Abstract
Based on molecular phylogenetic analyses of a multigene matrix of partial nuSSU-ITS-LSU rDNA, cal, his, ms204, rpb1, rpb2, tef1 and tub2 sequences, recent European and Iranian collections of Melanconium pterocaryae from the type host, Pterocarya fraxinifolia, are shown to be distinct from the Japanese Melanconis pterocaryae from Pterocarya rhoifolia, and both are confirmed as closely related members of the recently described genus Juglanconis. Therefore, the new name Juglanconis japonica is proposed for Melanconis pterocaryae. As no type collection could be traced, Melanconium pterocaryae (syn. J. pterocaryae) is neotypified, described and illustrated, and it is recorded for Europe for the first time. During field surveys in natural stands of P. fraxinifolia in Guilan province (Iran), Juglanconis pterocaryae was consistently isolated from tissues affected by branch and trunk cankers, twig dieback and wood necrosis, indicating that it is the causal agent of these diseases. The external and internal symptoms associated with these trunk diseases are described and illustrated.
Keywords: Ascomycota, Juglanconidaceae, Molecular phylogeny, Pathogen, Systematics, 1 new name
Introduction
The Diaporthales (Ascomycota, Sordariomycetes) comprise important plant pathogens, but the species diversity and host range of many phytopathologically important lineages are still imperfectly known. Recently, substantial progress was made to tackle the species diversity of several diaporthalean lineages involved in plant diseases by the application of multi-gene phylogenies in combination with morphological studies, e.g. in Coniella (Alvarez et al. 2016), Cytospora (Lawrence et al. 2018), Diaporthe (Guarnaccia et al. 2018) and Harknessia (Marin-Felix et al. 2019). These studies revealed a number of undescribed species on various plant hosts of economic importance in silvi-, agri- and horticulture, but also improved our knowledge on the circumscription and host range of already described species.
Based on morphology and molecular phylogenies, the genus Pterocarya is the closest relative of the genus Juglans in tribe Juglandinae, Juglandaceae (Manos et al. 2007; Xiang et al. 2016). The genus Pterocarya currently comprises about six accepted species, of which five occur in Eastern Asia (Vietnam, China, Korea and Japan), while one species, P. fraxinifolia, occurs widely disjunct in Western Asia from Anatolia via the southern Caucasus area to the Caspian forest of Iran (also known as Northern Iran) and Azerbaijan (Rix 2007). In Iran, P. fraxinifolia grows wildly in the three northern provinces Golestan, Guilan and Mazandaran, but in recent years, small populations have also been reported in two other western provinces, Lorestan (in the Zagros Mountains) and Ilam (bordering Iraq) (Nabavi et al. 2008). For a long time, native and local people have used young leaves of this tree as an anaesthetic agent for catching fish (Sadighara et al. 2009), for dyeing and as an antifungal agent (Hadjmohammadi and Kamyar 2006; Ebrahimzadeh et al. 2008, 2009). Various parts of this plant are rich in phenolic and flavonoid compounds (Ebrahimzadeh et al. 2008; Nabavi et al. 2008) and may therefore provide interesting bioactive compounds. Although P. fraxinifolia is currently of little economic importance in forestry, it has been planted as an ornamental tree throughout Europe mainly in large parks (Forrest 2006). So far, although Pterocarya species represent important components of Western and Eastern Asian forest ecosystems and are widely planted as ornamental trees, their mycobiota are poorly known and largely understudied.
Voglmayr et al. (2017) recently described the new genus Juglanconis for four Melanconis species on hosts of tribe Juglandinae, viz. three species (Juglanconis appendiculata, J. juglandina, J. oblonga) on various Juglans species and one (J. pterocaryae) from Pterocarya spp. During these investigations, the taxonomy of J. pterocaryae proved to be a complex issue that could not be resolved with certainty, as it involved asexual and sexual morphs described from two different Pterocarya hosts, i.e. P. fraxinifolia and P. rhoifolia from Western Asia and Japan, respectively. As first species, the asexual Melanconium pterocaryae was described by Kuschke (1913) from P. fraxinifolia collected in the Georgian Republic (Abkhazia). The species apparently was not recollected again until Riedl and Ershad (1977) published a record from the same host from Iran. No sexual morph is known from this host, and no specimens or cultures were available for morphological investigations and sequencing. Based on a holomorphic collection from P. rhoifolia collected in Japan, Kobayashi (1970) described Melanconis pterocaryae, and he considered that his species represented the sexual morph of Melanconium pterocaryae, based on similar conidial sizes of the Japanese collection and the original description of M. pterocaryae by Kuschke (1913). This synonymy was also accepted by Voglmayr et al. (2017), who accordingly combined the older Melanconium pterocaryae into their new genus Juglanconis. However, at that time, this synonymy could only be based on morphological evidence, because DNA data were only available for the ex-type culture of the Japanese Melanconis pterocaryae, but not for isolates from P. fraxinifolia, the type host of the basionym.
Recently, fresh collections from the type host of Melanconium pterocaryae, P. fraxinifolia, were made in Austria, the Czech Republic and Iran. This enabled us to perform detailed morphological investigations as well as pure culture isolation for sequencing and molecular phylogenetic analyses to resolve the taxonomic status of Melanconium pterocaryae and Melanconis pterocaryae, the results of which are reported here.
Materials and methods
Field survey and sample collection
During 2013–2017, natural forests in Guilan province (Northern Iran) were surveyed for endophytic fungal pathogens associated with trunk diseases of Pterocarya fraxinifolia. Symptomatic branches (1–4 samples from each tree) from trees showing canker and dieback were collected randomly from Asalem (Talesh), Chobar (Shaft), Jirdeh (Shaft), Masal, Rezvanshar (Talesh), Rudbar, Shaft and Talesh. Cross sections of symptomatic branches were examined in order to investigate development of wood necrosis in the wood and the type of necrosis was recorded. For fungal isolations, small wood fragments (5–8 mm) were cut from the margin between healthy and affected wood tissues. Wood discs were surface disinfected by immersion in 2% sodium hypochlorite (NaOCl) for 2 min and rinsed twice in sterile distilled water (SDW). Then they were dried under sterile airflow in the laminar hood and were placed on Petri dishes containing malt extract agar (MEA: 2% malt extract, Merck, Darmstadt, Germany) supplemented with 100 mg/l streptomycin sulphate (MEAS). Petri dishes were incubated at 25 °C for 5–15 days. Growth of endophytic fungi from the tissue segments were subcultured onto fresh MEA plates and incubated at 25 °C. In most cases, cankers and twigs with dieback symptoms were covered with black conidiomata (acervuli). Fungal isolations were made also from conidiomata formed on cankers and twigs. During 2017–2018, cankered branches of P. fraxinifolia bearing black conidiomata were also collected in landscape parks in Austria and the Czech Republic and pure cultures isolated from conidia.
Sample sources
Of the 12 isolates of Juglanconis pterocaryae from P. fraxinifolia included in the morphological and molecular phylogenetic analyses, 10 originated from conidia of fresh specimens and 2 were isolated from diseased host tissues (IRNHM-K116 = IRNHM-JP116 and IRNHM-K151 = IRNHM-JP151). Details of the strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the Westerdijk Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS culture collection). Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. Herbarium acronyms are according to Thiers (2018). Specimens have been deposited in the Fungarium of the Department of Botany and Biodiversity Research, University of Vienna (WU).
Table 1.
Strains and NCBI GenBank accessions used in the phylogenetic analyses of the combined multigene matrix of Juglanconis; accessions of J. pterocaryae for which only the ITS-LSU was sequenced were not included in the phylogenetic analyses. Sequences formatted in bold were generated during the present study
| Taxon | Strain | Culture collection | Herbarium | Origin | Host | GenBank accession no. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITS-LSU | cal | his | ms204 | rpb1 | rpb2 | tef1 | tub2 | ||||||
| Juglanconis appendiculata | D140 | WU 35956 | Greece | Juglans regia | KY427138 | – | – | KY427157 | – | KY427188 | KY427207 | KY427226 | |
| D96 | WU 35954 | Austria | Juglans nigra | KY427139 | – | – | – | – | KY427189 | KY427208 | – | ||
| D96A | WU 35954 | Austria | Juglans nigra | KY427140 | – | – | KY427158 | – | KY427190 | KY427209 | – | ||
| MC | WU 32010 | Greece | Juglans regia | KY427141 | KY427242 | – | KY427159 | KY427174 | KY427191 | KY427210 | KY427227 | ||
| MC2 | WU 35957 | Spain | Juglans regia | KY427142 | KY427243 | – | KY427160 | KY427175 | KY427192 | KY427211 | KY427228 | ||
| MC4 | WU 35958 | Spain | Juglans regia | KY427143 | KY427244 | – | KY427161 | KY427176 | KY427193 | KY427212 | KY427229 | ||
| ME17, W.J.1665, A.R.3581 | CBS 123194 | WU 35951, BPI 840932 | Austria | Juglans regia | KY427144 | KY427245 | – | KY427162 | KY427177 | KY427194 | KY427213 | KY427230 | |
| Juglanconis juglandina | D142 | WU 35960 | Austria | Juglans regia | KY427145 | – | – | – | – | KY427195 | KY427214 | – | |
| MC1 | WU 35967 | Austria | Juglans regia | KY427146 | KY427246 | KY427128 | KY427163 | KY427178 | KY427196 | KY427215 | KY427231 | ||
| MC3 | WU 35968 | Spain | Juglans regia | KY427147 | KY427247 | KY427129 | KY427164 | KY427179 | KY427197 | KY427216 | KY427232 | ||
| ME16, W.J.1450, A.R.3420 | CBS 121083 | BPI 843622 | Austria | Juglans regia | KY427148 | KY427248 | KY427130 | KY427165 | KY427180 | KY427198 | KY427217 | KY427233 | |
| ME22, W.J.1500, A.R.3860 | CBS 133343 | WU 35959 | Austria | Juglans regia | KY427149 | KY427249 | KY427131 | KY427166 | KY427181 | KY427199 | KY427218 | KY427234 | |
| ME23 | WU 35965 | Austria | Juglans nigra | KY427150 | KY427250 | KY427132 | KY427167 | KY427182 | KY427200 | KY427219 | KY427235 | ||
| Juglanconis oblonga | ME14, A.R.4413 | CBS 133344 | – | USA | Juglans cinerea | KY427151 | KY427251 | KY427133 | KY427168 | KY427183 | KY427201 | KY427220 | KY427236 |
| ME15, A.R.4529 | CBS 133330 | – | USA | Juglans cinerea | KY427152 | KY427252 | KY427134 | KY427169 | KY427184 | KY427202 | KY427221 | KY427237 | |
| ME18, M4–1 | MAFF 410216 | TFM FPH 2623 | Japan | Juglans ailanthifolia | KY427153 | KY427253 | KY427135 | KY427170 | KY427185 | KY427203 | KY427222 | KY427238 | |
| ME19, M4–10 | MAFF 410217 | TFM FPH 3599, TFM FPH 3601 | Japan | Juglans ailanthifolia | KY427154 | KY427254 | KY427136 | KY427171 | KY427186 | KY427204 | KY427223 | KY427239 | |
| Juglanconis japonica | ME20, LFP-M4–8 | MAFF 410079 | TFM FPH 3373 | Japan | Pterocarya rhoifolia | KY427155 | KY427255 | KY427137 | KY427172 | KY427187 | KY427205 | KY427224 | KY427240 |
| Juglanconis pterocaryae | D272 | CBS 144326 | WU 39981 | Austria | Pterocarya fraxinifolia | MK229175 | MK238308 | MK238312 | MK238314 | MK238319 | MK238324 | MK238332 | MK238338 |
| Juglanconis pterocaryae | D275 | WU 39983 | Austria | Pterocarya fraxinifolia | MK229176 | – | – | – | – | MK238325 | MK238333 | – | |
| Juglanconis pterocaryae | D281 | WU 39982 | Austria | Pterocarya fraxinifolia | MK229177 | MK238309 | MK238313 | MK238315 | MK238320 | MK238326 | MK238334 | MK238339 | |
| Juglanconis pterocaryae | D267a | IRNHM-JP1 | WU 39985 | Iran | Pterocarya fraxinifolia | MK229168 | – | – | – | – | MK238321 | MK238329 | – |
| Juglanconis pterocaryae | D267b | IRNHM-JP8 | WU 39985 | Iran | Pterocarya fraxinifolia | MK229169 | – | – | – | – | – | – | – |
| Juglanconis pterocaryae | D268a | IRNHM-JP3 | WU 39986 | Iran | Pterocarya fraxinifolia | MK229170 | – | – | – | – | – | – | – |
| Juglanconis pterocaryae | D268b | IRNHM-JP5 | WU 39986 | Iran | Pterocarya fraxinifolia | MK229171 | – | – | – | – | – | – | – |
| Juglanconis pterocaryae | D268c | CBS 143631 = IRNHM-JP6 | WU 39986 | Iran | Pterocarya fraxinifolia | MK229172 | – | – | – | MK238318 | MK238322 | MK238330 | MK238337 |
| Juglanconis pterocaryae | D269a | IRNHM-JP4 | WU 39987 | Iran | Pterocarya fraxinifolia | MK229173 | – | – | – | – | MK238323 | MK238331 | – |
| Juglanconis pterocaryae | D269b | IRNHM-JP7 | WU 39987 | Iran | Pterocarya fraxinifolia | MK229174 | – | – | – | – | – | – | – |
| Juglanconis pterocaryae | K116 | IRNHM-JP116 | – | Iran | Pterocarya fraxinifolia | MK229178 | MK238310 | – | MK238316 | – | MK238327 | MK238335 | MK238340 |
| Juglanconis pterocaryae | K151 | IRNHM-JP151 | – | Iran | Pterocarya fraxinifolia | MK229179 | MK238311 | – | MK238317 | – | MK238328 | MK238336 | MK238341 |
| Melanconis stilbostoma | D143 | WU 35970 | Poland | Betula pendula | KY427156 | – | – | KY427173 | – | KY427206 | KY427225 | KY427241 | |
| Melanconis stilbostoma | MS | CBS 121894 | – | Austria | Betula pendula | JQ926229 | – | – | – | – | – | JQ926302 | JQ926368 |
Morphology
Microscopic observations were made in tap water except where noted. Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 equipped with a Nikon DS-U2 digital camera, and Nomarski differential interference contrast (DIC) using a Zeiss Axio Imager.A1 compound microscope equipped with a Zeiss Axiocam 506 colour digital camera. Images and data were gathered using the NIS-Elements D v. 3.22.15 or Zeiss ZEN Blue Edition software packages. Measurements are reported as maxima and minima in parentheses, and the range representing the mean plus and minus the standard deviation of a number of measurements given in parentheses. Due to poor or untypical sporulation in pure culture, conidial and conidiophore morphology was only studied in detail from natural substrates.
Culture preparation, DNA extraction, PCR and sequencing
Single conidium isolates were prepared and grown on MEA or on 2% corn meal agar plus 2% w/v dextrose (CMD). Growth of liquid culture and extraction of genomic DNA was performed as reported previously (Voglmayr and Jaklitsch 2011; Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany).
The following eight loci were amplified and used for phylogenetic analyses: partial nuSSU-ITS-LSU rDNA, cal, his, ms204, rpb1, rpb2, tef1 and tub2; for details on loci and primers see Table 2. PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr and Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) and the PCR primers; in addition, primers ITS4, LR2R-A and LR3 were used as internal sequencing primers for the ITS-LSU rDNA region and TEF1_INTF and TEFD_iR for tef1 (Table 2). Sequencing was performed on an automated DNA sequencer (ABI 3730xl Genetic Analyser, Applied Biosystems).
Table 2.
Details of the loci and primers used in the molecular study
| Locus1 | Primer | Primer sequence (5′–3′) | Orientation | Amplicon size | Reference |
|---|---|---|---|---|---|
| SSU-ITS-LSU | V9G | TTAAGTCCCTGCCCTTTGTA | Forward | de Hoog and Gerrits van den Ende (1998) | |
| LR5 | TCCTGAGGGAAACTTCG | Reverse | ca 1.6 kb | Vilgalys and Hester (1990) | |
| ITS42 | TCCTCCGCTTATTGATATGC | Reverse | White et al. (1990) | ||
| LR32 | CCGTGTTTCAAGACGGG | Reverse | Vilgalys and Hester (1990) | ||
| LR2R-A2 | CAGAGACCGATAGCGCAC | Forward | Voglmayr et al. (2012) | ||
| cal | CAL-228F | GAGTTCAAGGAGGCCTTCTCCC | Forward | Carbone and Kohn (1999) | |
| CAL-737R | CATCTTTCTGGCCATCATGG | Reverse | 458 bp | Carbone and Kohn (1999) | |
| his | CYLH3F | AGGTCCACTGGTGGCAAG | Forward | (Crous et al. (2004) | |
| H3-1b | GCGGGCGAGCTGGATGTCCTT | Reverse | 438 bp | Glass and Donaldson (1995) | |
| ms204 | MSE1F1n1 | AAGGGNACYCTSGAGGGCCAC | Forward | Voglmayr and Mehrabi (2018) | |
| MS-E5R2n | CCASAGCATGGTGGTRCCRTC | Reverse | ca 1 kb | Voglmayr and Mehrabi (2018) | |
| rpb1 | RPB1-Af | GARTGYCCDGGDCAYTTYGG | Forward | Stiller and Hall (1997) | |
| RPB1-6R1asc | ATGACCCATCATRGAYTCCTTRTG | Reverse | ca 1.2 kb | Hofstetter et al. (2007) | |
| rpb2 | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | Forward | Liu et al. (1999) | |
| fRPB2-7cR | GGGGWGAYCAGAAGAAGGC | Reverse | ca 1.2 kb | Liu et al. (1999) | |
| dRPB2-5f | GAYACNGAYGAYCGWGAYCAYTTYGG | Forward | Voglmayr et al. (2016) | ||
| dRPB2-7r | AANCCCATDGCYTGYTTDCCCAT | Reverse | ca 1.2 kb | Voglmayr et al. (2016) | |
| tef1 | EF1-728F | CATCGAGAAGTTCGAGAAGG | Forward | Carbone and Kohn (1999) | |
| TEF1-LLErev | AACTTGCAGGCAATGTGG | Reverse | ca 1.3 kb | Jaklitsch et al. (2005) | |
| TEF1_INTF2 | CCGTGAYTTCATCAAGAACATG | Forward | Jaklitsch (2009) | ||
| TEFD_iR2 | GTCTGGCCATCCTTGGAGAT | Reverse | Voglmayr et al. (2018) | ||
| tub2 | T1 | AACATGCGTGAGATTGTAAGT | Forward | O’Donnell and Cigelnik (1997) | |
| BtHV2r | CATCATRCGRTCNGGGAACTC | Reverse | ca 1 kb | Voglmayr et al. (2017) | |
| T1D | CAANATGCGTGAGATTGTRAGT | Forward | This study | ||
| T22D | CATCATRCGRTCNGGGAACTC | Reverse | ca 1.6 kb | This study |
1SSU-ITS-LSU, partial nuclear 18S rDNA, internal transcribed spacers and intervening 5.8S rDNA and 28S rDNA amplified and sequenced as a single fragment; cal, calmodulin; his, histone H3; ms204, guanine nucleotide-binding protein subunit beta; rpb1, DNA-directed RNA polymerase II largest subunit; rpb2, DNA-directed RNA polymerase II second largest subunit; tef1, translation elongation factor 1-alpha; tub2, β-tubulin
2Internal sequencing primers
Data analysis
The newly generated sequences were aligned to the sequence alignments of Voglmayr et al. (2017), and a combined matrix of the eight loci (partial SSU-ITS-LSU rDNA, cal, his, ms204, rpb1, rpb2, tef1 and tub2) was produced for phylogenetic analyses, with two accessions of Melanconis stilbostoma added as the outgroup. The GenBank accession numbers of sequences used in these analyses are given in Table 1.
Sequence alignments for phylogenetic analyses were produced with the server version of MAFFT (http://mafft.cbrc.jp/alignment/server/), checked and refined using BioEdit v. 7.2.6 (Hall 1999). The combined data matrix contained 8441 characters; viz. 1600 nucleotides of SSU-ITS-LSU, 460 nucleotides of cal, 449 nucleotides of his, 1037 nucleotides of ms204, 711 nucleotides of rpb1, 1160 nucleotides of rpb2, 1400 nucleotides of tef1 and 1624 nucleotides of tub2.
Maximum parsimony (MP) analyses were performed with PAUP v. 4.0a163 (Swofford 2002). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to MINBRLEN. MP analysis of the combined multilocus matrix was done using 1000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). Bootstrap analyses with 1000 replicates were performed in the same way, but using 10 rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate.
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI 1.5 (Silvestro and Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMA substitution model with 1000 bootstrap replicates. The matrix was partitioned for the different gene regions.
Results
Field survey and isolation
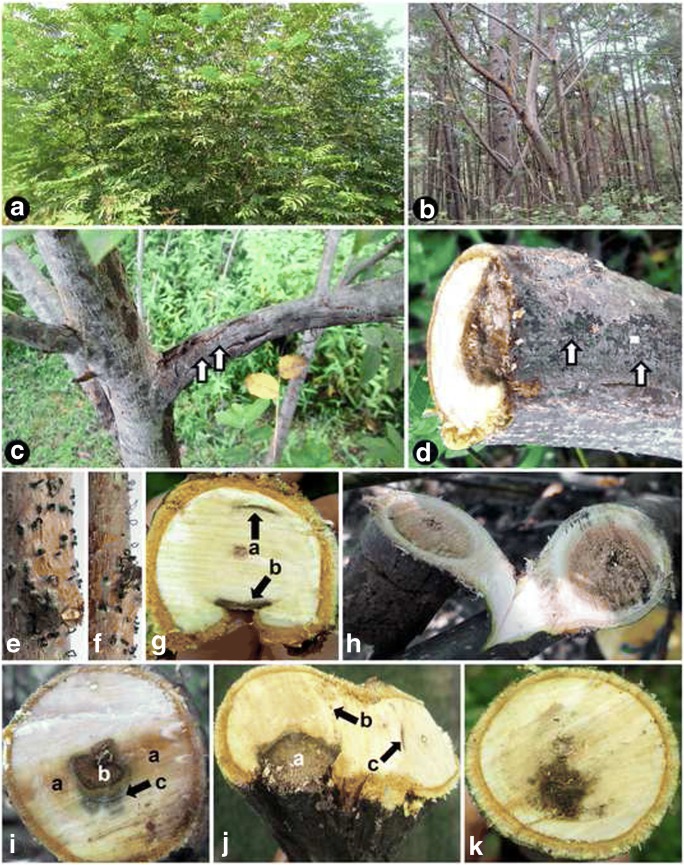
In the field surveys in the natural forests in Guilan province (Iran), declining trees of P. fraxinifolia showed branch and trunk canker, extensive dieback of terminal and lateral branches and death (Fig. 1b, c). Examination of branches from symptomatic trees revealed seven types of wood discolouration in cross sections: brown to black wood streaking, black spots, arch-shaped necrosis, central necrosis, irregular wood necrosis, water necrosis and wedge-shaped necrosis (Fig. 1g–k). Some collected samples showed multiple lesion types on the same sample in cross sections (Fig. 1g, i, j). A fungus morphologically resembling the genus Juglanconis (Voglmayr et al. 2017) was consistently isolated from wood lesions of affected trees (eight isolates). Among those isolates, seven (i.e. one from each different wood lesion type) were selected as representative isolates for further detailed studies. All of these isolates showed the same pure culture, conidioma and conidial characters. Two of these isolates, IRNHM-JP116 and IRNHM-JP151, were also selected for molecular studies. IRNHM-JP116 was isolated from infected tissue of a tree from Masal showing dieback and irregular wood necrosis in cross section, while IRNHM-JP151 was isolated from a tree from Asalem (Talesh) showing branch canker and irregular wood necrosis in cross section. During this work, 24 Iranian and three Austrian isolates were also recovered from conidiomata produced on twigs showing dieback (Fig. 1d–f). All these isolates had the same pure culture, conidioma and conidial characters like the isolates from lesions. In addition to Juglanconis, two isolates of Phaeoacremonium alvesii (Kazemzadeh Chakusary et al. 2017) and five isolates of Lasiodiplodia mahajangana (Kazemzadeh Chakusary et al. 2019) were isolated from affected trees. The field observations indicate that J. pterocaryae plays a major role in the decline of P. fraxinifolia in the forests of Northern Iran.
Fig. 1.
External and internal symptoms associated with trunk diseases of Pterocarya fraxinifolia in Asalem and Talesh (Guilan province, Northern Iran), from which Juglanconis pterocaryae was isolated. a Healthy tree. b Trees showing severe decline symptoms. c Tree showing canker and branch dieback covered by acervuli of J. pterocaryae (arrows). d Cross section of a branch showing wedge-shaped necrosis, arrows showing acervuli of J. pterocaryae. e, f Dead branches with J. pterocaryae acervuli, some with conidial cirrhi (spore tendrils). g Co-occurrence of arch-shaped necrosis (a) and young wedge-shaped necrosis (b). h Extensive central necrosis. i Co-occurrence of watery necrosis (a), irregular necrosis (b) and black wood streaking (c). j Co-occurrence of wedge-shaped necrosis (a), black spots (b) and arch-shaped necrosis (c). k Irregular wood necrosis
Molecular phylogeny
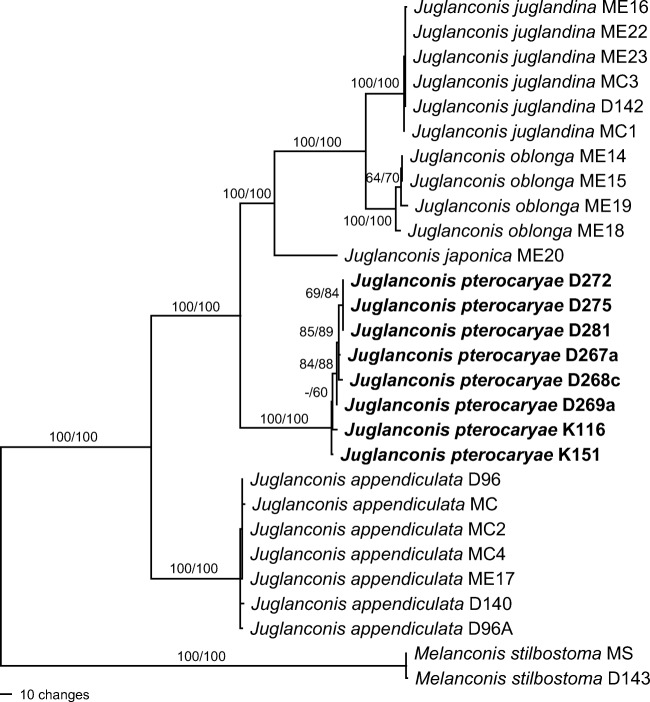
The combined multilocus matrix used for phylogenetic analyses comprised 8441 characters, of which 748 were parsimony informative (112 from SSU-ITS-LSU, 41 from cal, 34 from his, 64 from ms204, 35 from rpb1, 178 from rpb2, 173 from tef1 and 111 from tub2). The MP analysis revealed 30 MP trees 1090 steps long, one of which is shown in Fig. 2. Tree topologies of all MP trees were identical except for minor differences within Juglanconis appendiculata and J. pterocaryae. The ML tree revealed by RAxML was identical to the MP tree shown. Melanconis pterocaryae from P. rhoifolia and J. pterocaryae from P. fraxinifolia were revealed as distinct species; the two species were not closest relatives, but the latter was placed basal to the clade containing M. pterocaryae, J. juglandina and J. oblonga with maximum support. Due to the same species epithet, a new name needs to be proposed for Melanconis pterocaryae. All five species of Juglanconis received maximum support in both analyses, as well as the relationships between the species.
Fig. 2.
Phylogram showing one of 30 MP trees of 1090 steps (CI = 0.945, RI = 0.978, RC = 0.924) revealed by PAUP from an analysis of the combined SSU-ITS-LSU-cal-his-ms204-rpb1-rpb2-tef1-tub2 matrix of Juglanconis, with Melanconis stilbostoma selected as outgroup. MP and ML bootstrap support above 50% are given at the first and second position, respectively, above or below the branches. Strain numbers are given following the taxon names; strains formatted in bold were isolated and sequenced in the present study
Taxonomy
Juglanconis japonica (Tak. Kobay.) Voglmayr & Jaklitsch, nom. nov.
MycoBank: MB 828925.
Replaced synonym. Melanconis pterocaryae Tak. Kobay., Bull. Govt Forest Exp. Stn Meguro 226: 24. 1970, non Melanconium pterocaryae Kuschke, Trudy Tiflissk. Bot. Sada 28: 25. 1913.
Etymology: referring to its occurrence in Japan.
Holotype: Japan, Shizuoka, Fuji, on corticated twigs of Pterocarya rhoifolia, 5 Aug. 1968, T. Kobayashi (TFM FPH2623!); ex-type culture MAFF 410079.
Notes: When describing Melanconis pterocaryae from P. rhoifolia collected in Japan, Kobayashi (1970) considered his species to represent the sexual morph of Melanconium pterocaryae from P. fraxinifolia, based on similar conidial sizes. This synonymy was also accepted by Voglmayr et al. (2017), who combined the older Melanconium pterocaryae into the new genus Juglanconis. However, the current molecular phylogenies reveal Melanconis pterocaryae to represent a clearly distinct species, which therefore needs a new name. Morphologically, the conidial size of J. japonica is similar to that of J. pterocaryae, with slightly narrower conidia (11–20 × 5–9 μm vs. 11–22 × 6–11 μm in J. pterocaryae); however, the conidia of J. japonica usually have in average a distinctly higher length/width ratio, (1.5–)2.0–2.5(−3.1), vs. (1.3–)1.5–2.1(−3.0) in J. pterocaryae. For a detailed description and illustrations of the holomorph of J. japonica from the holotype, see Voglmayr et al. (2017; as J. pterocaryae).
Juglanconis pterocaryae (Kuschke) Voglmayr & Jaklitsch, in Voglmayr, Castlebury & Jaklitsch, Persoonia 38: 150 (2017), emend. Fig. 3.
Fig. 3.
Juglanconis pterocaryae. a Conidiomata in surface view. b, c Transverse (b) and vertical (c) sections of conidiomata, showing central column. d Culture (CMD, 25 d, 16 °C). e–h Conidiophores (annellides; in e, g with young conidia). i–e1 Vital conidia with gelatinous sheath. f1 Squashed conidium showing the densely verruculose inner conidial wall. All in water (a–c, i–m, f1 WU 39981, neotype; d WU 39983; e, f, n–x WU 39982; g, h WU 39985b; y–d1 WU 39986b; e1 WU 39987a). Scale bars a 500 μm; b, c 200 μm; e–e1 10 μm; f1 5 μm
Basionym. Melanconium pterocaryae Kuschke, Trudy Tiflissk. Bot. Sada 28: 25. 1913.
Sexual morph unknown. Conidiomata on natural substrate acervular, 0.8–2.2 mm diam, embedded in bark tissues, blackish, inconspicuous, scattered, with central or eccentric conical olivaceous grey stromatic column 300–850 μm wide at the base; at maturity covered by blackish discharged conidial masses forming black spots 0.2–2.5 mm diam or sometimes long cirrhi on the cortex. Conidiophores (11–)17–30(−48) × (3.0–)3.5–4.7(−5.5) μm (n = 74), narrowly cylindrical, simple or branched at the base, smooth, subhyaline to pale brown. Conidiogenous cells annellidic with distinct annellations, integrated. Conidia (11.2–)13.3–16.8(−22.3) × (6.0–)7.5–9.3(−11.0) μm, l/w = (1.3–)1.5–2.1(−3.0) (n = 980), unicellular, hyaline when immature, medium to dark brown when mature, variable in shape, ellipsoid to elongate, sometimes pip-shaped, often truncate with an abscission scar at the base, densely multiguttulate, thick-walled; wall ca. 0.5–0.8 μm, with distinct ornamentation on the inside of the wall consisting of small irregular confluent verrucae 0.3–0.7 μm diam, with ca. 0.5–1 μm wide gelatinous sheath.
Culture: Colony on CMD at 22 °C reaching 70 mm diam after 7 days; first white, turning cream to greyish brown in the centre, with irregular concentric zones and tufts of woolly aerial mycelium, margin uneven, wavy. Conidial pustules formed on tufts of aerial mycelium after ca 3 weeks, up to 4 mm diam, containing numerous branched conidiophores produced on subhyaline to brown aerial hyphae. Conidia similar to those produced on natural substrate except for slightly smaller size, (8.2–)10.5–13.0(−15.2) × (5.5–)6.8–8.2(−8.8) μm, l/w = (1.2–)1.4–1.8(−2.2) (n = 67).
Habitat and host range: Dead corticated trunks, twigs and branches of Pterocarya fraxinifolia.
Distribution: Europe and Western Asia (known from Austria, Czech Republic, Georgian Republic, Iran).
Typification: Austria, Oberösterreich, Bad Hall, Kurpark, on corticated twigs of Pterocarya fraxinifolia, 20 Oct. 2017, W. Jaklitsch (WU 39981, neotype of Melanconium pterocaryae here proposed; ex neotype culture D272 = CBS 144326).
Additional specimens examined (all on corticated twigs of Pterocarya fraxinifolia): Austria, Niederösterreich, Bruck an der Leitha, Harrachpark, 25 Mar. 2018, H. Voglmayr (WU 39982; culture D281); Steiermark, Graz, Geidorf, Botanical Garden of the University of Graz (HBG), 5 Feb. 2018, H. Voglmayr (WU 39983; culture D275). Czech Republic, Morava, Lednice landscape park, 1 May 2018, H. Voglmayr (WU 39984). Iran, Shaft, Chobar, 28 Apr. 2017, H. Mohammadi (WU 39988); Shaft, Jirdeh, 25 Apr. 2017, H. Mohammadi (WU 39985a, b; cultures D267a, b); Talesh, Rezvanshar, 2 May 2017, M. Kazemzadeh Chakusary (WU 39986a, b, c; cultures D268a, b, c = CBS 143631); Talesh, 2 May 2017, M. Kazemzadeh Chakusary (WU 39987a, b; cultures D269a, b).
Notes: The basionym, Melanconium pterocaryae, was described by Kuschke (1913) from the Georgian Republic (Abkhazia) from P. fraxinifolia, but until recently, no collections from the original host were available for morphological investigations and for DNA sequencing, and therefore no material from that host could be included in the investigations of Voglmayr et al. (2017). The conidial sizes given in the protologue of Melanconium pterocaryae (14–19 × 8–12 μm) are slightly wider than those revealed in the current study (11–22 × 6–11 μm), which is in line with Riedl and Ershad (1977), who also reported narrower conidia (12–15.5 × 6.5–9.5 μm) in their Iranian collection. The conidial size and shape of J. pterocaryae can be quite variable between collections but also within the same specimen, probably depending on the environmental conditions during development; we observed slightly smaller conidia in the Iranian collections ((11.2–)12.0–15.5(−19.2) × (6.0–)7.5–9.0(−10.8) μm, l/w = (1.3–)1.5–1.9(−2.6) (n = 567)) than in the Central European ones ((11.5–)14.5–17.8(−22.3) × (6.3–)7.8–9.5(−11.0) μm, l/w = (1.3–)1.6–2.2(−3.0) (n = 413)). However, as the sequences of the Central European and Iranian collections are (almost) identical, this variation is confirmed to represent intraspecific variability. In contrast to the other described Juglanconis species, no sexual morph is known for J. pterocaryae.
Despite extensive enquiries, no type collection of Melanconium pterocaryae could be traced in Russian or Georgian herbaria. In the apparent lack of an extant type, we here propose a well-developed Austrian collection, for which a culture and sequences are available, as neotype. Although the neotype collection does not originate from the area from where the species was described, we consider this justified, as the P. fraxinifolia accessions (and therefore also its associated Juglanconis) grown in Central Europe likely originate from the Caucasus area, the conidial sizes of the neotype collection and the protologue agree well, and the conspecific Austrian and Iranian Juglanconis accessions confirm a wide distribution of the species that likely corresponds with the distribution of its host.
Discussion
Previous molecular phylogenetic analyses had shown that Melanconis species on Juglans and Pterocarya form a highly supported lineage that is distinct from Melanconis sensu stricto, and the new genus Juglanconis was established for them (Voglmayr et al. 2017), which was classified in the new family Juglanconidaceae. However, in this previous study, only a single Eastern Asian isolate from Pterocarya rhoifolia could be included, but none from the Western Asian P. fraxinifolia. The current molecular phylogenetic analyses (Fig. 2) clearly show that Juglanconis accessions from P. fraxinifolia and P. rhoifolia represent two distinct species, J. pterocaryae and J. japonica, respectively. This is not surprising, as high host specificity in combination with vicariant speciation has been commonly reported in Diaporthales on woody hosts, e.g. in Coryneum (Jiang et al. 2018), Cryptosporella (Mejía et al. 2008, 2011a), Melanconiella (Voglmayr et al. 2012), Melanconis (Fan et al. 2016), Plagiostoma (Mejía et al. 2011b; Walker et al. 2014), Stegonsporium and Stilbospora (Voglmayr and Jaklitsch 2008, 2014). In many of these lineages, morphological species identification can be difficult due to lack of a clear morphological distinction, while molecular data but also host ranges are highly diagnostic on the species level. However, in the Juglanconis species on Juglans, host specificity was shown to be rather on the genus than on the species level, as both European species, J. appendiculata and J. juglandina, were reported from various hosts (the indigenous Juglans regia as well as the naturalised North American J. nigra), and the North American and Eastern Asian Juglanconis oblonga was likewise confirmed to occur on several Juglans species. It remains unclear whether the Juglanconis species on Pterocarya potentially have wider host ranges, their different host ranges and geographic areas being rather the result of the highly disjunct distribution of their hosts than of host specificity. Interestingly, Melanconis/Melanconium spp. have also been recorded from China on Pterocarya stenoptera (Farr and Rossman 2018), which has a wide distribution in Eastern Asia, occurring in China, Korea and Japan and is also widely cultivated as a shade tree (Lu et al. 1999). Investigation of isolates from this host could help to shed light on this question.
According to Kazemzadeh Chakusary (2017), J. pterocaryae is suspected to be one of the most important fungal agents of P. fraxinifolia dieback in Guilan province in Northern Iran. Seven kinds of wood lesions were associated with P. fraxinifolia showing decline symptoms in Iran. Similar observations were reported in previous studies conducted on trunk diseases of fruit (Van Niekerk et al. 2011, Cloete et al. 2011, Sami et al. 2014) and ornamental and forest trees (Hashemi and Mohammadi 2016; Kazemzadeh Chakusary et al. 2017). Iranian isolates were recovered from all kinds of wood lesions recorded on P. fraxinifolia. Moreover, a large number of acervuli of J. pterocaryae were observed on the surface of cankers and twigs showing dieback symptoms. During this study, several Iranian isolates of J. pterocaryae were isolated from necrotic wood tissues of P. fraxinifolia trees. We did not determine the pathogenicity of these isolates on this woody plant. Therefore, pathogenicity studies will be necessary to evaluate and confirm the importance of this species in trunk diseases of P. fraxinifolia.
It is remarkable that J. pterocaryae has apparently not been previously reported from Europe, considering its conspicuous symptoms which are similar to those of the well-known black pustular dieback disease of walnut (Juglans) species caused by closely related Juglanconis species (Graves 1923; Belisario 1999). This may be due to the fact that, compared to Juglans spp., Pterocarya fraxinifolia has little economic impact and is rather infrequently grown, mainly in botanical gardens, arboreta and large landscape parks. In one Austrian site (Harrachpark), it was found abundantly on large cut as well as recently wind-broken branches, the ejected conidial pustules covering their entire length. This indicates that J. pterocaryae, like other Diaporthales, may be commonly present as a latent pathogen in living host tissues, enabling a massive development following the death of the host tissue. Juglanconis pterocaryae represents another example of a tree pathogen co-occurring with its hosts in old arboreta and parks far outside their natural distribution; similar cases were, e.g. reported for North American and Southern European Stegonsporium spp. following their maple (Acer) hosts grown in Central and Western European parks (Voglmayr and Jaklitsch 2014). As these pathogens can have a long latent phase in living host tissue, they are difficult to detect and can be distributed over wide distances with the transport of symptomless but yet infected living trees. Therefore, parks and arboreta are a potential source for the introduction and establishment of alien fungal diseases of trees, and should therefore be regularly monitored especially for problem pathogens of forest trees.
Funding information
Open access funding provided by Austrian Science Fund (FWF). HV received financial support from the Austrian Science Fund (FWF; project P27645-B16). Hamid Mohammadi was supported by Grant Number 93027879 from the Iran National Science Foundation (INSF).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alvarez LV, Groenewald JZ, Crous PW. Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Stud Mycol. 2016;85:1–34. doi: 10.1016/j.simyco.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisario A. Cultural characteristics and pathogenicity of Melanconium juglandinum. Eur J For Pathol. 1999;29:317–322. doi: 10.1046/j.1439-0329.1999.00165.x. [DOI] [Google Scholar]
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.2307/3761358. [DOI] [Google Scholar]
- Cloete M, Fourie PH, Damm U, Crous PW, Mostert L (2011) Fungi associated with dieback symptoms of apple and pear trees with a special reference to grapevine trunk disease pathogens. Phytopathol Mediterr 50 (Suppl.):S176–S190
- Crous PW, Groenewald JZ, Risede JM, Hywel-Jones NL. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Stud Mycol. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog GS, Gerrits van den Ende AHG. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008;7:3188–3192. [Google Scholar]
- Ebrahimzadeh MA, Nabavi SF, Nabavi SM. Essential oil composition and antioxidant activity of Pterocarya fraxinifolia. Pak J Biol Sci. 2009;12:957–963. doi: 10.3923/pjbs.2009.957.963. [DOI] [PubMed] [Google Scholar]
- Fan X, Du Z, Liang Y, Tian C. Melanconis (Melanconidaceae) associated with Betula spp. in China. Mycol Prog. 2016;15:40. doi: 10.1007/s11557-016-1163-2. [DOI] [Google Scholar]
- Farr DF, Rossman AY (2018) Fungal databases - fungus-host distributions, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved august 18, 2018, from http://nt.ars-grin.gov/fungaldatabases/
- Forrest M. Landscape trees and shrubs: selection, use and management. Wallingford: CABI; 2006. [Google Scholar]
- Glass NL, Donaldson G. Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AH. The Melanconis disease of the butternut (Juglans cinerea L.) Phytopathology. 1923;13:411–435. [Google Scholar]
- Guarnaccia V, Groenewald JZ, Woodhall J, Armengol J, Cinelli T, et al. Diaporthe diversity and pathogenicity revealed from a broad survey of grapevine diseases in Europe. Persoonia. 2018;40:135–153. doi: 10.3767/persoonia.2018.40.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjmohammadi MR, Kamyar K. Determination of juglone (5-hydroxy 1, 4-naphthoquinone) in Pterocarya fraxinifolia by RP-HPLC. Iran J Chem Eng. 2006;25:73–76. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis. Program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hashemi H, Mohammadi H. Identification and characterization of fungi associated with internal wood lesions and decline disease of willow and poplar trees in Iran. For Pathol. 2016;46:341–352. doi: 10.1111/efp.12269. [DOI] [Google Scholar]
- Hofstetter V, Miądlikowska J, Kauff F, Lutzoni F. Phylogenetic comparison of protein-coding versus ribosomal RNA-coding sequence data: a case study of the Lecanoromycetes (Ascomycota) Mol Phylogenet Evol. 2007;44:412–426. doi: 10.1016/j.ympev.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. European species of Hypocrea part I. The green-spored species. Stud Mycol. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, Druzhinina IS. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.1080/15572536.2006.11832743. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Voglmayr H, Tian CM (2018) New species and records of Coryneum from China. Mycologia. 10.1080/00275514.2018.1516969 [DOI] [PMC free article] [PubMed]
- Kazemzadeh Chakusary M (2017) Etiology and distribution of forest trees decline in Guilan province with emphasis on isolation and identification of Phaeoacremonium and Botryosphaeriaceae species and the potential role of woody debris on the survival of the pathogens. Ph.D. Dissertation. Shahid Bahonar University of Kerman Kerman, Iran, 300 pp
- Kazemzadeh Chakusary M, Mohammadi H, Khodaparast SA. Decline-associated Phaeoacremonium spp. occurring on forest trees in the north of Iran. For Pathol. 2017;47:e12368. doi: 10.1111/efp.12368. [DOI] [Google Scholar]
- Kazemzadeh Chakusary M, Mohammadi H, Khodaparast SA (2019) Diversity and pathogenicity of Botryosphaeriaceae species on forest trees in the north of Iran. Eur J For Res
- Kobayashi T. Taxonomic studies of Japanese Diaporthaceae with special reference to their life-histories. Bull Gov For Exp Stn Meguro. 1970;226:1–242. [Google Scholar]
- Kuschke G. Mycoflorae Caucasicae Novitates. Viestnik Tiflisskago Botanicheskago Sada. 1913;28:23–26. [Google Scholar]
- Lawrence DP, Holland LA, Nouri MT, Travadon R, Abramians A, et al. Molecular phylogeny of Cytospora species associated with canker diseases of fruit and nut crops in California, with the descriptions of ten new species and one new combination. IMA Fungus. 2018;9:333–370. doi: 10.5598/imafungus.2018.09.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Lu A, Stone DE, Grauke LJ. Juglandaceae. In: Flora of China Editorial Committee, editor. Flora of China 4. St. Louis: Science Press, Beijing, and Missouri Botanical Garden; 1999. pp. 277–285. [Google Scholar]
- Manos PS, Soltis PS, Soltis DE, Manchester SR, Oh SH, et al. Phylogeny of extant and fossil Juglandaceae inferred from the integration of molecular and morphological data sets. Syst Biol. 2007;56:412–430. doi: 10.1080/10635150701408523. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Wingfield MJ, Akulov A, Carnegie AJ, et al. Genera of phytopathogenic fungi: GOPHY 2. Stud Mycol. 2019;92:47–133. doi: 10.1016/j.simyco.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF. Phylogenetic placement and taxonomic review of the genus Cryptosporella and its synonyms Ophiovalsa and Winterella (Gnomoniaceae, Diaporthales) Mycol Res. 2008;112:23–35. doi: 10.1016/j.mycres.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Mejía LC, Rossman AY, Castlebury LA, White JF., Jr New species, phylogeny, host-associations and geographic distribution of genus Cryptosporella (Gnomoniaceae, Diaporthales) Mycologia. 2011;103:379–399. doi: 10.3852/10-134. [DOI] [PubMed] [Google Scholar]
- Mejía LC, Castlebury LA, Rossman AY, Sogonov MV, White JF., Jr A systematic account of the genus Plagiostoma (Gnomoniaceae, Diaporthales) based on morphology, host-associations, and a four-gene phylogeny. Stud Mycol. 2011;68:211–235. doi: 10.3114/sim.2011.68.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SM, Ebrahimzadeh MA, Nabavi SF. Evaluation of antioxidant activity of methanolic extract of leaf and branch bark of Pterocarya fraxinifolia (Lam.) Spach. Seasonal. Iran J Med Aromat Plants. 2008;24:374–384. [Google Scholar]
- O’Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Riedl H, Ershad D. Mykologische Ergebnisse einer Sammelreise in den Iran im Frühjahr 1974. - I. Sydowia. 1977;29:155–169. [Google Scholar]
- Rix M. Pterocarya macroptera var. insignis, Juglandaceae. Curtis’s. Bot Mag. 2007;24:180–185. [Google Scholar]
- Sadighara P, Ashrafihelan J, Barin A, Aliesfahani T. Histopathology and cholinergic assessment of Pterocarya fraxinifolia on chicken embryo. Interdiscip Toxicol. 2009;2:254–256. doi: 10.2478/v10102-009-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami S, Mohammadi H, Heydarnejad J. Phaeoacremonium species associated with necrotic wood of pome fruit trees in Iran. J Plant Pathol. 2014;96:487–495. [Google Scholar]
- Silvestro D, Michalak I. raxmlGUI: a graphical front-end for RAxML. Org Divers Evol. 2012;12:335–337. doi: 10.1007/s13127-011-0056-0. [DOI] [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stiller JW, Hall BD. The origin of red algae: implications for plastid evolution. Proc Natl Acad Sci U S A. 1997;94:4520–4525. doi: 10.1073/pnas.94.9.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL (2002) PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland
- Thiers B (2018) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/
- Van Niekerk JM, Bester W, Halleen F, Crous PW, Fourie PH. The distribution and symptomatology of grapevine trunk disease pathogens are influenced by climate. Phytopathol Mediterr. 2011;50(Suppl):S98–S111. [Google Scholar]
- Vilgalys R, Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Prosthecium species with Stegonsporium anamorphs on Acer. Mycol Res. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Divers. 2011;46:133–170. doi: 10.1007/s13225-010-0078-5. [DOI] [Google Scholar]
- Voglmayr H, Jaklitsch WM. Stilbosporaceae resurrected: generic reclassification and speciation. Persoonia. 2014;33:61–82. doi: 10.3767/003158514X684212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Mehrabi M. Molecular phylogeny and a new Iranian species of Caudospora (Sydowiellaceae, Diaporthales) Sydowia. 2018;70:67–80. doi: 10.12905/0380.sydowia70-2018-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, Jaklitsch W. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales) Fungal Divers. 2012;57:1–44. doi: 10.1007/s13225-012-0175-8. [DOI] [Google Scholar]
- Voglmayr H, Akulov OY, Jaklitsch WM. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycol Prog. 2016;15:921. doi: 10.1007/s11557-016-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Castlebury LA, Jaklitsch W. Juglanconis gen. nov. on Juglandaceae, and the new family Juglanconidaceae (Diaporthales) Persoonia. 2017;38:136–155. doi: 10.3767/003158517X694768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Friebes G, Gardiennet A, Jaklitsch WM. Barrmaelia and Entosordaria in Barrmaeliaceae (fam. nov., Xylariales), and critical notes on Anthostomella-like genera based on multi-gene phylogenies. Mycol Prog. 2018;17:155–177. doi: 10.1007/s11557-017-1329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Lawrence BR, Wooten JA, Rossman AY, Castlebury LA. Five new species of the highly diverse genus Plagiostoma (Gnomoniaceae, Diaporthales) from Japan. Mycol Prog. 2014;13:1057–1067. doi: 10.1007/s11557-014-0993-z. [DOI] [Google Scholar]
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Xiang X-G, Wang W, Li RQ, Lin L, Liu Y, et al. Large-scale phylogenetic analyses reveal fagalean diversification promoted by the interplay of diaspores and environments in the Paleogene. Perspect Plant Ecol Evol Syst. 2016;16:101–110. doi: 10.1016/j.ppees.2014.03.001. [DOI] [Google Scholar]