Abstract
Objective
Our aim was to determine topographical variations in zonal properties of articular cartilage over the medial tibia in an experimental osteoarthritis (OA) model using 7-T magnetic resonance imaging (MRI).
Materials and methods
An anterior cruciate ligament (ACL)-transection canine model was subjected to study at 8 (six) and 12 (seven) weeks after the surgery. Each medial tibia was divided into five topographical locations. For each specimen, T2 relaxation (at 0° and 55°) was quantified at microscopic resolution. The imaging data grouped the five locations into two topographical areas (meniscus-covered and -uncovered).
Results
The T2 (55°) bulk values from the meniscus-covered area were significantly lower than those from the uncovered area. The total cartilage thicknesses on the meniscus-covered area were significantly thinner than those on the meniscus-uncovered area. Significant differences in the T2 (0°) values were observed in most thicknesses of the four subtissue zones and whole-tissue from the uncovered area, while the same significant changes were detected in the superficial zone from the meniscus-covered area.
Conclusion
By quantifying high-resolution imaging data both topographically and depth-dependently (zonal-wise), this study demonstrates that the rate of disease progression varies topographically over the medial tibia. Future correlation with OA pathology could lead to better detection of early OA.
Keywords: Microscopic MRI (μMRI), Cartilage, T2 relaxation time, Osteoarthritis, Anterior cruciate ligament
Introduction
Osteoarthritis (OA) is the most common type of arthritis and affects a major portion of the adult population [1, 2]. The disease is characterized by proteoglycan loss, osteophyte formation, synovitis, and subchondral bone changes. Slow progression of the disease causes cartilage to have different pathological characteristics and localizations at different times after the onset of degradation [3], which contributes to the difficulty of establishing an accurate diagnostic standard. Magnetic resonance imaging (MRI) has been widely used in clinical research and diagnosis of OA. High-field systems (≥7 T) with advanced coil technology significantly improve the ability of quantitative high-resolution morphological, biochemical, and molecular features and functional imaging of musculoskeletal tissues [4–6]. Several MRI parameters and protocols, such as T2 and T1ρ relaxations, sodium imaging, and the use of contrast agents, have been developed to quantify water motion, proteoglycan (PG) concentration, and collagen structure in cartilage matrices [7–9]. Among these MRI techniques, T2 relaxation time is sensitive to molecular interactions related to integrity, orientation, and anisotropy of the extracellular matrix (ECM) in cartilage [7, 8, 10–12] and is reported to have higher values with the advancement of OA pathology [13–16].
Since biochemical properties of healthy cartilage are known to be different at different locations on the knee surface [13, 17], early OA must vary topographically (variation in surface sites) and depth dependently (zonal variations) within a single knee joint. Quantitative measurement of the clinically important and topographically complicated joint structures such as the knee, however, is largely lacking in the literature due to the fact that an adequate description of the zonal properties of articular cartilage require microscopic resolution on imaging. In this regard, high-field microscopic MRI (μMRI) using animal models has a unique value in clinical OA research because it bypasses the resolution limitation in clinical MRI of human cartilage. In addition, onset of the disease can be initiated in an animal model, which can be subsequently monitored during the degradation process.
The surgical procedure of anterior cruciate ligament transection (ACLT) in dogs is the best characterized and most commonly used animal model in which induced instability eventually leads to complete loss of cartilage during a process that mimics human OA progression [18, 19]. Since the canine cartilage is thinner than that in humans, high spatial resolution in imaging becomes extremely critical. In this project, we used high-field μMRI to study the intact canine cartilage-bone specimens from five different locations of the medial tibial surface. We hypothesized that zonal distributions of tissue thickness and T2 would vary topographically during progression of cartilage degradation in the animal model. This project is significant because it puts a set of already validated, clinically important, and highly sensitive parameters into use in the first comprehensive investigation of the complex progression due to ACLT-induced OA at microscopic resolution.
Materials and methods
Cartilage specimens
With the approval of local institutional review committees, 13 skeletally mature dogs underwent ACL transection in one knee joint. The contralateral knee served as the non-operated control. Six animals were sacrificed 8 weeks postsurgery (one female and five males, 22–30 kg); the other seven animals were sacrificed 12 weeks postsurgery (three females and four males, 18–29 kg). Within 24 h after sacrifice, the medial tibial plateau from each knee was sectioned into five rectangular specimens (3.0 × 2.5 × 4–5 mm3), as shown in Fig. 1. Each specimen included the full thickness of the articular cartilage attached to the underlying bone. Prior to the μMRI experiments, specimens were equilibrated in 1-mM gadolinium solution (Magnevist, Berlex, NJ, USA) for a minimum of 10 h (at 4 °C) and sealed in precision nuclear magnetic resonance (NMR) tubes with an internal diameter of 4.34 mm (Wilmad Glass, Buena, NJ, USA). A total of 130 specimens from the 26 medial tibias were imaged; specimens were never frozen.
Fig. 1.
Tibia surface with topographical locations of five specimens from the medial tibia used in imaging experiments. Imaging slice locations were transverse at the center of each tissue block. Anterior medial tibia (AMT), exterior medial tibia (EMT), and posterior medial tibia (PMT) are covered by meniscus, whereas central medial tibia (CMT) and interior medial tibia (IMT) are not
High-field μMRI protocols
T2-weighted imaging experiments were performed using a Bruker AVANCE II 300 NMR spectrometer with a vertical-bore magnet (7 T/89 mm), which has a microimaging accessory (Bruker Instrument, Billerica, MA, USA) and a homemade 5-mm solenoid coil. Using a magnetization-prepared T2 imaging sequence [7], T2-weighted images of the specimen were acquired at two orientations of the specimen in the magnet—when the normal surface axis of the tissue block was 0° and 55° to the direction of the magnetic field B0. (It should be noted that for clinical MRI of the human knee with a horizontal magnet, the tibial cartilage is also oriented in small angles around 0°. The small variation comes mainly from the natural contour of a tibial surface.) Echo time of the leading contrast segment (TEc) in the pulse sequence had five increments (2, 8, 20, 40, 80, or 90 ms), and echo time of the imaging sequence (TE) was 7.2 ms. T2 relaxation time in cartilage was calculated pixel by pixel using a single exponential fitting on MatLab (MathWorks, Natick, MA, USA). The field of view (FOV) was 0.45 × 0.45 cm; imaging matrix size was 256 × 256; slice thickness was 0.8 mm; in-plane pixel resolution was 17.6 μm. Other experimental details have been described previously [17].
Image analysis
Quantitative T2 images of each specimen were analyzed by applying a ten-pixel-wide region of interest (ROI) to each 2D image, which generated a 1D profile that covered the entire tissue thickness. Since the averaging never occurred along the tissue depth, the depth-wise pixel resolution remained 17.6 μm. The total cartilage thickness of each specimen was measured from the T2 proton images at the 55° orientation. The zonal boundaries inside cartilage were determined from the T2 profiles at 0°, according to criteria established and validated previously [17, 20]. Four subtissue zones were defined across the depth of cartilage: superficial zone (SZ), transitional zone (TZ), and radial zone (RZ), which was divided equally into two halves (upper RZ as RZ1 and lower RZ as RZ2). Bulk and zonal T2 values were averaged, respectively, in all subtissue zones. When the T2 (0°) profile was not the typical bell shape, zones were divided using the averaged relative zonal thickness at each location from the bell-shaped T2 (0°) profiles (Table 1).
Table 1.
Topographical map of different parameters at bulk and each subtissue zone
 |
Statistical analysis
Six animals at 8 weeks and seven animals at 12 weeks were studied and analyzed and referenced to data reported earlier from seven healthy animals [17]. Each joint was evaluated using five predefined specimen locations (Fig. 1), where each specimen had four spatially resolved zonal profiles (SZ, TZ, RZ1, RZ2). Imaging data were averaged into the four individual zonal areas and also grouped into one bulk group. To simplify analysis of the complex data, the five specimens were grouped into two topographical regions: the meniscus-covered and the meniscus-uncovered area [17]. Results are expressed as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) with Bonferroni correction was used to compare the five locations and both OA time points independently. Kruskal–Wallis rank sum test was used to compare the meniscus-covered and -uncovered areas at each postoperative week. Paired Student’s t-test was used to compare contralateral and OA joints at each postoperative week. P values <0.05 were considered significant.
Results
Morphological observations
In all 8-week OA (8X) joints, medial menisci were partly torn, medial femoral condyles showed signs of partial cartilage erosion, and multiple osteophytes were found around the joint periphery. The articular cartilage of the 8X joints appeared more opaque compared with contralateral normal (N) joints, which appeared translucent. All 8X joints had lesions or fibrillation at uncovered areas [central medial tibia (CMT) and interior medial tibia (IMT)], and two 8X joints had severe lesions at covered areas [anterior medial tibia (AMT) and posterior medial tibia (PMT)]. All joints had excessive bone overgrowth at the posterior region and the far medial side of the tibia (AMT). All 12-week OA (12X) medial tibias were completely covered with a fibrillated meniscus. The lesions were clearly seen after opening the joints, with the meniscus being loose and appearing fibrillated. On the other hand, contralateral joints appeared to have little or no damage on the surface of the tissue. Menisci were intact and had the normal crescent shape. The only visual lesions observed were around the central region of the medial tibia.
Figure 2 shows a set of T2 (0° and 55°) images from PMT of N, 8-week contralateral (8C), and OA (8X), as well as 12-week contralateral (12C) and OA (12X) specimens. At 0°, all specimens had a typical laminar appearance seen in cartilage. Compared with normal specimens, the low-intensity band underneath the articular surface appeared thicker at 8X and thinner at 12X, and total cartilage thickness was greater in the OA specimens (Fig. 2a). (This visual observation was confirmed later in quantitative measurements in μMRI at microscopic resolution.) At the magic angle (55°), all 8C, 12C, and most 8X and 12X cartilages showed similar homogeneous appearance as N cartilage, while some 8X and 12X cartilage surfaces were visibly fibrillated on MRI.
Fig. 2.
Quantitative T2 microscopic magnetic resonance imaging (μMRI) at two orientations (0° and 55°) for five specimens: N normal, 8C 8 weeks contralateral, 8X 8 weeks osteoarthritis (OA), 12C 12 weeks contralateral, 12X 12 weeks OA. Angle was defined as the angle between the normal axis of the articular surface and the direction of the magnetic field (B0). The display Limits of all images are on the same intensity scale
Figure 3 shows the averaged total thickness of both meniscus-covered and -uncovered specimens of OA and contralateral knees. Total thickness of the covered area was significantly thinner than the uncovered area at all time points. For the covered area, total cartilage thickness remained relatively constant at N and 8X but increased significantly at 12X. For the uncovered area, total thickness increased significantly at 8X then decreased at 12X.
Fig. 3.
Average total thickness versus osteoarthritis (OA) progression (N–8X–12X and N–8C–12C) for meniscus-covered and -uncovered areas with Kruskal-Wallis rank sum test results. N normal, 8X 8 weeks osteoarthritis (OA), 12X 12 weeks OA, 8C 8 weeks contralateral, 12C 12 weeks contralateral
Depth-dependent profiles of T2 in cartilage
Figure 4 shows a set of T2 profiles from the N, 8X, and 12X specimens at both 0° (a) and 55° (b), which are plotted on the relative depth scale from the articular surface at 0 to the cartilage–bone interface at 1. T2 (0°) profiles of N (as well as 8C and 12C, not shown) and most 8X had a typical bell-shape curve, which was lost in 12X, with its T2 values increased significantly at SZ. The insert in Fig. 4a expands the superficial and transitional zones of the T2 profile, which has three types of T2 (0°) characteristics: a typical bell-shape curve (solid line), a non-bell-shaped curve (dotted line), and a linearly decreasing curve (dash line). Changes in these shapes correspond with OA progression, which implies increasing damage to the articular surface. The linearly decreasing trends were found in ten of 35 specimens for 12X, two of 30 for 8X, one of 35 for 12C, and one of 30 for 8C. These trends were found in both the meniscus-covered (seven) and the -uncovered (three) areas for 12X, at PMT and CMT for 8X, and at the uncovered area only for both 8C and 12C. T2 (55°) profiles (Fig. 4b) for N, 8X, and 12X covered specimens showed that T2 (55°) values increased with OA progression from N to 12X.
Fig. 4.
A representative set of 1D T2 profiles for N, 8X, and 12X specimens at 0° (a) and 55° (b) from the PMT location, plotted using a relative depth (0 articular surface, 1 cartilage bone interface). Inset in a shows the upper one third of the same data, together with the fitted T2 profiles (crosses and solid line normal, open squares and dotted line 8X, solid dots and dashed line 12X). Changes in the surface portion of T2 profiles are clear. N normal, 8X 8 weeks osteoarthritis (OA), 12X 12 weeks OA,
Topographical variations of zonal properties of OA cartilage
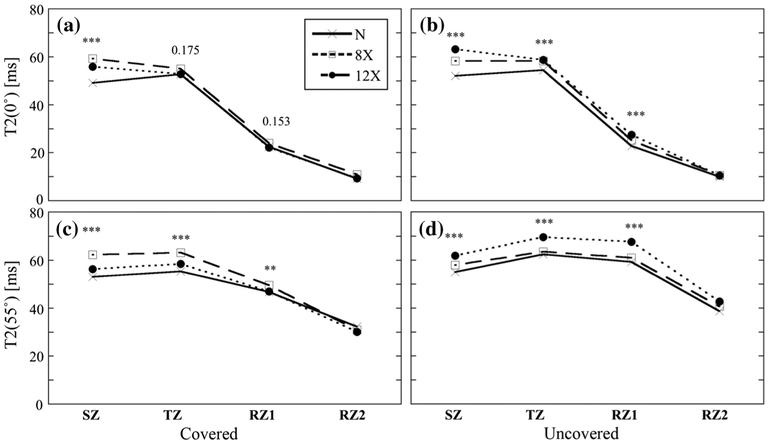
The entire depth of cartilage (as shown in Fig. 4) was divided into four subtissue zones (SZ, TZ, RZ1, and RZ2), where the zonal averaged T2 and thickness were quantitatively compared. Zonal division was based on the T2 (0°) profile (Fig. 4a). Table 1 summarizes zonal averaged topographical variations of both thickness and T2 in articular cartilage from the medial tibia at all OA time points. All zonal data were also grouped into bulk values in this table. Zonal T2 and mean values with SDs are summarized in Fig. 5 comparing covered and uncovered specimens for all OA time points. Several conclusions can be drawn from Fig. 5 and the tables.
Fig. 5.
T2 values at both 0° and 55°: 130 specimens are divided into different subgroups according to osteoarthritis (OA) time points and topographical areas (meniscus-covered and -uncovered) at each subtissue zone as well as the bulk. (Note that previous data from 70 normal healthy specimens were compared together [17] in this plot.). Each box represents mean value and standard deviation of T2 values. The 95 and 5 percentiles were cut off the top and bottom 5 % for each group
Tissue thickness. Total thickness of both normal and OA cartilage at the uncovered area was significantly thicker than the covered area. Zone wise, surface zones (SZ and TZ) at the uncovered area were thinner than those at the covered area and vice versa for radial zones (RZ1 and RZ2). For the covered area, the relative SZ and TZ increased until reaching a maximum at 12C, then decreased at 12X. The trend was opposite for the relative radial zones. For the uncovered area, the relative SZ and TZ decreased from 8 to 12 weeks, then vice versa for the relative radial zones, but showed no significance.
T2 (0°). Averaged T2 (0°) values showed three trends: (1) The typical bell-shape curve (TZ > SZ > RZ1 > RZ2) for both 8C and 12C (AMT, EMT, and IMT); (2) non-bell-shape curve (TZ ~ SZ > RZ1 > RZ2) for 8C and 12C(PMT, CMT), 8X (AMT, IMT), and 12X (CMT); (3) linearly decreasing trend (SZ > TZ > RZ1 > RZ2) for 8X (EMT, PMT, CMT) and 12X (AMT, EMT, PMT, and IMT). In most cases, uncovered tissues had higher T2 values than covered tissues, except in the SZ and RZ2 for 12X. Trend in the bulk T2 (0°) was the opposite at 12 weeks for both 12C and 12X: covered tissues had higher T2 (0°) values compared with uncovered tissues. T2 (0°) values increased with OA at all four zones at both covered and uncovered areas, except RZ1 (both covered and uncovered) and RZ2 (covered) at 12 weeks. The highest increase in T2 (0°; 10.7 ± 1.2 ms) with OA progression was found at SZ from the covered area at 12 weeks.
T2 (55°). Values of T2 (55°) were more homogeneous than those of T2 (0°) at all OA time points. In most cases, the uncovered tissues had higher T2 values than the covered ones at each zone, except in the SZ for 12X. The trend in bulk T2 (55°) was the same: 12C had the lowest T2 (55°) values in all zones, as well as bulk, among all OA time points. For both covered and uncovered tissues, T2 (55°) values increased with OA. At 8 weeks, uncovered tissue increased (p < 0.001) more than covered tissue (p > 0.05) with OA, and vice versa at 12 weeks. The most T2 (55°) with OA increase was found in the SZ of covered tissue at 12 weeks (p < 0.001).
Comparison of T2 values among normal, 8X, and 12X tissues can be seen more clearly in Fig. 6, as the zonal-averaged T2 profiles for the covered (Fig. 6a, c) and uncovered (Fig. 6b, d) areas, together with results of the Kruskal–Wallis rank sum test. T2 values among all OA time points showed statistically significant difference in each zone and bulk, except T2 (0°) in TZ and RZ1 for the covered area. For T2 (0°) profiles, the largest local changes were between SZ and TZ, where the increase at SZ resulted in loss of the bell-shaped curve (Fig. 4a for the high-resolution profiles). The smallest local changes of T2 occurred in the deep cartilage, where dipolar interaction was the strongest. Minimization of dipolar interaction at the magic angle allowed detection of T2 in the deep cartilage, as shown in Fig. 6c, d. Statistical analysis for T2 (0°) in RZ2 was not carried out, since T2 in the deep cartilage was low. The strong dipole–dipole interaction caused poor signal to noise ratio (SNR) in the deep cartilage.
Fig. 6.
Averaged zonal-dependent T2 profiles with Kruskal–Wallis rank sum test results between normal (N) and osteoarthritis (OA) specimens (8C and 12C are not shown) for the meniscus-covered [a T2 (0°), c T2 (55°)] and uncovered area [b T2 (0°), d T2 (55°)] at all subtissue zones. RZ2 was not tested in statistics due to its strong dipole–dipole interaction. 8C 8 weeks contralateral, 12C 12 weeks contralateral
Discussion
Although the ACLT model of OA has been studied extensively using many tools, including MRI, this high-field μMRI project puts a set of quantitative parameters into use in the first microscopic resolution investigation of the complex role of ACLT during the progression of early OA. We were able to resolve quantitatively the topographical distributions of cartilage thickness and T2 alterations in canine tibial cartilage as the function of OA degradation. Studying 20 animals (including the previous data on seven healthy animals [17]), we observed significant differences in T2 (0°) in most subtissue zones and in bulk on the meniscus-uncovered area. The same changes on the meniscus-covered area could only be observed in SZ. Additionally, throughout OA progression, there were variation patterns in zonal and bulk tissue thickness between covered and uncovered areas. These differences demonstrate that the rate of disease progression in the covered area is slower than in the uncovered area. This may be due the lack of a loose meniscal body at the uncovered area, which allows prevalent healing there, while the loose body limits healing until complete degeneration of the meniscus at 12 weeks.
It should be pointed out that our bulk and zonal-averaged data from μMRI are fundamentally different from clinical MRI measurements at low resolution. In our approach, we used high-resolution imaging to resolve variations in tissue profiles; we then averaged the spatially resolved profile—based on the established zonal division criteria—into four zonal data to mimic accurately the low-resolution imaging and bulk measurement. Consequently, depth wise, our data averaging only occurs within each structural zone. In contrast, any low-resolution imaging will average within each voxel, different cartilage zones, and even between cartilage and other surrounding tissues (e.g., meniscus, fluid, tendon), inevitably reducing detection sensitivity to the lesion.
Topographical variations in bulk properties of OA cartilage
This study compared thickness and T2 of articular cartilage between meniscus-covered and -uncovered areas during the early development of OA. As reported in the literature, many tissue properties can vary with OA progression, such as water content, collagen-fibril orientation, stiffness, optical properties, tissue volume, and thickness in the knee joint [17, 21–24]. Over any single knee surface, the thickness of healthy articular cartilage is known to have significant topographical variations [17, 25].
We can identify several detectable signs for early OA progression, which include an increase in total thickness in medial tibia cartilage that was topographically distributed. The meniscus-covered area had the maximum total thickness and the highest bulk values of T2 (55°) at 12X, while the meniscus-uncovered area had the maximum of those values at 8X (Fig. 3). Since early changes in OA include the increase in interstitial water mobility in cartilage [26, 27], our observation of an increase in T2 as OA progresses supports this understanding. Furthermore, Lusse et al. [28] demonstrated a positive correlation between increases in T2 and water content in ex vivo cartilage from OA patients who underwent total knee replacement surgery. The tissue initially swelled then decreased with OA progression [29]. Our results demonstrated that the meniscus protects the meniscus-covered area [29–33] and identified the topographical variations of OA progression on the media tibial surface.
Topographical variations in zonal properties of OA cartilage
All properties of articular cartilage vary depth dependently. Subtissue zonal properties best illustrate the various changes in cartilage at different tissue depths due to OA progression. Measuring these properties is technically demanding, requiring not only high spatial resolution but also many other technical factors, such as high SNR, precise specimen location on the joint surface, and knowledge of healthy cartilage characteristics. Topographical variations in zonal properties of cartilage have been reported for healthy tibial tissue [17]. Zonal thickness data at all OA time points in Table 1 (the first four rows) show clearly that the relative zonal thickness of SZ and TZ at all OA time points were higher in the covered area, which resulted in the lower relative thickness of the two radial zones, similar to healthy cartilage [17]. Since each pixel in our microscopic imaging is smaller than the thickness of the thinnest subtissue zone—verified previously by several correlation studies between μMRI and optical imaging [20, 34]—zonal averaging and comparison can be carried out individually and independently in each zone. Since the high resolution in μMRI allows differentiation of individual zones, parameters within each subtissue zone can be quantified, where each zone has a different thickness.
In this study, many topographical variations in zonal properties of the diseased cartilage have been quantified. Most differences in T2 (55°) between covered and uncovered areas are statistically significant at 8X and 12X, while most differences in T2 (0°) are not significantly different at most OA time points, illustrating the subtle influence of the nuclear dipolar interaction in the quantification and interpretation of T2 data on cartilage MRI. It is interesting to note that topographical variations of zonal properties match variations in bulk properties for T2 (55°). Zonal distributions of T2 (0°) values, which are strongly influenced by the dipole–dipole interaction, had a less clear matching. We showed that changes in T2 at different subtissue zones and total thickness with OA could be detected by μMRI. This knowledge could help the design of clinically effective protocols for early OA patients.
It should be pointed out that the zonal averaged numbers in Table 1 reflect the profile differences in μMRI. For example, for both OA time points of the contralateral cartilage (8C and 12C), T2 (0°) in SZ are lower than T2 (0°) in TZ. In comparison, cartilages at both OA time points (8X and 12X) had an SZ T2 (0°) higher than TZ T2 (0°). This reversed trend illustrates a distinct difference between the bell-shaped T2 (0°) profiles in healthy cartilage and the non-bell-shaped T2 (0°) profiles in degraded cartilage (insert in Fig. 4a). This difference can be interpreted based on orientational changes in collagen fibril structure during OA progression. When tissue is healthy, SZ collagen fibers are densely packed and oriented parallel with the surface. This structure results in the typical bell-shaped T2 (0°) profile, where the SZ T2 is lower than the TZ T2 [7, 18]. In contrast, OA cartilage can have a number of early changes, including fibrillation (disorganization) of the SZ collagen network, influx of water, and swelling, which can modulate the T2 (0°) profile into a non-bell-shaped curve.
Experimental issues
The high-resolution μMRI experiments in this project were carried out at room temperature and used a vertical-bore 7-T magnet, which is different from common clinical conditions (1.5- to 3-T horizontal magnet, body temperature). In addition, the microscopic resolution in this project is currently not translatable to the clinical setting. However, the fact that μMRI and clinical MRI share the same physics principles and engineering architectures ensures a potential translational pathway from μMRI study of animal OA to clinical detection and intervention [35]. Since it is not possible to initiate joint degradation in humans, the use of the animal model in biomedical research offers the only avenue by which to study/follow disease progression to obtain precise knowledge of when and how the disease begins. Once we understand tissue degradation from the beginning to end, step-by-step, in animal studies, intervention in the early stages before the point of no return may eventually be developed to save human joints.
Conclusion
In summary, this study characterized topographical and zonal variations in cartilage thickness and T2 as the function of OA progression using high-field, high-resolution MRI. Our data indicate that the rate of disease progression in the meniscus-covered area is slower compared with the meniscus-uncovered area. Observations of several topographically distributed characteristic changes in both T2 and tissue thickness in an experimental model of OA provided the detailed knowledge of the disease-modified cartilage properties. Although biomolecular and pathological specifics of an animal OA are likely to be different from those in humans, quantitative findings in this μMRI study have the potential to contribute to the development of sensitive diagnostic protocols to detect early lesions in human cartilage, benefitting future clinical management and intervention of OA.
Acknowledgments
Y. Xia is grateful to the National Institutes of Health for the R01 grant (AR 052353). The authors thank Dr. Cliff Les (Michigan State University, Lansing, MI, USA), Mr. David Kahn and Mr. Daniel Mittelstaedt (Oakland University) for constructive comments and suggestions, Dr. Nian Wang (Duke University, Durham, NC, USA) for technical help, Ms. Janelle Spann (Michigan Resonance Imaging, Rochester Hills, MI, USA) for providing the contrast agent, and Ms. Carol Searight (Oakland University) for editorial comments on the manuscript.
Funding This work was supported by a R01 grant from the National Institutes of Health USA (AR 052353).
Footnotes
Conflict of interest The authors declare that they have no conflicts of interests.
Ethical standards All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent For this type of study formal consent is not required.
References
- 1.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, Dragomir A, Kalsbeek WD, Luta G, Jordan JM (2008) Lifetime risk of symptomatic knee osteoarthritis. Arthritis Care Res 59:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yelin E, Callahan LF (1995) The economic cost and social and psychological impact of musculoskeletal conditions. National Arthritis Data Work Groups. Arthritis Rheum 38:1351–1362 [DOI] [PubMed] [Google Scholar]
- 3.McDevitt C, Muir H (1976) Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J Bone Joint Surg Br 58:94–101 [DOI] [PubMed] [Google Scholar]
- 4.Regatte RR, Schweitzer ME (2007) Ultra-high-field MRI of the musculoskeletal system at 7.0 T. J Magn Reson Imaging 25:262–269 [DOI] [PubMed] [Google Scholar]
- 5.Trattnig S, Bogner W, Gruber S, Szomolanyi P, Juras V, Robinson S, Zbyn S, Haneder S (2015) Clinical applications at ultrahigh field (7 T). Where does it make the difference? NMR. doi: 10.1002/nbm.3272 [DOI] [PubMed] [Google Scholar]
- 6.Trattnig S, Zbyn S, Schmitt B, Friedrich K, Juras V, Szomolanyi P, Bogner W (2012) Advanced MR methods at ultra-high field (7 T) for clinical musculoskeletal applications. Eur Radiol 22:2338–2346 [DOI] [PubMed] [Google Scholar]
- 7.Xia Y (1998) Relaxation anisotropy in cartilage by NMR microscopy (μMRI) at 14-μm resolution. Magn Reson Med 39:941–949 [DOI] [PubMed] [Google Scholar]
- 8.Fragonas E, Mlynarik V, Jellus V, Micali F, Piras A, Toffanin R, Rizzo R, Vittur F (1998) Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthr Cartil 6:24–32 [DOI] [PubMed] [Google Scholar]
- 9.Xia Y, Elder K (2001) Quantification of the graphical details of collagen fibrils in transmission electron micrographs. J Microsc 204:3–16 [DOI] [PubMed] [Google Scholar]
- 10.Welsch GH, Apprich S, Zbyn S, Mamisch TC, Mlynarik V, Scheffler K, Bieri O, Trattnig S (2011) Biochemical (T2, T2* and magnetisation transfer ratio) MRI of knee cartilage: feasibility at ultra-high field (7 T) compared with high field (3 T) strength. Eur Radiol 21:1136–1143 [DOI] [PubMed] [Google Scholar]
- 11.Welsch GH, Mamisch TC, Zak L, Mauerer A, Apprich S, Stelzeneder D, Marlovits S, Trattnig S (2011) Morphological and biochemical T2 evaluation of cartilage repair tissue based on a hybrid double echo at steady state (DESS-T2d) approach. J Magn Reson Imaging 34:895–903 [DOI] [PubMed] [Google Scholar]
- 12.Roemer FW, Crema MD, Trattnig S, Guermazi A (2011) Advances in imaging of osteoarthritis and cartilage. Radiology 260:332–354 [DOI] [PubMed] [Google Scholar]
- 13.Alhadlaq HA, Xia Y, Moody JB, Matyas JR (2004) Detecting structural changes in early experimental osteoarthritis of tibial cartilage by microscopic magnetic resonance imaging and polarised light microscopy. Ann Rheum Dis 63:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahl R, Blumenkrantz G, Carballido-Gamio J, Zhao S, Munoz T, Hellio Le Graverand-Gastineau MP, Li X, Majumdar S, Link TM (2007) MRI-derived T2 relaxation times and cartilage morphometry of the tibio-femoral joint in subjects with and without osteoarthritis during a 1-year follow-up. Osteoarthr Cartil 15:1225–1234 [DOI] [PubMed] [Google Scholar]
- 16.Jones EF, Schooler J, Miller DC, Drake CR, Wahnishe H, Siddiqui S, Li X, Majumdar S (2012) Characterization of human osteoarthritic cartilage using optical and magnetic resonance imaging. Mol Imaging Biol 14:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Badar F, Kahn D, Matyas J, Qu X, Chen CT, Xia Y (2014) Topographical variations of the strain-dependent zonal properties of tibial articular cartilage by microscopic MRI. Connect Tissue Res 55:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhadlaq HA, Xia Y (2004) The structural adaptations in compressed articular cartilage by microscopic MRI (microMRI) T2 anisotropy. Osteoarthr Cartil 12:887–894 [DOI] [PubMed] [Google Scholar]
- 19.Teeple E, Jay GD, Elsaid KA, Fleming BC (2013) Animal models of osteoarthritis: challenges of model selection and analysis. Am Assoc Pharm Sci J 15:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Moody JB, Burton-Wurster N, Lust G (2001) Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthr Cartil 9:393–406 [DOI] [PubMed] [Google Scholar]
- 21.Hayes WC, Keer LM, Herrmann G, Mockros LF (1972) A mathematical analysis for indentation tests of articular cartilage. J Biomech 5:541–551 [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Moody JB, Alhadlaq H, Hu J (2003) Imaging the physical and morphological properties of a multi-zone young articular cartilage at microscopic resolution. J Magn Reson Imaging 17:365–374 [DOI] [PubMed] [Google Scholar]
- 23.Jurvelin JS, Arokoski JP, Hunziker EB, Helminen HJ (2000) Topographical variation of the elastic properties of articular cartilage in the canine knee. J Biomech 33:669–675 [DOI] [PubMed] [Google Scholar]
- 24.Nissi MJ, Rieppo J, Toyras J, Laasanen MS, Kiviranta I, Nieminen MT, Jurvelin JS (2007) Estimation of mechanical properties of articular cartilage with MRI—dGEMRIC, T2 and T1 imaging in different species with variable stages of maturation. Osteoarthr Cartil 15:1141–1148 [DOI] [PubMed] [Google Scholar]
- 25.Kiviranta I, Tammi M, Jurvelin J, Helminen HJ (1987) Topographical variation of glycosaminoglycan content and cartilage thickness in canine knee (stifle) joint cartilage. Application of the microspectrophotometric method. J Anat 150:265–276 [PMC free article] [PubMed] [Google Scholar]
- 26.Buckwalter JA, Mankin HJ, Grodzinsky AJ (2005) Articular cartilage and osteoarthritis. Instr Course Lect 54:465–480 [PubMed] [Google Scholar]
- 27.Mankin HJ, Buckwalter JA (1996) Restoration of the osteoar-throtic joint. J Bone Joint Surg Am 78:1–2 [DOI] [PubMed] [Google Scholar]
- 28.Lusse S, Knauss R, Werner A, Grunder W, Arnold K (1995) Action of compression and cations on the proton and deuterium relaxation in cartilage. Magn Reson Med 33:483–489 [DOI] [PubMed] [Google Scholar]
- 29.Arokoski JP, Lammi MJ, Hyttinen MM, Kiviranta I, Parkkinen JJ, Jurvelin JS, Tammi MI, Helminen HJ (2001) Etiopathogenesis of osteoarthritis. Duodecim 117:1617–1626 [PubMed] [Google Scholar]
- 30.Friedrich KM, Shepard T, Chang G, Wang L, Babb JS, Schweitzer M, Regatte R (2010) Does joint alignment affect the T2 values of cartilage in patients with knee osteoarthritis? Eur Radiol 20:1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Rocca Vieira R, Pakin SK, de Albuquerque Cavalcanti CF, Schweitzer M, Regatte R (2007) Three-dimensional spin-lock magnetic resonance imaging of the shoulder joint at 3 T: initial experience. Skelet Radiol 36:1171–1175 [DOI] [PubMed] [Google Scholar]
- 32.Regatte RR, Schweitzer ME, Jerschow A, Reddy R (2007) Magic sandwich echo relaxation mapping of anisotropic systems. Magn Reson Imaging 25:433–438 [DOI] [PubMed] [Google Scholar]
- 33.Ling W, Regatte RR, Schweitzer ME, Jerschow A (2006) Behavior of ordered sodium in enzymatically depleted cartilage tissue. Magn Reson Med 56:1151–1155 [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Xia Y (2013) Quantitative zonal differentiation of articular cartilage by microscopic magnetic resonance imaging, polarized light microscopy, and Fourier-transform infrared imaging. Microsc Res Tech 76:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y (2007) Resolution ‘scaling law’ in MRI of articular cartilage. Osteoarthr Cartil 15:363–365 [DOI] [PMC free article] [PubMed] [Google Scholar]