 1989; Li et al., 2008). We plotted spline graphs on the arithmetic scale (Rothman et al., 2011).
We stratified final models by maternal age (< 30 vs. ≥ 30 years) and parity (nulliparous vs. parous) to assess the extent to which the relation between PCE exposure and delayed TTP is stronger among older or nulliparous couples, potentially less fertile subgroups. We conducted a sensitivity analysis restricting to first pregnancies only, out of concern that pregnancies to more fertile women were over-represented in our sample. Finally, we conducted a sensitivity analysis excluding pregnancies that ended after 1980, when flushing and bleeding began, out of concern that exposure misclassification was more likely than in earlier pregnancies.
We generated five imputation data sets using a Markov chain Monte Carlo method (Schafer, 1997) that assumes multivariate normality to impute missing outcome and covariate data (Donders et al., 2006; Schafer, 1999; Sterne et al., 2009). TTP data were missing for 2.4% of pregnancies. Missingness for covariates ranged from 0% (gravidity, parity) to 2.5% (cycle regularity).
TTP may not be reported accurately, especially over long time periods of recall (Baird et al., 1991; Cooney et al., 2009; Joffe et al., 1993; Jukic et al., 2016; Radin et al., 2015). Participants reported TTP for each of their pregnancies, with a median recall of 24 years (range 12–53 years). To assess the effect of outcome misclassification on our results, we conducted a probabilistic bias analysis on all five imputation data sets (Lash et al., 2009). Prior analyses demonstrate that women did not know their true PCE exposure levels (Aschengrau et al., 2009b); therefore, we assumed that TTP misclassification was non-differential with respect to exposure. We defined trapezoidal distributions for sensitivity and specificity of TTP > 12 months based on prior literature (Jukic et al., 2016). Trapezoidal distributions specify lower and upper modes, between which the probability density is uniform, as well as minimum and maximum values, to which the probability density decreases linearly to zero. For sensitivity (93% in the Jukic et al. validation study), we assigned minimum, lower mode, upper mode, and maximum values of 0.86, 0.88, 0.98, and 1.00, respectively; for specificity (96% in the Jukic et al. validation study), these values were 0.92, 0.94, 0.98, and 1.00, respectively. We sampled 1000 times from these distributions (separately for exposed and unexposed women, but assuming a correlation of 0.8) and converted sensitivity and specificity to positive and negative predictive values. We calculated 1000 corrected data sets by sampling true outcome values from a binary distribution with probabilities of success equal to the positive and negative predictive values, and estimated RRs for each of these data sets. From the distribution of correct RRs, we present the median, 2.5th, and 97.5th percentiles to determine the measure of central tendency and magnitude of bias.
3. Results
The 1565 women in the analytic sample were mostly white (96.3%), educated (80.0% with some college education) and had partners working white collar jobs (51.2%). Women reported 3826 planned pregnancies between 1949 and 1990. Overall, 20.7% of women contributed one pregnancy to the analysis, 40.5% contributed two pregnancies, and 38.8% contributed three or more pregnancies (maximum = 10 pregnancies). Mean maternal and paternal ages for all pregnancies were 26.7 (standard deviation (SD) = 4.7) and 29.7 (SD = 5.8) years, respectively. The prevalence of cigarette smoking or alcohol consumption in the first trimester was 27.1% and 37.5%, respectively. Nineteen percent of women drank at least two caffeinated beverages per day during the pregnancy, and 3.6% smoked marijuana. Most women reported regular or somewhat regular menstrual cycles (86.7%) and had cycle lengths ranging from 25 to 40 days (88.7%).
Most study pregnancies (65.3%) took < 3 months to conceive; however, 15.8%, 7.2%, and 11.3% of pregnancies took 3–6, 7–12, and > 12 months to conceive, respectively. Longer TTP was positively associated with older maternal and paternal age, lower maternal education, non-white maternal race, first trimester cigarette smoking, caffeine intake during pregnancy, pre-pregnancy history of several gynecologic conditions (including pelvic inflammatory disease, ovarian cysts, endometriosis, and amenorrhea), and irregular, short (< 25 days) or long (> 40 days) menstrual cycles.
Some PCE exposure occurred before 42.4% of pregnancies; only 34.2% of pregnancies were exposed during the year the pregnancy attempt began. Among the exposed, the median (range) exposure was 26.8 g (0.001–3826.7) for cumulative PCE exposure and 0.6 g (0.0001–84.5) for average monthly PCE exposure. The two exposures were highly correlated (Spearman correlation coefficient = 0.82). On average, exposed women were older than unexposed women (Table 1). They were also more likely to have births later in the study period, to have a history of pregnancy or live birth, and to have a history of specific gynecologic conditions, although these differences are likely confounded by time (i.e., cumulative exposure, probability of pregnancy, and risk of specific gynecologic conditions all increase with time).
Table 1.
Distribution of sociodemographic, lifestyle, and pregnancy-related characteristics by PCE exposures among 3826 planned pregnancies.
| Cumulative PCE exposure |
Average monthly PCE exposure |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristica | Unexposed | Exposed < 10g | Exposed 10–99g | Exposed ≥100g | Unexposed | Exposed < 1.0 g | Exposed 1.0–2.4 g | Exposed ≥ 2.5 g |
| Number of pregnancies | 2202 | 546 | 663 | 415 | 2518 | 798 | 215 | 295 |
| Maternal age (years), mean | 25.7 | 27.5 | 27.8 | 29.2 | 26.2 | 28.1 | 27.3 | 27.3 |
| Paternal age (years), mean | 29.5 | 30.3 | 30.1 | 30.5 | 29.5 | 27.0 | 26.9 | 26.9 |
| Year of pregnancy pre-1975, % | 37.1 | 15.2 | 15.5 | 12.0 | 34.5 | 48.2 | 12.8 | 25.3 |
| Year of pregnancy, post-1980, % | 26.0 | 45.3 | 37.6 | 44.3 | 29.7 | 48.2 | 26.7 | 23.3 |
| Pregnancy number, mean | 2.2 | 2.4 | 2.5 | 2.4 | 2.2 | 2.4 | 2.3 | 2.2 |
| Maternal education ≤ high school graduate, % | 19.5 | 19.4 | 20.1 | 20.7 | 19.6 | 20.1 | 17.9 | 22.3 |
| Paternal blue collar occupation, % | 29.8 | 32.0 | 35.4 | 31.0 | 30.2 | 33.7 | 36.6 | 28.7 |
| Maternal white race, % | 96.6 | 95.6 | 95.6 | 99.1 | 96.7 | 95.2 | 95.9 | 98.5 |
| Any first trimester smoking, % | 29.0 | 24.0 | 22.2 | 24.7 | 28.3 | 22.7 | 25.4 | 27.6 |
| Any first trimester alcohol use, % | 39.1 | 33.9 | 36.8 | 32.8 | 37.8 | 35.5 | 38.4 | 37.0 |
| > 2 cups/day caffeine during pregnancy, % | 19.6 | 16.0 | 18.7 | 22.5 | 19.0 | 16.7 | 19.4 | 26.1 |
| Any marijuana use in pregnancy, % | 3.3 | 3.3 | 4.2 | 3.6 | 3.5 | 4.3 | 2.2 | 3.3 |
| Gravid, % | 61.2 | 71.6 | 74.5 | 76.5 | 63.8 | 73.2 | 71.3 | 68.6 |
| Parous, % | 54.7 | 65.8 | 69.3 | 72.9 | 57.5 | 67.7 | 65.6 | 63.1 |
| Pre-pregnancy pelvic inflammatory disease, % | 1.5 | 1.7 | 1.1 | 1.5 | 1.4 | 1.8 | 2.4 | 0.9 |
| Pre-pregnancy uterine leiomyomata, % | 3.1 | 3.4 | 3.5 | 4.7 | 3.2 | 3.5 | 4.9 | 3.5 |
| Pre-pregnancy ovarian cysts, % | 6.9 | 7.9 | 6.6 | 9.7 | 6.9 | 7.3 | 11.3 | 8.4 |
| Pre-pregnancy endometriosis, % | 3.1 | 3.3 | 4.3 | 4.8 | 3.1 | 4.5 | 4.2 | 3.7 |
| Pre-pregnancy PCOS, % | 0.4 | 1.1 | 0.4 | 0.6 | 0.5 | 0.8 | 0.5 | 0.3 |
| Pre-pregnancy gonorrhea or chlamydia, % | 1.2 | 1.6 | 1.5 | 0.9 | 1.1 | 1.9 | 2.6 | 0.3 |
| Irregular cycles, % | 11.7 | 13.6 | 12.5 | 11.4 | 12.1 | 12.6 | 13.2 | 11.9 |
| Cycle length < 25 days, % | 8.7 | 9.2 | 13.3 | 11.4 | 9.0 | 11.0 | 14.4 | 7.9 |
| Cycle length > 40 days, % | 1.8 | 1.6 | 1.0 | 2.0 | 1.8 | 1.1 | 1.9 | 1.5 |
| Pre-pregnancy amenorrhea, % | 8.0 | 8.4 | 7.7 | 7.7 | 7.9 | 8.7 | 10.1 | 5.4 |
| OC use in 3 months before conception, % | 13.6 | 13.9 | 17.0 | 16.5 | 13.9 | 14.4 | 16.1 | 18.1 |
OC = oral contraceptive; PCE = perchloroethylene; PCOS = polycystic ovarian syndrome.
Characteristics are standardized to maternal age using a SAS macro.
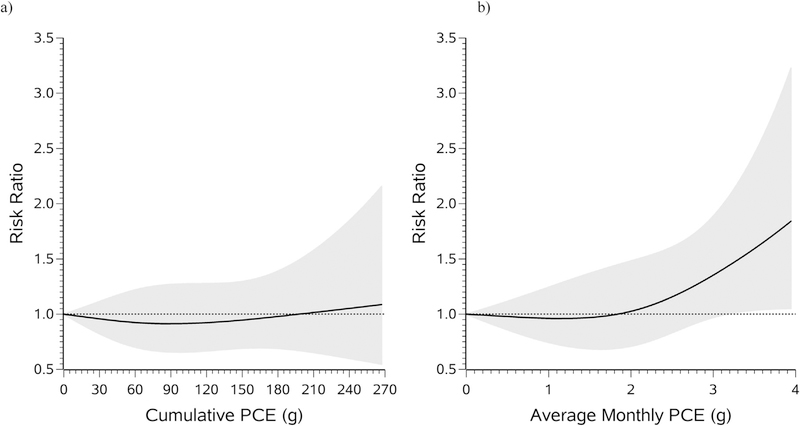
Women with any PCE exposure before the pregnancy attempt had 15% lower risk of TTP > 12 months than unexposed women (Table 2). When categorizing exposure, women in each of the exposed categories had a lower risk of TTP > 12 months relative to unexposed women (for example, for cumulative PCE exposure of > 0- < 10 g vs. unexposed, RR = 0.76, 95% CI: 0.58–1.00). However, women with the highest average monthly PCE exposure had an increased risk of TTP > 12 months compared with unexposed women (RR = 1.36, 95% CI: 1.06–1.76). In the restricted cubic spline analysis, risk of TTP > 12 months was not substantially related to cumulative PCE exposure (Fig. 1a), but increased approximately linearly from 2 to 4 g for average monthly PCE exposure (Fig. 1b). We did not have sufficient data to draw conclusions on risk at exposure levels above 4 g. Results were similar, but weaker, when examining risk of TTP > 6 months (Table 3).
Table 2.
PCE exposure and risk of TTP > 12 months.
| Exposure | Cases/N | Crude RR | Adjusteda RR (95% CI) | Adjustedb RR (95% CI) |
|---|---|---|---|---|
| Cumulative PCE exposure (grams) | ||||
| None | 272/2202 | Reference | Reference | Reference |
| Any | 174/1624 | 0.97 (0.81, 1.16) | 0.81 (0.67, 0.98) | 0.85 (0.70, 1.03) |
| None | 272/2202 | Reference | Reference | Reference |
| > 0– < 10 | 50/546 | 0.84 (0.64, 1.09) | 0.57 (0.32, 0.99) | 0.76 (0.58, 1.00) |
| 10– < 100 | 71/663 | 1.02 (0.80, 1.29) | 0.84 (0.63, 1.12) | 0.91 (0.70, 1.17) |
| ≥ 100 | 53/415 | 1.08 (0.80, 1.46) | 0.92 (0.71, 1.18) | 0.90 (0.66, 1.24) |
| Average monthly PCE exposure (grams) | ||||
| None | 306/2518 | Reference | Reference | Reference |
| Any | 140/1308 | 0.97 (0.81, 1.16) | 0.87 (0.73, 1.05) | 0.89 (0.73, 1.07) |
| None | 306/2518 | Reference | Reference | Reference |
| > 0– < 1 | 63/798 | 0.73 (0.57, 0.93) | 0.64 (0.50, 0.82) | 0.66 (0.51, 0.85) |
| 1– < 2.5 | 31/215 | 1.15 (0.84, 1.59) | 1.05 (0.75, 1.45) | 1.03 (0.74, 1.45) |
| ≥ 2.5 | 46/295 | 1.50 (1.16, 1.95) | 1.40 (1.08, 1.81) | 1.36 (1.06, 1.76) |
Adjusted for maternal age.
Adjusted for maternal age, paternal age, year of pregnancy, maternal education, paternal occupation, first trimester cigarette smoking, first trimester alcohol intake, and pregnancy number.
Fig. 1.
Association between cumulative (a) and average monthly (b) PCE exposure and risk of TTP > 12 months, fit using restricted cubic splines. The reference level for the RR is 0 g (unexposed). The curves are adjusted for maternal age, paternal age, year of pregnancy, maternal education, paternal occupation, first trimester cigarette smoking, first trimester alcohol intake, and pregnancy number. The solid lines represent the RR, and the shaded area represents the 95% confidence band. The splines are trimmed at the 95th percentile (due to the highly right-skewed distributions) and have three knot points each at the 50th, 75th, and 90th percentiles.
Table 3.
PCE exposure and risk of TTP > 6 months.
| Exposure | Cases/N | Crude RR (95% CI) | Adjusteda RR (95% CI) | Adjustedb RR (95% CI) |
|---|---|---|---|---|
| Cumulative PCE exposure (grams) | ||||
| None | 435/2202 | Reference | Reference | Reference |
| Any | 288/1624 | 0.97 (0.84, 1.11) | 0.83 (0.72, 0.96) | 0.87 (0.75, 1.01) |
| None | 435/2202 | Reference | Reference | Reference |
| > 0– < 10 | 90/546 | 0.89 (0.73, 1.08) | 0.79 (0.65, 0.96) | 0.82 (0.67, 1.01) |
| 10– < 100 | 118/663 | 1.03 (0.86, 1.23) | 0.89 (0.74, 1.07) | 0.94 (0.77, 1.13) |
| ≥ 100 | 80/415 | 0.99 (0.78, 1.26) | 0.81 (0.63, 1.03) | 0.84 (0.65, 1.07) |
| Average monthly PCE exposure (grams) | ||||
| None | 491/2518 | Reference | Reference | Reference |
| Any | 232/1308 | 0.97 (0.85, 1.11) | 0.89 (0.78, 1.02) | 0.91 (0.79, 1.05) |
| None | 491/2518 | Reference | Reference | Reference |
| > 0– < 1 | 121/798 | 0.85 (0.72, 1.00) | 0.76 (0.62, 0.91) | 0.79 (0.66, 0.95) |
| 1– < 2.5 | 47/215 | 1.11 (0.88, 1.40) | 1.03 (0.81, 1.30) | 1.02 (0.80, 1.29) |
| ≥ 2.5 | 64/295 | 1.19 (0.96, 1.48) | 1.12 (0.91, 1.39) | 1.10 (0.89, 1.36) |
Adjusted for maternal age.
Adjusted for maternal age, paternal age, year of pregnancy, maternal education, paternal occupation, first trimester cigarette smoking, first trimester alcohol intake, and pregnancy number.
Results were similar when we assessed TTP as a 4-level outcome (Table 4), with some evidence of an inverse relationship with cumulative PCE exposure, as well as a positive association between the highest level of average monthly PCE exposure and risk of having TTP > 12 vs. < 3 months (RR for ≥ 2.5 g vs. unexposed = 1.26, 95% CI: 1.01, 1.58).
Table 4.
PCE exposure and time-to-pregnancy.
| TTP 3–6 months vs. < 3 months |
TTP 7–12 months vs. < 3 months |
TTP > 12 months vs. < 3 months |
||||
|---|---|---|---|---|---|---|
| Exposure | Events/N | Adjusteda RR (95% CI) | Events/N | Adjusteda RR (95% CI) | Events/N | Adjusteda RR (95% CI) |
| Cumulative PCE exposure (grams) | ||||||
| None | 335/2202 | Reference | 163/2202 | Reference | 272/2202 | Reference |
| Any | 270/1624 | 0.96 (0.83, 1.12) | 114/1624 | 0.89 (0.71, 1.13) | 174/1624 | 0.85 (0.71, 1.01) |
| None | 335/2202 | Reference | 163/2202 | Reference | 272/2202 | Reference |
| > 0– < 10 | 90/546 | 0.96 (0.78, 1.17) | 40/546 | 0.90 (0.65, 1.23) | 50/546 | 0.80 (0.63, 1.00) |
| 10– < 100 | 110/663 | 0.96 (0.79, 1.16) | 47/663 | 0.96 (0.72, 1.28) | 71/663 | 0.89 (0.70, 1.11) |
| ≥ 100 | 70/415 | 0.99 (0.77, 1.27) | 27/415 | 0.78 (0.52, 1.16) | 53/415 | 0.86 (0.64, 1.15) |
| Average monthly PCE exposure (grams) | ||||||
| None | 392/2518 | Reference | 185/2518 | Reference | 306/2518 | Reference |
| Any | 213/1308 | 0.95 (0.83, 1.10) | 92/1308 | 0.91 (0.74, 1.13) | 140/1308 | 0.87 (0.74, 1.02) |
| None | 392/2518 | Reference | 185/2518 | Reference | 306/2518 | Reference |
| > 0– < 1 | 132/798 | 0.96 (0.81, 1.13) | 58/798 | 0.94 (0.73, 1.21) | 63/798 | 0.67 (0.54, 0.83) |
| 1– < 2.5 | 33/215 | 0.94 (0.73, 1.21) | 16/215 | 1.03 (0.70, 1.53) | 31/215 | 0.98 (0.73, 1.31) |
| ≥ 2.5 | 48/295 | 0.96 (0.76, 1.22) | 18/295 | 0.78 (0.50, 1.21) | 46/295 | 1.26 (1.01, 1.58) |
Adjusted for maternal age, paternal age, year of pregnancy, maternal education, paternal occupation, first trimester cigarette smoking, first trimester alcohol intake, and pregnancy number.
The positive association between average monthly PCE exposure and risk of TTP > 12 months was stronger among women ≥ 30 years old (RR = 1.60, 95% CI: 1.09, 2.35) than among women < 30 years old (RR = 1.16, 95% CI: 0.81, 1.66), but was similar among nulliparous and parous women. The inverse association between cumulative PCE exposure and risk of TTP > 12 months was stronger among women < 30 years old and parous women (Supplemental Tables 1 and 2). When we examined the association among first pregnancies only (n = 1297), we found little evidence of an inverse association between cumulative PCE exposure and risk of TTP > 12 months; the point estimate comparing average monthly PCE exposure of ≥ 2.5 g vs. unexposed (RR = 1.32, 95% CI: 0.86, 2.04) was similar to the main analysis, but results were less precise. Treating TTP as a continuous variable and assigning values of 2, 5, 10, and 18 months to each woman reporting TTP in the four ranked categories, respectively, resulted in a downward shift in RRs (RR comparing average monthly PCE exposure of ≥ 2.5 g with no exposure was 1.24 (95% CI: 0.95–1.62)). When we restricted to pregnancies that ended before the flushing and bleeding began, the inverse association between cumulative PCE and TTP > 12 months was slightly stronger (RR = 0.79, 95% CI: 0.61, 1.01). The positive association between average monthly PCE ≥ 2.5 g vs. unexposed and TTP > 12 months was weaker (RR = 1.15, 95% CI: 0.82, 1.63).
In the probabilistic bias analysis, the median RR comparing risk of TTP > 12 months among women with any vs. no cumulative PCE exposure was 0.85, with a 95% simulation interval of 0.65–0.98 (observed RR = 0.85). The median RR comparing average monthly PCE exposure ≥ 2.5 g with no exposure was 1.39, with a 95% simulation interval of 0.84–1.72 (observed RR = 1.36).
3.1. Discussion
We found little evidence for long-term, cumulative adverse effects of PCE exposure on TTP, but PCE exposure around the time of the pregnancy attempt was associated with longer TTP. The spline analysis indicated a threshold effect; associations between average monthly PCE exposure and risk of TTP > 12 months were only present at exposure levels ≥ 2.5 g per month. Results were stronger among women aged ≥ 30 years, but were similar for nulliparous and parous women. Conversely, cumulative PCE exposure was associated with lower risk of TTP > 12 months, regardless of exposure level, although subsequent sensitivity analyses call into question the validity of this finding.
Our finding of an increased risk of TTP > 12 months among women with the highest average monthly exposure is consistent with studies examining TTP among female dry cleaning workers. Danish women currently exposed to dry-cleaning chemicals had a 60% higher risk of infertility compared with unexposed women (Rachootin and Olsen, 1983). Likewise, a study of Finnish women occupationally exposed to organic solvents found that current dry cleaning work was associated with reduced fecundability (fecundability ratio (FR), the probability of pregnancy in exposed compared with unexposed women = 0.44); FRs for low and high exposure to PCE were 0.63 and 0.69, respectively (Sallmen et al., 1995). Female exposure to other volatile organic solvents, including acetone, toluene, and ethylene glycol ethers, has also been associated with reduced fecundability and increased infertility risk in occupational studies (Chen et al., 2002; Correa et al., 1996; Eskenazi et al., 1995; Plenge-Bonig and Karmaus, 1999; Sallmen et al., 2006; Smith et al., 1997; Wennborg et al., 2001).
Studies of male exposure are relevant to the present study because our PCE exposure metric was based on residence, and the vast majority of couples planning a pregnancy reside at the same address. Therefore, we were unable to distinguish between female and male exposure and our results could reflect an association with PCE exposure to either sex. Prior studies have found that male exposure to PCE or other organic solvents may be associated with longer TTP (Cherry et al., 2001; Eskenazi et al., 1991a; Sallmen et al., 2006), in vitro fertilization (IVF) failure (Tielemans et al., 2000), and poor semen quality (Chia et al., 1996; Eskenazi et al., 1991b; Tielemans et al., 1999). An occupational study in California found subtle changes in semen quality among dry cleaning workers compared with laundry workers (Eskenazi et al., 1991b); the wives of dry cleaning workers also had lower fecundability (FR = 0.54) than the wives of laundry workers (Eskenazi et al., 1991a). In a study of 726 Dutch couples undergoing IVF, couples where the male was exposed to organic solvents had lower implantation rates than unexposed men (Tielemans et al., 2000). A Finnish study conducted as part of a biologic monitoring program of men occupationally exposed to organic solvents found increased TTP in first pregnancies among men with organic solvent exposure (Sallmen et al., 1998).
The difference in results for cumulative PCE exposure and average monthly PCE exposure could speak to potential biologic mechanisms, if the association is causal. Because we observed an increased risk of longer TTP among women with the highest average monthly PCE exposure, but not among women with the highest cumulative exposure, PCE exposure around the time of conception, rather than long-term exposure, may be more temporally relevant. In the short-term, PCE exposure may influence menstrual function, hormone levels, or sperm quality (Zielhuis et al., 1989). It may cause an increased risk of early fetal loss (Agnesi et al., 1997; Doyle et al., 1997; Kolstad et al., 1990; Kyyronen et al., 1989; Lindbohm et al., 1990; Olsen et al., 1990; Windham et al., 1991), either by affecting implantation, placental function, or increasing risk of congenital anomalies (Bove et al., 1995; Goldberg et al., 1990; Khattak et al., 1999; Lagakos et al., 1986; McMartin et al., 1998). If the early fetal loss is not clinically recognized, it would result in an apparently longer TTP. Our analysis of cumulative PCE exposure indicate that it is unlikely that long-term PCE exposure depletes ovarian reserve or harms oocyte quality.
An unusual feature of this study design for our research question is that we only had information on pregnancy attempts that were successful (i.e., resulted in a clinically-recognized pregnancy). The Cape Cod Family Health Study was designed to examine the effects of prenatal PCE exposure on neonatal and childhood health, so only women who had a live birth during the study period were eligible, and we did not collect information on the timing of unsuccessful pregnancy attempts. Conditioning on pregnancy precluded estimation of absolute TTP among exposed and unexposed women and therefore restricted our analysis to relative measures of association. In addition, it likely resulted in some selection bias. If PCE exposure does lengthen TTP, its effect would be underestimated in this study because the women who never conceived are not represented in our study population.
Our analytic sample was restricted to planned pregnancies. However, we observed similar PCE concentrations in planned and unplanned pregnancies, indicating that selection into the study by pregnancy planning status is unlikely to result in substantial selection bias.
We attempted to quantify the extent of outcome misclassification on our findings by repeatedly sampling from sensitivity and specificity distributions defined using external validation data and calculating corrected RRs for each simulation. The bias analysis results for cumulative PCE exposure (exposed vs. unexposed) suggest that while there is some uncertainty regarding the size of the association, it is unlikely that outcome misclassification obscured a true positive effect. The 95% simulation interval for RR values ranged from 0.65 to 0.98, providing little indication that any plausible misclassification obscured a meaningful positive effect. In contrast, for average monthly PCE exposure (≥ 2.5 g vs. unexposed), the bias analysis results suggested a wide range of plausible true values (95% simulation interval 0.84–1.72), from a weak inverse to a strong positive association.
Because individual exposure measurements at the time of each pregnancy attempt were not available, we relied on a leaching and transport model to estimate historical PCE exposures (Webler and Brown, 1993). To the extent that the model assumptions were incorrect, or the model inputs were measured with error, exposure will be misclassified as a result. A validation study comparing model-based PCE exposure estimates with PCE concentrations in historical water samples showed reasonable correlation (Spearman correlation coefficient = 0.65) (Gallagher et al., 2011). The model estimates the annual amount of PCE that entered the residence, but does not account for individual variation in water consumption habits. A previous study in this population, however, found that conducting a more detailed personal exposure assessment that incorporated information on bathing, showering and drinking habits did not substantially change the exposure ranking of subjects or measures of association (Vieira et al., 2005). This may reflect poor recall of or lack of variability in water-related behaviors or may indicate that personal exposures were driven by residence, not behaviors. The model also does not account for non-residential drinking water exposures. Because older women may spend more time at home than younger women, their exposure misclassification due to non-residential sources of drinking water is likely lower. This difference could explain the stronger association we observed among women ≥ 30 years old compared with women < 30 years old. However, we expected the same pattern to hold among parous women, who also spend more time at home, but we did not observe differences between parous and nulliparous women.
Exposure misclassification may also have resulted from imperfect information on when women started trying to conceive. The study collected information on TTP as a categorical variable, which made it difficult to identify the month women started trying to conceive, in particular among women who reported trying for > 12 months, where the range of possible values is wide. Our sensitivity analysis that converted the categorical variable into a continuous one, with TTP values of 2, 5, 10, and 18 for each category of TTP, showed that exposure estimates were relatively sensitive to our choice of continuous TTP. Assigning longer continuous TTP values resulted in a downward shift in RRs, due to the generally lower exposures associated with beginning the pregnancy attempt at an earlier date. This analysis reduced exposures for the infertile group the most, which explains the downward shift in RRs.
We did not collect information on behaviors during the preconception period that could affect infertility risk. Instead, we relied on pregnancy behaviors as a proxy for preconception behaviors. However, given the irregular pattern of pipe installation on Cape Cod, very few behavioral or demographic characteristics were associated with exposure, and it is unlikely that unmeasured confounding explained our results.
Confounding by time was likely in our analysis because pregnancies ended between 1949 and 1990, and both PCE exposure and TTP were correlated with time. Cumulative exposure, by definition, increases or stays constant over time and average monthly PCE exposure was 0 from 1949 to 1968, increased from 1969 to 1972, stayed relatively constant from 1973 to 1981, then decreased from 1982 onwards. Pregnancies that occurred later in the study period were to women who were older and had demonstrated fecundity. If the most fertile women were having pregnancies later in the study period, when their cumulative exposure was highest, this could account for the observed inverse association between cumulative PCE exposure and longer TTP. We attempted to control for these temporal patterns by adjusting for maternal age, year of pregnancy, and pregnancy number. We also conducted a sensitivity analysis restricting the analytic sample to first pregnancies, and observed a much weaker inverse association between cumulative PCE exposure and risk of TTP > 12 months (RR = 0.92 compared with 0.85 in the main analysis). Residual confounding by time may have contributed to the observed inverse association. We observed a similar association between the highest average monthly PCE exposure in all pregnancies (RR = 1.36) and first pregnancies (RR = 1.32).
Exposures were based on the annual mass of PCE that entered the home. This mass was diluted in approximately 90,000 gallons of water, the average annual usage of households in Massachusetts (Massachusetts Water Resources Authority, 2003). We observed an increased risk of TTP > 12 months at average monthly PCE levels of ≥ 2.5 g, which corresponds to average concentrations of approximately ≥ 88 μg/L for the year. Our results indicate that adverse effects on fertility are unlikely to be experienced by the general population at levels below the current EPA regulations, but higher levels of exposure that may occur in occupationally-exposed populations or from environmental contamination could lengthen TTP.
Supplementary Material
Acknowledgements
We thank Mr. Michael Winter for his assistance with data management; Dr. Matthew Fox for his guidance on epidemiologic methods; and Dr. Anne Marie Jukic for her critical review of this manuscript.
Funding source
This work was supported by the National Institute of Environmental Health Sciences Superfund Research Program (5P42-ES007381) and the National Institutes of Health (T32-HD052458). The funding source did not play a role in study design; collection, analysis, or interpretation of data; in writing of this report; or in the decision to submit the article for publication.
The institutional review boards of Boston University Medical Center and the Massachusetts Department of Public Health approved the study protocol. All participants provided informed consent.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.envres.2018.07.012.
References
- Agency for Toxic Substances and Disease Registry, 1997. Toxicological Profile for Tetrachloroethylene. US Department of Health and Human Services, Atlanta. [PubMed] [Google Scholar]
- Agnesi R, et al. , 1997. Risk of spontaneous abortion and maternal exposure to organic solvents in the shoe industry. Int. Arch. Occup. Environ. Health 69, 311–316. [DOI] [PubMed] [Google Scholar]
- Aral MM, et al. , 1996. Estimating exposure to volatile organic compounds from municipal water-supply systems: use of a better computational model. Arch. Environ. Health 51, 300–309. [DOI] [PubMed] [Google Scholar]
- Aschengrau A, et al. , 2016. Long-term neurotoxic effects of early-life exposure to tetrachloroethylene-contaminated drinking water. Ann. Glob. Health 82, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschengrau A, et al. , 2003. Perchloroethylene-contaminated drinking water and the risk of breast cancer: additional results from Cape Cod, Massachusetts, USA. Environ. Health Perspect 111, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschengrau A, et al. , 2008. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of adverse birth outcomes. Environ. Health Perspect 116, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschengrau A, et al. , 2009a. Exposure to tetrachloroethylene-contaminated drinking water and the risk of pregnancy loss. Water Qual. Expo. Health 1, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschengrau A, et al. , 2009b. Prenatal exposure to tetrachloroethylene-contaminated drinking water and the risk of congenital anomalies: a retrospective cohort study. Environ. Health 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DD, et al. , 1991. Reporting errors in time-to-pregnancy data collected with a short questionnaire. Impact on power and estimation of fecundability ratios. Am. J. Epidemiol 133, 1282–1290. [DOI] [PubMed] [Google Scholar]
- Beliles RP, et al. , 1980. Teratogenic-Mutagenic Risk of Workplace Contaminants: Trichloroethylene, Perchloroethylene, and Carbon Disulfide (210–77-0047). National Institute for Occupation Safety and Health, Cincinnati, OH. [Google Scholar]
- Berger T, Horner CM, 2003. In vivo exposure of female rats to toxicants may affect oocyte quality. Reprod. Toxicol 17, 273–281. [DOI] [PubMed] [Google Scholar]
- Bove FJ, et al. , 1995. Public drinking water contamination and birth outcomes. Am. J. Epidemiol 141, 850–862. [DOI] [PubMed] [Google Scholar]
- Chen PC, et al. , 2002. Prolonged time to pregnancy in female workers exposed to ethylene glycol ethers in semiconductor manufacturing. Epidemiology 13, 191–196. [DOI] [PubMed] [Google Scholar]
- Cherry N, et al. , 2001. Occupational exposure to solvents and male infertility. Occup. Environ. Med 58, 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SE, et al. , 1996. Semen parameters in workers exposed to trichloroethylene. Reprod. Toxicol 10, 295–299. [DOI] [PubMed] [Google Scholar]
- Cooney MA, et al. , 2009. Validity of self-reported time to pregnancy. Epidemiology 20, 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, et al. , 1996. Ethylene glycol ethers and risks of spontaneous abortion and subfertility. Am. J. Epidemiol 143, 707–717. [DOI] [PubMed] [Google Scholar]
- Demond AH, 1982. A Source of Tetrachloroethylene in the Drinking Water of New England: An Evaluation of Toxicity of Tetrachloroethyelen and the Prediction of Its Leaching Rates from Vinyl-lines Asbestos Cement Pipe. Massachusetts Institute of Technology, Cambridge, MA. [Google Scholar]
- Donders AR, et al. , 2006. Review: a gentle introduction to imputation of missing values. J. Clin. Epidemiol 59, 1087–1091. [DOI] [PubMed] [Google Scholar]
- Doyle P, et al. , 1997. Spontaneous abortion in dry cleaning workers potentially exposed to perchloroethylene. Occup. Environ. Med 54, 848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrleman S, Simon R, 1989. Flexible regression models with cubic splines. Stat. Med 8, 551–561. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency, 2012. Toxicological Review of Tetrachloroethylene (Perchloroethylene). U.S. Environmental Protection Agency, Washington, DC. [Google Scholar]
- Eskenazi B, et al. , 1991a. A study of the effect of perchloroethylene exposure on the reproductive outcomes of wives of dry-cleaning workers. Am. J. Ind. Med 20, 593–600. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. , 1995. Prospective assessment of fecundability of female semiconductor workers. Am. J. Ind. Med 28, 817–831. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. , 1991b. A study of the effect of perchloroethylene exposure on semen quality in dry cleaning workers. Am. J. Ind. Med 20, 575–591. [DOI] [PubMed] [Google Scholar]
- Gallagher LG, et al. , 2011. Risk of breast cancer following exposure to tetrachloroethylene-contaminated drinking water in Cape Cod, Massachusetts: reanalysis of a case-control study using a modified exposure assessment. Environ. Health 10, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MD, et al. , 1998. Exposure to trihalomethanes and adverse pregnancy outcomes. Epidemiology 9, 484–489. [PubMed] [Google Scholar]
- Goldberg SJ, et al. , 1990. An association of human congenital cardiac malformations and drinking water contaminants. J. Am. Coll. Cardiol 16, 155–164. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2014. IARC Monographs on the evaluation of carcinogenic risks to humans, vol 106. Some chlorinated solvents and their metabolites. IARC, Lyon. [Google Scholar]
- Joffe M, et al. , 1993. Long-term recall of time-to-pregnancy. Fertil. Steril 60, 99–104. [DOI] [PubMed] [Google Scholar]
- Jukic AM, et al. , 2016. Long-term recall of time to pregnancy. Epidemiology 27, 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak S, et al. , 1999. Pregnancy outcome following gestational exposure to organic solvents: a prospective controlled study. JAMA 281, 1106–1109. [DOI] [PubMed] [Google Scholar]
- Kolstad HA, et al. , 1990. Chlorinated solvents and fetal damage. Spontaneous abortions, low birth weight and malformations among women employed in the dry-cleaning industry. Ugeskr. Laege 152, 2481–2482. [PubMed] [Google Scholar]
- Kyyronen P, et al. , 1989. Spontaneous abortions and congenital malformations among women exposed to tetrachloroethylene in dry cleaning. J. Epidemiol. Community Health 43, 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagakos SW, et al. , 1986. An analysis of contaminated well water and health effects in Woburn, Massachusetts. J. Am. Stat. Assoc 81, 583–596. [Google Scholar]
- Larson CD, et al. , 1983. Tetrachloroethylene leached from lined asbestos-cement pipe into drinking water. JAWWA 75, 184–190. [Google Scholar]
- Lash TL, et al. , 2009. Applying Quantitative Bias Analysis to Epidemiologic Data. Springer-Verlag, New York. [Google Scholar]
- Li R, et al. , 2008. The SAS GLMCURV9 Macro. Channing Laboratory, Boston, MA. [Google Scholar]
- Lindbohm ML, et al. , 1990. Spontaneous abortions among women exposed to organic solvents. Am. J. Ind. Med 17, 449–463. [DOI] [PubMed] [Google Scholar]
- Maslia ML, et al. , 1996. Use of computational models to reconstruct and predict trichloroethylene exposure. Toxicol. Ind. Health 12, 139–152. [PubMed] [Google Scholar]
- Massachusetts Water Resources Authority, 2003. MWRA Annual Report on Your Drinking Water. Vol. 2005. [Google Scholar]
- McMartin KI, et al. , 1998. Pregnancy outcome following maternal organic solvent exposure: a meta-analysis of epidemiologic studies. Am. J. Ind. Med 34, 288–292. [DOI] [PubMed] [Google Scholar]
- Olsen J, et al. , 1990. Low birthweight, congenital malformations, and spontaneous abortions among dry-cleaning workers in Scandinavia. Scand. J. Work Environ. Health 16, 163–168. [DOI] [PubMed] [Google Scholar]
- Plenge-Bonig A, Karmaus W, 1999. Exposure to toluene in the printing industry is associated with subfecundity in women but not in men. Occup. Environ. Med 56, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachootin P, Olsen J, 1983. The risk of infertility and delayed conception associated with exposures in the Danish workplace. J. Occup. Med 25, 394–402. [PubMed] [Google Scholar]
- Radin RG, et al. , 2015. Maternal recall error in retrospectively reported time-to-pregnancy: an assessment and bias analysis. Paediatr. Perinat. Epidemiol 29, 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif JS, et al. , 2003. Neurobehavioral effects of exposure to trichloroethylene through a municipal water supply. Environ. Res 93, 248–258. [DOI] [PubMed] [Google Scholar]
- Rossman LA, 1994. EPANET Users Manual. R. R. E. L. U.S. Environmental ProtectionAgency, Cincinnati, OH. [Google Scholar]
- Rothman KJ, et al. , 2008. Modern Epidemiology, 3rd ed. Lippincott, Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Rothman KJ, et al. , 2011. Should graphs of risk or rate ratios be plotted on a log scale? Am. J. Epidemiol 174, 376–377 (discussion 377). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmen M, et al. , 2006. Fertility and exposure to solvents among families in the Agricultural Health Study. Occup. Environ. Med 63, 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmen M, et al. , 1998. Time to pregnancy among the wives of men exposed to organic solvents. Occup. Environ. Med 55, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmen M, et al. , 1995. Reduced fertility among women exposed to organic solvents. Am. J. Ind. Med 27, 699–713. [DOI] [PubMed] [Google Scholar]
- Schafer JL, 1997. Analysis of Incomplete Multivariate Data. CRC Press, Boca Raton, FL. [Google Scholar]
- Schafer JL, 1999. Multiple imputation: a primer. Stat. Methods Med. Res 8, 3–15. [DOI] [PubMed] [Google Scholar]
- Sherlach KS, et al. , 2011. Quantification of perchloroethylene residues in dry-cleaned fabrics. Environ. Toxicol. Chem 30, 2481–2487. [DOI] [PubMed] [Google Scholar]
- Smith EM, et al. , 1997. Occupational exposures and risk of female infertility. J. Occup. Environ. Med 39, 138–147. [DOI] [PubMed] [Google Scholar]
- Sterne JA, et al. , 2009. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338, b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz CH, et al. , 2003. Historical reconstruction of wastewater and land use impacts to groundwater used for public drinking water: exposure assessment using chemical data and GIS. J. Expo. Anal. Environ. Epidemiol 13, 403–416. [DOI] [PubMed] [Google Scholar]
- Tielemans E, et al. , 1999. Occupationally related exposures and reduced semen quality: a case-control study. Fertil. Steril 71, 690–696. [DOI] [PubMed] [Google Scholar]
- Tielemans E, et al. , 2000. Paternal occupational exposures and embryo implantation rates after IVF. Fertil. Steril 74, 690–695. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency, 2009. National Primary Drinking Water Regulations. List of Contaminants and their Maximum Contaminant Levels (MCLs).
- Vieira V, et al. , 2005. Impact of tetrachloroethylene-contaminated drinking water on the risk of breast cancer: using a dose model to assess exposure in a case-control study. Environ. Health 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webler T, Brown HS, 1993. Exposure to tetrachloroethylene via contaminated drinking water pipes in Massachusetts: a predictive model. Arch. Environ. Health 48, 293–297. [DOI] [PubMed] [Google Scholar]
- Wennborg H, et al. , 2001. Solvent use and time to pregnancy among female personnel in biomedical laboratories in Sweden. Occup. Environ. Med 58, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, et al. , 1991. Exposure to organic solvents and adverse pregnancy outcome. Am. J. Ind. Med 20, 241–259. [DOI] [PubMed] [Google Scholar]
- Wu KL, Berger T, 2007. Trichloroethylene metabolism in the rat ovary reduces oocyte fertilizability. Chem. Biol. Interact 170, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, et al. , 2004. Exposure to trichloroethylene and its metabolites causes impairment of sperm fertilizing ability in mice. Toxicol. Sci 82, 590–597. [DOI] [PubMed] [Google Scholar]
- Zielhuis GA, et al. , 1989. Menstrual disorders among dry-cleaning workers. Scand. J. Work Environ. Health 15, 238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.