Abstract
Fournier’s gangrene is classically associated with diabetes mellitus and alcohol use disorder. While it is associated with chemotherapy, there are few case reports of Fournier’s gangrene as the initial presentation of acute myelogenous leukemia. A 38-year-old male presented with progressive scrotal swelling and hematochezia. Blood cell count showed depression of all cell lines without myeloblasts. He received broad-spectrum antibiotics and underwent surgical debridement once. Urgent bone marrow biopsy confirmed acute promyelocytic leukemia. The patient was started on chemotherapy. He was discharged without relapse of the infection. This is the fourth case of acute myelogenous leukemia presenting as Fournier’s gangrene in the literature and the only case to have survived. This brings forth a possible diagnostic consideration in patients without obvious predisposing risk factors for Fournier’s gangrene, particularly in those with pancytopenia. Coordination with surgical services as well as hematology/oncology specialists is imperative to survival of these dual diagnosis patients.
Keywords: Fournier’s gangrene, leukemia, acute myeloid leukemia, acute myelogenous leukemia, necrotizing fasciitis
Introduction
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineum that is thought to arise from a synergistic combination of obliterative endarteritis and vascular thrombosis with infection by both anaerobic and aerobic bacteria.1,2 Multiple risk factors have been identified that predispose an individual to developing FG including diabetes mellitus, alcohol use disorder, chronic heart failure, systemic lupus erythematosus, Addison’s disease, immunosuppression, malignancy, liver disease, and renal disease.2–6 All of these conditions are associated with impaired host immunity. In addition, it is estimated that 95% of cases have an identifiable cause, such as local trauma, genitourinary infection, perianal infection, or anorectal infection.2–4 There is an observed male predominance in cases of FG, with estimates of male-to-female ratio ranging from 10:1 up to 40:1.4,6,7 The microbiology of FG is often polymicrobial, with a predominance of Gram-negative pathogens and other organisms colonizing the perineum, including Escherichia coli, Streptococcus spp., and Bacteroides spp.3–6 Bacteroides spp. appear important in the pathogenesis of FG as they inhibit phagocytosis of aerobic bacteria.3
The clinical presentation of FG typically begins with non-specific symptoms such as induration, pruritis, edema, and erythema of the affected tissues in the first 24 to 48 h.4 Over the subsequent days, inflammation spreads and tissues enter the necrotic phase. Necrosis can spread as quickly as 2–3 cm/h2,3 with risk of abdominal wall invasion and septic shock.3 Pain can disappear as ischemia from tissue necrosis leads to nerve death.2,4 Case series mortality rate has been reported as high as 88% but is generally felt to be 20%–40%,3 although two population-based studies reviewing American inpatients estimated a case mortality rate of 6%–7%.8 Multiple rounds of surgical debridement are frequently needed to establish source control.7
FG is known to be associated with hematologic cancer, frequently in the setting of active chemotherapy.9 There are few case reports of FG as the presentation for hematologic cancers. All case reports were with subtypes of acute myelogenous leukemia (AML) and all were fatal. While rare, this disease should be considered a possible concurrent diagnosis in a patient without an obvious risk factor for FG. Here, we discuss a case of FG as the presentation for acute promyelocytic leukemia (APML). Written informed consent was obtained to share patient information.
Case presentation
A 38-year-old otherwise healthy man presented for evaluation of painful scrotal swelling. He was afebrile; other vital signs were unremarkable. On examination, it was tender and without active drainage, erythema, or evidence of abscess formation. A diagnosis of folliculitis was made. He was discharged with instructions to complete a week of cephalexin. On the last day of this course, he returned to the emergency department with progressive non-painful perineal swelling as well as hematochezia. Examination showed an edematous area measuring approximately 3 cm in diameter with drainage of feculent material and serosanguinous fluid. He was febrile to 102.5°F with tachycardia. Bloodwork was obtained and was notable for hemoglobin 9.5 g/dL, platelets of 38 × 103/mm3, and leukocytes 1 × 103/mm3 with a differential of 19% neutrophils (absolute neutrophil count of 100), 72% lymphocytes, 7% monocytes, and 2% metamyelocytes. A pelvic computed tomography (CT) was obtained due to concern for necrotizing soft tissue infection. It demonstrated foci of air in the perineum and inferior scrotum consistent with FG (Figure 1). He was initiated on vancomycin, piperacillin/tazobactam, and clindamycin before undergoing emergent wound exploration and debridement. The feculent-appearing material was found to be necrotic connective tissue requiring scrotal exploration performed by a urologist and surgical debridement performed by a general surgeon. Debridement extended from scrotal base posteriorly toward the anus without involvement of the anus to an area of 12 cm × 5 cm. Rectal examination was without induration, fluctuance, masses, or connection to the perineal wound. There was no evidence of involvement of bowel, penis, or tissue deep to the dartos fascia. Negative pressure wound therapy was initiated with thrice-weekly dressing changes.
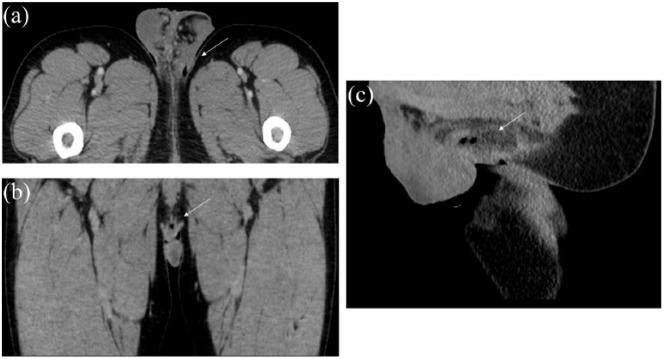
Figure 1.
Images from computed tomography scan of perineal region demonstrating foci of air along with an ill-defined region of peripheral hyperattenuation with central hypoattenuation consistent with focal infection/abscess in the area of noted perineal wound between scrotum and anus: (a) transverse plane, (b) coronal plane, and (c) sagittal plane.
Hematology was consulted the next day for evaluation of pancytopenia. As his peripheral smear was without myeloblasts, a bone marrow biopsy was performed (Figure 2). A diagnosis of APML was established via fluorescence in situ hybridization, chromosomal analysis, and promyelocytic leukemia/retinoic acid receptor-alpha quantitative polymerase chain reaction (PCR) of the bone marrow. He was presumptively started on half-dose all-trans-retinoic acid (ATRA) therapy on hospital day 2, then started on definitive chemotherapy when this diagnosis was confirmed on hospital day 4. Definitive therapy dosing was ATRA 22.5 mg/m2 by mouth twice daily. On hospital day 5, he was also started on arsenic trioxide (ATO) 0.15 mg/kg intravenous (IV) daily on weekdays based on regimens described previously in low-to-intermediate-risk APML.10 Both agents were continued until day 26 of induction therapy when complete hematologic remission was confirmed by a bone marrow biopsy performed the week prior. He was then started on and completed consolidation therapy.
Figure 2.
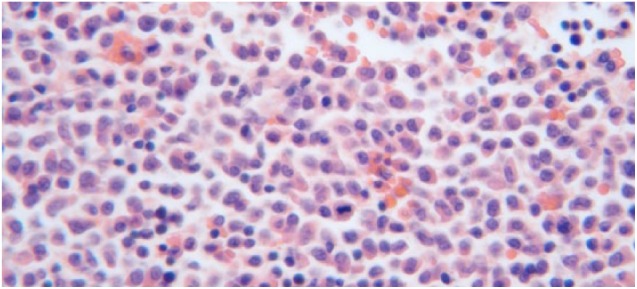
Patient’s bone marrow biopsy demonstrating hypercellularity greater than expected for age. Marked myeloid hyperplasia with left shift accounting for 85% of bone marrow cellularity. Maturing myeloid elements nearly absent. Maturing erythroid elements moderately to markedly decreased. Megakaryocytes markedly decreased. No significant lymphocytosis or plasmacytosis.
His surgical cultures grew E. coli, Enterococcus faecalis, Bacteroides thetaiotaomicron, Streptococcus agalactiae, Clostridium clostridioforme, as well as Gram-positive bacilli resembling diphtheroids and many anaerobic Gram-positive cocci that were not identified. Blood cultures remained negative throughout admission. Antibiotic therapy was changed to vancomycin, cefepime, and metronidazole after development of a rash on the initial empiric therapy, and culture data did not support any further adjustment to IV therapy. He was continued on IV antibiotics until 48 h after resolution of his neutropenia, after which an oral regimen of levofloxacin and metronidazole was initiated to complete a total antibiotic course of 2 weeks duration. Hematochezia resolved during hospitalization and was attributed to disseminated intravascular coagulation (DIC) secondary to APML. He improved and was discharged with a wound vacuum-assisted closure device that lost seal 9 days after discharge and was removed. In follow-up, his wound was left to heal by secondary intention with wet to dry dressings. He did not require a skin graft and has not experienced urologic complications or had recurrence of either FG or APML.
Discussion
In our literature search, we found three cases of perineal gangrene as the presentation of acute leukemia. Two patients were adult males and had evidence of scrotal edema and scrotal tenderness prior to the development of gangrene.10,11 The third case was in a 4-day-old female who developed FG while in the hospital.12 All cases were patients presenting with AML and all cases were fatal (Table 1).
Table 1.
Case reports of acute leukemia presenting as Fournier’s gangrene: demographics, blood counts, culture data, leukemia with time of diagnosis, treatment course, and outcomes.
| Age | Sex | Blood counts | Culture data | Leukemia details | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| 50 years | Male | WBC: 10.5 × 103/mm3
Hgb: 7.7 g/dL Plt: 17 × 103/mm3 Myeloblasts: 49% |
Surgical (E. coli) Blood (negative) Urine (negative) |
Acute promyelocytic Postmortem |
Ciprofloxacin Clindamycin Penicillin G |
Death | Oiso et al.13 |
| 33 years | Male | WBC: 1.9 × 103/mm3
Hgb: 8 g/dL Plt: 50 × 103/mm3 Myeloblasts: NR |
Surgical (Bacteroides fragilis) Blood (B. fragilis) |
Acute myelomonocytic After antibiotic initiation by 1 week |
Antibiotics NR Chemotherapy |
Death with chemotherapy induction | Islamoglu et al.12 |
| 4 days | Female | WBC: 2.1 → 18.5 × 103/mm3
Hgb: 8.8 g/dL Plt: 21 × 103/mm3 Myeloblasts: 0% → 52% |
Surgical (Pseudomonas aeruginosa) Blood (P. aeruginosa) |
Acute myelogenous After antibiotic initiation, timing NR |
Meropenem Vancomycin Chemotherapy declined |
Death | Mosayebi et al.14 |
WBC: white blood cells; Hgb: hemoglobin; Plt: platelets; NR: not reported.
One other case report of genital infection, without progression to necrosis, as the first sign of AML has been reported in the literature in a 51-year-old patient following perineal trauma.14 His blood counts showed a normal total leukocyte count, but the differential was notable for 83% myeloblast predominance. A simple incision and drainage was performed. Cultures of the drainage fluid were notable for Corynebacterium spp., representing cutaneous contamination. He received 2 weeks of broad-spectrum antibiotic therapy prior to chemotherapy initiation for AML. This case further illustrates that genitourinary infection can serve as the first presenting sign of AML.
Our case is consistent with aspects of the presentation of FG in the setting of AML. In our case, a male patient with evidence of antecedent perianal folliculitis developed necrotizing fasciitis over the course of a week. This timeline is consistent with typical progression of FG. He presented with pancytopenia that was concerning for both a severe infection and an underlying malignancy although without evidence of myeloblasts on review of a peripheral blood smear. Multiple organisms were isolated, including two common pathogens associated with FG, E. coli and Bacteroides spp., consistent with synergistic infection by aerobic and anaerobic bacteria. In addition, our case had the potential for severe consequences if the patient’s underlying hematologic malignancy had not been rapidly diagnosed and treatment initiated. Remarkably, he only required a single surgical procedure, perhaps due to his neutropenia and his diminished ability to mount an inflammatory response in addition to correction of his only comorbidity.
In this case, infection was possibly induced and likely propagated through DIC caused by APML, as evidenced by his reported hematochezia prior to admission. This is the fourth report of AML presenting in the necrotic stage of FG and the only one to have survived. As both FG and APML can be rapidly fatal, it is important to involve multiple disciplines including surgery, urology, and hematology/oncology to optimize patient outcomes. This outcome was likely due to timely recognition and treatment of both the infection and leukemia. These cases illustrate the need to fully explore risk factors for FG and recognize that hematologic malignancy can present acutely as FG despite a lack of myeloblasts in the peripheral circulation.
Conclusion
FG can be the presenting feature for acute leukemia, with all reported cases being in the myeloid lineage, even without the presence of circulating myeloblasts in the peripheral circulation. This infection may arise from decreased integrity of perineal mucosa due to DIC, allowing urogenital flora to translocate into soft tissues. Early recognition without extensive tissue involvement contributed to a favorable outcome in this case. Other factors contributing to this good outcome include an otherwise healthy patient, immediate workup of pancytopenia, chemotherapy regimen with relatively low association with hematologic toxicity, and effective collaboration between multiple surgical and medical specialties. In this patient, without any other reason for immunosuppression, initiation of an appropriate APML treatment regimen likely improved his immune function and ability to fight infection. In addition, antecedent treatment with cephalexin for presumed folliculitis likely delayed progression and prompted close patient follow-up and workup due to failure of outpatient therapy.
Acknowledgments
The authors acknowledge the physicians and nursing staff involved in the care of this patient. Without their efforts and expertise, a favorable outcome would not have been possible.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent in was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Anahita Mostaghim  https://orcid.org/0000-0002-5790-9288
https://orcid.org/0000-0002-5790-9288
References
- 1. Stevens DL, Bryant A. Necrotizing soft-tissue infections. N Engl J Med 2017; 377(23): 2253–2265. [DOI] [PubMed] [Google Scholar]
- 2. Misiakos EP, Bagias G, Patapis P, et al. Current concepts in the management of necrotizing fasciitis. Front Surg 2014; 1: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shyam DC, Rapsang AG. Fournier’s gangrene. Surgeon 2013; 11(4): 222–232. [DOI] [PubMed] [Google Scholar]
- 4. Voelzke BB, Hagedorn JC. Presentation and diagnosis of Fournier Gangrene. J Urol 2017; 114: 8–13. [DOI] [PubMed] [Google Scholar]
- 5. Tang LM, Su YJ, Lai YC. The evaluation of microbiology and prognosis of Fournier’s gangrene in past five years. Springerplus 2015; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ioannidis O, Loukiani K, Tatsis D, et al. Fournier’s gangrene: lessons learned from multimodal and multidisciplinary management of perineal necrotizing fasciitis. Front Surg 2017; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorensen MD, Krieger JN. Fournier’s gangrene: epidemiology and outcomes in the general U.S. population. Urol Int 2016; 97(3): 249–259. [DOI] [PubMed] [Google Scholar]
- 8. Osbun N, Hampson LA, Hold SK, et al. Low-volume vs high-volume centers and management of Fournier’s gangrene in Washington State. J Am College Surg 2017; 224(3): 270–275. [DOI] [PubMed] [Google Scholar]
- 9. Arena GD, Pietrantuono G, Buccino E, et al. Fournier’s gangrene complicating hematologic malignancies: a case report and review of literature. Med J Hematol Infect Dis 2013; 5(1): e2013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013; 369(2): 111–121. [DOI] [PubMed] [Google Scholar]
- 11. Faber HJ, Cirbes ARJ, Daenen S. Fournier’s gangrene as first presentation of promyelocytic leukemia. Leukemia Res 1998; 22(5): 473–476. [DOI] [PubMed] [Google Scholar]
- 12. Islamoglu K, Serdaroglu I, Ozgentas E. Co-occurrence of Fournier’s gangrene and pancytopenia may be the first sign of acute myelomonocytic leukemia. Ann Plastic Surg 2001; 47(3): 352–353. [DOI] [PubMed] [Google Scholar]
- 13. Oiso N, Rai S, Kawara S, et al. Genital infection as a first sign of acute myeloid leukemia. Case Rep Dermatol 2010; 2(1): 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mosayebi Z, Omidian A, Movahedia AH, et al. Fournier’s gangrene in a neonate with acute myeloid leukemia: a case report. Iran J Pediatr 2016; 26(3): e4537. [DOI] [PMC free article] [PubMed] [Google Scholar]