Abstract
Purpose:
To collect information about the needs of families affected by childhood-onset dystrophinopathies residing in the United States.
Methods:
Individuals with an eligible dystrophinopathy were identified by the Muscular Dystrophy Surveillance, Tracking, and Research network. Between September 2008 and December 2012, 272 caregivers completed a 48-item survey about needs related to information, healthcare services, psychosocial issues, finances, caregiver demographics, and the individual’s functioning.
Results:
Overall, at least 80% of the survey items were identified as needs for more than one-half of caregivers. Among the needs identified, physical health and access to information were currently managed for most caregivers. Items identified as needed but managed less consistently were funding for needs not covered by insurance and psychosocial support.
Conclusions:
Healthcare providers, public health practitioners, and policymakers should be aware of the many needs reported by caregivers, and focus on addressing gaps in provision of needed financial and psychosocial services.
Keywords: Becker muscular dystrophy, Duchenne muscular dystrophy, dystrophinopathy, caregivers, needs assessment, survey
Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD), herein referred to as dystrophinopathies, are allelic X-linked disorders caused by a mutation in the DMD gene.1 Currently, there are no curative treatments. Oral corticosteroids are associated with improved outcomes, including prolonged walking and delayed orthopedic, cardiac, and respiratory complications in DMD.2–16 In addition, monitoring and intervention on aspects of cardio-pulmonary manifestations has likely improved longevity.17,18 More recently, use of medications, such as Eteplirsen, may modify disease progression in a subset of males with specific DMD mutations amenable to exon 51 skipping.2–15
Although available treatments modify disease course, declines in muscle integrity and multi-system functional deterioration continue to be significant problems requiring long-term management.19–21 Consequently, family needs associated with a dystrophinopathy diagnosis remain and may increase as diagnosed individuals survive longer.20 Studies on the quality of life of individuals diagnosed with a dystrophinopathy and on the burden to their families have reported lower adaptability among those individuals with increasing health demands.22–31 Although the measurement of burden and adaptation have varied across studies, adequate practical and psychological resources have been shown to reduce the effect of these demands on the diagnosed individual and their families.23,26,27,32,33
To assist healthcare providers, public health practitioners, and policymakers in gaining a better understanding of the needs of caregivers and families of individuals with a dystrophinopathy diagnosis, we administered a comprehensive survey to caregivers of individuals with a childhood-onset dystrophinopathy identified from a multi-site, population-based cohort. The purposes of our survey were to describe the needs of caregivers and diagnosed individuals, the association of needs with stages of disease, and family demographics, and to identify gaps in care.
Methods
The Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) is a retrospective surveillance cohort of eligible individuals diagnosed with a childhood-onset dystrophinopathy. MD STARnet methods have been described elsewhere.34,35 Briefly, starting in 2004, the MD STARnet retrospectively ascertained individuals with childhood-onset dystrophinopathy who were born since 1 January 1982, diagnosed by age 21 years, and resided in an MD STARnet site (Arizona (AZ), Colorado (CO), Iowa (IA), and the western 12 counties of New York State [wNY]). Georgia (GA) and Hawaii (HI) joined the MD STARnet in 2005 and 2008, respectively. Since the initiation of surveillance, medical record abstraction was completed annually for all retrospectively ascertained individuals and newly diagnosed individuals through 31 December 2011 (for individuals identified before September 2011), 31 December 2012 (for individuals identified after September 2011), or until death or migration out of an MD STARnet site. Trained medical abstractors reviewed medical records and entered data into an electronic surveillance application. Abstracted data included sociodemographic data, clinical signs and symptoms, diagnostic/clinical tests, family history of dystrophinopathy, medical treatments received, and medical complications experienced. Data on schooling, rehabilitation, mobility, and neurobehavioral problems were also collected.
Needs assessment survey
The survey was developed by the MD STARnet Needs Assessment workgroup, comprising representatives from each MD STARnet site. The survey expanded on an initial needs assessment funded by the Centers for Disease Control and Prevention (CDC) and piloted in Iowa in 2005–2006. Questions included caregiver sociodemographic information (marital status (single, never married; married or living as married; widowed; separated; divorced), race (American Indian or Alaska Native; Asian; Black or African American; Native Hawaiian or Other Pacific Islander; White), ethnicity (Hispanic or Latino; Not Hispanic or Latino), education (grade school; junior high; high school, no diploma; high school with diploma; some college, no diploma; technical college or associate degree; bachelor’s degree; graduate or professional degree), and annual household income (<US$10,000; US$10,000–US$19,999; US$20,000–US$29,999; US$30,000–US$39,999, US$40,000–US$49,999; US$50,000–US$59,999; US$60,000–US$69,999;US$70,000–US$79,999; US$80,000–US$89,999; US$90,000–US$99,999; [>US$100,000]), household composition (number), physical disabilities of family members in the household (yes/no), caregiving provided by family members (yes/no), with whom the child resided (both parents, same household; one parent; both parents, different households; relative; foster home; care facility or group home; independently; spouse or partner; other), current ambulation (walking without help; walking with help; manual wheelchair, full-time; power scooter, full-time; power wheelchair, full-time; cannot operate power mobility) and respiratory function (no assistance; bi-pap or c-pap with sleep; daytime bi-pap or c-pap; bi-pap or c-pap with sleep and sip-n-puff mouthpiece, breath-stacking during the day; tracheostomy with part-time ventilation; trachesotomy with full-time ventilation), assistance with activities of daily living (yes/no), and health-related services received (available and helpful; frequency).
The survey consisted of 48 items across 5 domains: home, community, and durable equipment (13 items); healthcare management of common morbidities (10 items); access to factual information about genetic testing, research, and planning (11 items); psychosocial issues for the individual (5 items); and psychosocial issues for the parent/caregiver and family (9 items). These items were chosen based on a literature review, clinical experience, and pilot data that included the opportunity for families to list needs not included among the item responses in the pilot survey. Each need was scored for management by the caregiver as “Currently being managed or has usually been managed,” “Is not currently or has not usually been managed,” and “Does not apply.” Finally, caregivers were asked to select their three highest unmanaged needs items from the 48 items listed.
Survey sample and data collection
The eligible sample comprised 520 primary caregivers of living individuals diagnosed with a dystrophinopathy, who had either a positive DNA or muscle biopsy test result or a positive family history of a dystrophinopathy and were identified from surveillance years 2004–2011 (see Figure 1). Among multiple-affected families, caregivers were asked to report on their oldest affected son. Surveys were administered from January 2008 through August 2012. Recruitment was initiated by mail either directly to the eligible caregiver (CO, HI, IA, NY, or GA) or through the primary healthcare provider (AZ). Following a pre-contact letter informing the caregiver about the study, an introductory packet—consisting of a detailed explanation of the study, a project brochure, information on frequently asked questions and rights of research subjects, US$20 compensation, and the survey—was mailed. A systematic follow-up procedure included reminder telephone calls every 2 weeks followed by a mailed reminder letter if contact could not be made by telephone, until the survey was returned, or a maximum of three unsuccessful follow-up attempts were completed. The final reminder mailing included an additional copy of the survey. The survey was available in English or Spanish and typically took 30 min to complete. Returned surveys were evaluated for completeness; caregivers were contacted if missing data were identified. Surveys were scanned using Teleform (Verity, Inc. Sunnyvale, CA, version 10.6, 2012), and scanned data were reviewed manually for accuracy by two reviewers. Local institutional review board approval to conduct survey data collection with caregivers was obtained at each MD STARnet site. Consent to participate was received by signature (CO, wNY) or implied through completion of the survey (AZ, GA, IA).
Figure 1.
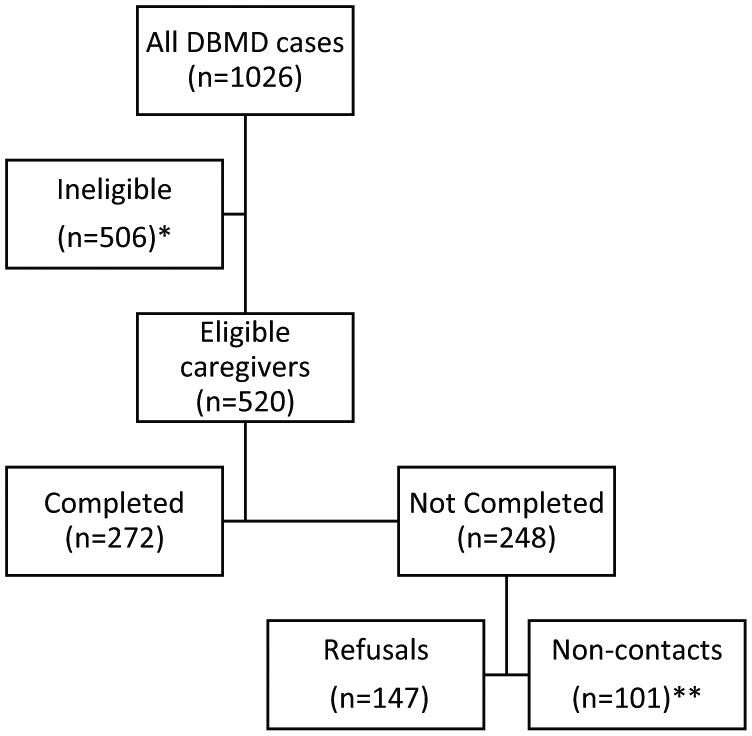
Flowchart of eligible caregivers and completed questionnaires.
*Ineligible caregivers included those of deceased males or those of males with possible or asymptomatic diagnoses, or affected females.
**Non-contacts include mailings to an unverified address that were not verified, returned mail with undeliverable address, and unverifiable address for mailings.
Statistical analysis
Item response categories were recoded in two steps in order to identify needs and the management of those needs. First, responses were recoded to reflect the presence of a need based on current or past management of an item (Not needed [0] = “Does not apply”; Needed [1] = “Currently being managed or has usually been managed” OR “Is not currently or has not usually been managed”). Missing items were excluded when recoding presence of needs. Second, a variable representing management of needs was created by recoding only those items for which management was reported (Not managed [0] = “Is not currently or has not usually been managed”; Managed [1] = “Currently being managed or has usually been managed”). “Does not apply” and missing responses were excluded when recoding management of needs.
Using available surveillance data, we compared selected characteristics for all eligible caregivers and those who responded to the survey. Analyses included item frequency counts for identified needs and reported management of each need. Chi-square analyses examined associations between identified needs and management of needs and ambulation status of the individual (ambulatory; non-ambulatory) and caregiver demographics (annual income [<US$30,000; ⩾US$30,000] and race/ethnicity (non-Hispanic white; minority race/ethnicity)). A p-value < 0.05 was used to determine statistically significant associations.
Results
The American Association for Public Opinion Research (AAPOR) calculator was used to estimate outcome rates; formulas that estimated the number of potentially eligible participants from the number of participants with undetermined eligibility were used.36 Survey responses from HI (n = 2) were excluded from analyses due to the limited follow-up conducted with primary caregivers. For the remaining sites, the response rate (number of completed surveys by caregivers divided by the number of eligible caregivers in the sample) was 59%. The contact rate (proportion of households in which the caregiver was reached) was 85%. The cooperation rate (proportion of all completed surveys from all caregivers where contact was verified) was 69%.
Compared to all eligible caregivers in the MD STARnet cohort, caregivers who completed the survey were more likely to be non-Hispanic White and have higher education than those who did not respond (data not shown). No other characteristics differed statistically between all eligible caregivers and those who responded to the survey. At the time of survey completion, the average age of the individual with DMD was 14.8 years (SD = 6.9); 56% were non-ambulatory, and 29% required respiratory assistance (Supplemental Table 1). Among caregivers, 78% were married, 50% had some college or higher, 62% had an annual income of or above US $30,000 with an average of four persons within a household supported by this income, and 15% had other persons in the household affected by a disability. For the affected individual, over 70% lived with both parents in the same household, over 50% used a wheelchair full-time, and nearly one-third required respiratory intervention. Over 90% of caregivers provided personal and health care functions for their child and nearly 70% paid others to assist.
Needs of families
The top 10 needs identified are shown by rank order in Table 1 together with the percentage of respondents who affirmed that this was a need (e.g. “Currently being managed or has usually been managed” OR “Is not currently or has not usually been managed”). Tables 2–4 display identified needs related to ambulation status, household income, and caregiver race/ethnicity. Overall, the top 10 needs by these subgroups were similar to those identified by the overall sample, although the ranking differed and different subgroups identified some unique needs (highlighted in tables).
Table 1.
Top 10 needs by all caregivers, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Needs | % needed |
|---|---|
| Information on MDA, PPMD or similar organizations | 96.3 |
| Access to research updates | 95.9 |
| Information about the course of the disease | 95.5 |
| Access to research participation | 95.2 |
| Information about financial resources (private, state, federal) | 94.1 |
| Information for talking to family/friends about health concerns | 92.6 |
| Balancing work, family, and caregiving | 91.1 |
| Finding time and energy for yourself | 89.9 |
| Finding time and energy for adult relationships | 88.8 |
| Suggestions for recreational activities alone or with family/friends | 88.4 |
MDA: muscular dystrophy association; PPMD: parent project muscular dystrophy.
Needs were recoded as not needed = 0 or needed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified an item as needed.
Table 2.
Top 10 needs by ambulation status, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Caregivers of non-ambulatory individuals | % needed | Caregivers of ambulatory individuals | % needed |
|---|---|---|---|
| Completing personal care activities (bathing, lifting, toileting) | 98.0 | Access to research updates | 96.5 |
| Dental care | 97.3 | Information on MDA, PPMD or similar organizations | 95.6 |
| Information on MDA, PPMD or similar organizations | 96.7 | Information about the course of the disease | 93.9 |
| Information about financial resources (private, state, federal) | 96.7 | Access to research participation | 93.8 |
| Information about the course of the disease | 96.6 | Information for talking to family/friends about health concerns | 93 |
| Access to research participation | 96.0 | Information about financial resources (private, state, federal) | 90.4 |
| Balancing work, family, and caregiving | 96.0 | Information about inheritance and carrier testing | 86.7 |
| Getting funding/insurance to pay for equipment | 96.0 | Balancing work, family, and caregiving | 83.9 |
| Access to research updates | 95.4 | Finding time and energy for yourself | 82.1 |
| Finding time and energy for yourself | 98.0 | Finding time and energy for adult relationships | 82.1 |
MDA: muscular dystrophy association; PPMD: parent project muscular dystrophy.
Needs were recoded as not needed = 0 or needed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified an item as needed. Gray cells identify unique needs across ambulation status. Needs are presented from highest to lowest order based on percentages of caregivers who reported the item as needed.
Table 3.
Top 10 needs by caregiver reported annual household income, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| <US$30,000 household income | % Needed | ⩾US$30,000 household income | % needed |
|---|---|---|---|
| Information on MDA, PPMD or similar organizations | 94.9 | Information on MDA, PPMD or similar organizations | 97.0 |
| Access to research updates | 93.8 | Access to research updates | 97.0 |
| Information about financial resources (private, state, federal) | 93.8 | Information about the course of the disease | 96.4 |
| Information about the course of the disease | 93.7 | Access to research participation | 96.4 |
| Access to research participation | 91.3 | Information for talking to family/friends about health concerns | 95.2 |
| Dental care | 91.1 | Information about financial resources (private, state, federal) | 94.7 |
| Joint contractures and muscle weakness | 91.0 | Balancing work, family, and caregiving | 92.3 |
| Suggestions for recreational activities alone or with family/friends | 89.6 | Finding time and energy for yourself | 91.6 |
| Information for talking to family/friends about health concerns | 88.8 | Finding time and energy for adult relationships | 91.0 |
| Balancing work, family, and caregiving | 88.6 | Making and maintaining age-appropriate friendships | 89.7 |
MDA: muscular dystrophy association; PPMD: parent project muscular dystrophy.
Needs were recoded as not needed = 0 or needed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified an item as needed. Gray cells identify unique needs across household income. Needs are presented from highest to lowest order based on percentages of caregivers who reported the item as needed.
Table 4.
Top 10 needs by caregiver race/ethnicity, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Minority caregivers | % needed | Non-minority caregivers | % needed |
|---|---|---|---|
| Information on MDA, PPMD or similar organizations | 94.8 | Information on MDA, PPMD or similar organizations | 97.6 |
| Access to research updates | 91.4 | Access to research updates | 97.6 |
| Balancing work, family, and caregiving | 91.4 | Information about the course of the disease | 97.6 |
| Dental care | 91.4 | Access to research participation | 96.6 |
| Access to research participation | 91.2 | Information about financial resources (private, state, federal) | 96.2 |
| Information for talking to family/friends about health concerns | 91.2 | Information for talking to family/friends about health concerns | 93.8 |
| Information about the course of the disease | 91.1 | Balancing work, family, and caregiving | 91.8 |
| Information about financial resources (private, state, federal) | 89.7 | Finding time and energy for yourself | 91.3 |
| Suggestions for recreational activities alone or with family/friends | 89.3 | Finding time and energy for adult relationships | 90.8 |
| Finding time and energy for yourself | 87.9 | Making and maintaining age-appropriate friendships | 89.2 |
MDA: muscular dystrophy association; PPMD: parent project muscular dystrophy.
Needs were recoded as not needed = 0 or needed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified an item as needed. Gray cells identify unique needs across race/ethnicity. Needs are presented from highest to lowest order based on percentages of caregivers who reported the item as needed.
Descriptive statistics for needs, as well as results for comparisons by individual ambulation status, and caregiver reported annual household income and race/ethnicity are presented in Supplemental Table 2. As expected, given the development of the survey, 80% or more of the survey items were identified as needs by more than 50% of all responding caregivers (Supplemental Table 2). Overall, 33 of the 48 queried needs were significantly different by ambulation status with needs more common among caregivers of non-ambulatory individuals.
Managing needs of families
The top 10 unmanaged needs (e.g. “Is not currently or has not usually been managed”) for all respondents that identified an item as a need are shown in Table 5. The majority of the unmet needs were in the realms of information, psychosocial support, and access to funding. Consistent with the data on identified needs, the unmet needs in subgroups (shown in Tables 6–8) were very similar, with a few unique unmet needs in each subgroup and differences in rank order.
Table 5.
Top 10 unmanaged needs for all caregivers, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Needs | % managed |
|---|---|
| Information about jobs/future planning for the male with DBMD | 29.7 |
| Information about transitioning to independent adult life | 32.5 |
| Finding funding for vehicle modifications | 37.3 |
| Counseling to help emotional adjustment of parent (depression, fear, loneliness) | 40.1 |
| Arranging or getting caregiver access to marriage or relationship counseling | 41.1 |
| Finding funding for home modifications | 44.3 |
| Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 45.4 |
| Finding or attending parent, spouse or sibling support groups | 46.8 |
| Information about financial resources (private, state, federal) | 47.1 |
| Counseling to help emotional adjustment of individual with DMD (depression, fear, loneliness) | 49.3 |
DBMD: Duchenne/Becker muscular dystrophy; DMD: Duchenne muscular dystrophy.
Management of needs was recoded as not managed = 0 or managed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified a need as managed.
Table 6.
Top 10 unmanaged needs by ambulation status, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Caregivers of non-ambulatory individuals | % managed | Caregivers of ambulatory individuals | % managed |
|---|---|---|---|
| Information about jobs/future planning for individual with DBMD | 32.8 | Finding funding for vehicle modifications | 16.7 |
| Information about transitioning to independent adult life | 34.8 | Information about jobs/future planning for individual with DBMD | 23.5 |
| Arranging or getting access to marriage or relationship counseling | 37.3 | Information about transitioning to independent adult life | 25.9 |
| Caregiver counseling for emotional adjustment (depression, fear, loneliness) | 39.8 | Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 38.8 |
| Finding funding for vehicle modifications | 43.0 | Finding funding for home modifications | 42.0 |
| Finding or attending parent, spouse or sibling support groups | 43.8 | Caregiver counseling for emotional adjustment (depression, fear, loneliness) | 42.4 |
| Finding funding for home modifications | 44.5 | Finding a builder/contractor for home modifications | 43.6 |
| Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 48.5 | Information about financial resources (private, state, federal) | 44.7 |
| Information about financial resources (private, state, federal) | 48.6 | Arranging or getting access to marriage or relationship counseling | 46.7 |
| Affected individual counseling for emotional adjustment (depression, fear, loneliness) | 48.7 | Finding or attending parent, spouse or sibling support groups | 48.7 |
DBMD: Duchenne/Becker muscular dystrophy.
Management of needs was recoded as not managed = 0 or managed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified a need as managed. Gray cells identify unique unmanaged needs across ambulation status. Unmanaged needs were selected based on percentage of caregivers reporting management of the need, for example, only 16.7% of caregivers of ambulatory males report finding funding for vehicle modifications as managed.
Table 7.
Top 10 unmanaged needs by caregiver reported annual household income, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| <US$30,000 Household income | % managed | ⩾US$30,000 Household income | % managed |
|---|---|---|---|
| Arranging or getting access to marriage or relationship counseling | 22.9 | Information about transitioning to independent adult life | 30.6 |
| Finding funding for vehicle modifications | 27.6 | Information about jobs/future planning for individual with DBMD | 30.7 |
| Information about jobs/future planning for individual with DBMD | 29.6 | Caregiver counseling for emotional adjustment (depression, fear, loneliness) | 42.0 |
| Finding or attending parent, spouse or sibling support groups | 32.1 | Finding funding for vehicle modifications | 43.7 |
| Finding funding for home modifications | 33.9 | Information about financial resources (private, state, federal) | 45.6 |
| Information about transitioning to independent adult life | 34.0 | Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 49.6 |
| Caregiver counseling for emotional adjustment (depression, fear, loneliness) | 35.3 | Arranging or getting access to marriage or relationship counseling | 50.0 |
| Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 36.1 | Finding or attending parent, spouse or sibling support groups | 51.1 |
| Finding a builder/contractor for home modifications | 37.5 | Finding funding for home modifications | 51.7 |
| Affected individual counseling for emotional adjustment (depression, fear, loneliness) | 38.9 | Affected individual counseling for emotional adjustment (depression, fear, loneliness) | 54.3 |
DBMD: Duchenne/Becker muscular dystrophy.
Management of needs was recoded as not managed = 0 or managed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified a need as managed. Gray cells identify unique unmanaged needs across household income. Unmanaged needs were selected based on percentage of caregivers reporting management of the need, for example, only 22.9% of caregivers from lower income households report getting access to marriage counseling as managed.
Table 8.
Top 10 unmanaged needs by caregiver race/ethnicity, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Minority caregivers | % managed | Non-minority caregivers | % managed |
|---|---|---|---|
| Finding funding for vehicle modifications | 20.9 | Information about jobs/future planning for the individual with DBMD | 28.5 |
| Information about transitioning to independent adult life | 32.5 | Information about transitioning to independent adult life | 32.0 |
| Information about jobs/future planning for individual with DBMD | 33.3 | Caregiver counseling for emotional adjustment (depression, fear, loneliness) | 40.7 |
| Finding a builder/contractor for home modifications | 36.8 | Arranging or getting access to marriage or relationship counseling | 41.7 |
| Arranging or getting access to marriage or relationship counseling | 37.0 | Finding funding for vehicle modifications | 42.4 |
| Finding funding for home modifications | 37.8 | Finding funding for home modifications | 46.3 |
| Caregiver counseling for emotional adjustment (depression, fear, loneliness) | 38.9 | Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 46.6 |
| Information about how to prepare advance directives: living wills and designating durable power of attorney for healthcare | 39.5 | Finding or attending parent, spouse or sibling support groups | 47.1 |
| Affected individual counseling for emotional adjustment (depression, fear, loneliness) | 42.5 | Information about financial resources (private, state, federal) | 47.3 |
| Patient discussion/support groups | 43.2 | Affected individual counseling for emotional adjustment (depression, fear, loneliness) | 51.6 |
DBMD: Duchenne/Becker muscular dystrophy.
Management of needs was recoded as not managed = 0 or managed = 1; thus, the percentage reported in the table represents the percentage of caregivers that identified a need as managed. Gray cells identify unique unmanaged needs across caregiver race/ethnicity. Unmanaged needs were selected based on percentage of caregivers reporting management of the need, for example, only 22.9% of caregivers from lower income households report getting access to marriage counseling as managed.
Descriptive statistics for management of needs, as well as results for comparisons by individual ambulation status, caregiver reported annual income and race/ethnicity are presented in Supplemental Table 3. Relatively few significant differences were found for comparisons of subgroups regarding management of needs. Only 12 of 48 comparisons were differed significantly by ambulation status and those were equally distributed across all domains. Similarly, the management of nine needs differed by annual income, with poorer management of home and vehicle modifications, and time and energy and relationships among household with annual incomes <US$30,000 compared to those with higher incomes. Only four comparisons were significant by race/ethnicity; poorer management of funding needs for home and vehicle modifications, and access to information was found among minority caregivers compared to non-minority.
Most unmanaged needs
In a further effort to identify high-priority needs, caregivers were asked to select the top 3 issues from the list of 48 survey items that they felt had been the most difficult to deal with or were (usually) not managed. Table 9 shows the items identified by responders, together with the percentage of caregivers who chose that item. Consistent with the overall analysis, unmanaged needs most commonly listed were related to the realms of financial and psychosocial supports.
Table 9.
Items listed as most unmanaged needs by caregivers, the Muscular Dystrophy Surveillance, Research, and Tracking network, 2008–2012 (n = 272).
| Most unmanaged needs | % of caregivers |
|---|---|
| Information about financial resources (private, state, federal, etc.) | 19.0 |
| Finding funding for home modifications | 16.8 |
| Counseling to help emotional adjustment of affected male | 16.1 |
| Finding funding for vehicle modifications | 15.0 |
| Information about jobs/future planning for the male with DBMD | 13.1 |
| Finding time and energy for yourself (personal time for the caregiver) | 12.4 |
| Making and maintaining age-appropriate friendships for the male with DBMD | 10.9 |
| Finding funding and available respite care so caregivers can be gone | 9.8 |
| Finding time and energy for adult relationships (spouse/significant other) | 9.5 |
DBMD: Duchenne/Becker muscular dystrophy.
Discussion
Our study systematically identified needs and management of these needs by caregivers and families of individuals diagnosed with a childhood-onset dystrophinopathy across a range of domains. The most commonly identified needs included access to information about support groups, research, disease progression, and balancing time and energy. Overall, although we found that families have many needs, caregivers responded that most medical and information needs were being managed. Gaps in meeting individual and family needs remained, however, primarily in the spheres of availability of information about future planning and transitions, financial assistance, and psychosocial supports for family members. Financial-related needs were unmanaged for over one-half of caregivers and were the most frequently selected unmanaged needs from all available items.
Our findings are in keeping with others who have documented the financial burden of DMD. Survey data from caregivers of individuals with DMD followed in Muscular Dystrophy Association (MDA) clinics reported costs for durable medical equipment are, on average, approximately US$1100, modifying motor vehicle US$1600, and home modification US$3050.29 Similarly, a Treat NeuroMuscular Disease (TREAT-NMD) DMD registry survey of caregivers residing in the United States reported total annual costs of approximately US$7900 for aids and devices and investments in home and vehicle modifications.28 Several analyses have reported that the healthcare costs for an individual with DMD are ~10x higher than the general population.37 These direct healthcare costs are only a part of the overall financial burden; indirect costs, such as loss of work and difficulty finding and funding alternative care providers, increases the financial burden on families.37
In our study, over one-half of caregivers identified unmanaged psychosocial issues experienced by the diagnosed individual. These needs included issues related to transitioning from childhood to adulthood. Caregiver responses showed gaps in management of age-appropriate discussion of issues relating to sexuality and information about transitioning to independent living, advance directives, and employment for the diagnosed individual. Many of these issues were addressed in a recent European Neuromuscular Centre (ENMC) workshop that aimed to identify gaps in knowledge about an emerging population of adults with DMD;21 considerations for management of this transitional period is also expanded in the updated DMD care considerations publication.38 Additional psychosocial issues identified by caregivers in our study included counseling for the diagnosed individual, which was identified as a need by nearly three-quarters of caregivers, overall, but only one-half reported this was managed. Internalizing (e.g. depression and anxiety) and externalizing (e.g. aggression, hyperactivity) neurobehavioral problems have been highlighted in several recent publications39–41 and have been identified for older individuals with DMD as an area of importance that needs further attention.21 The failure to meet adequately the mental health needs of these individuals might be due to a lack of recognition and referral on the part of medical professionals, but might also reflect the national need for mental health service providers.42
Caregivers in our study also responded to having unmanaged needs for their own support. Family support groups and personal counseling were identified as needs by over two-thirds of caregivers, but less than one-half adequately managed these needs. The lack of adequate supports, both financial and emotional, may contribute to greater perceived burden and psychological distress for caregivers of individuals with a dystrophinopathy.23,26,30 A survey completed by 1238 women in the United States who were caring for someone with DMD reported an age-adjusted rate of serious psychological stress of 12.4% among caregivers, significantly higher than the 7.1% in the general population of mothers;23 almost one-half of these women reported high levels of caregiving demands. More advanced disease is often associated with increased caregiver stress.26,27,43,44 Consistent with this observation, counseling for caregivers was identified as a need for a significantly higher proportion of caregivers when the diagnosed individual was non-ambulatory. Social support, resiliency, increased perceived control, and healthy family identity are factors that have been associated with less caregiver stress.23,27,45 Our study, along with others, suggests that recommendations for the management of psychosocial issues have not been effectively translated at point of care,46,47 despite evidence that adequate resources have been shown to protect against related stresses.27,45
Our study is not without limitations. The perceived needs and their management were from the perspective of the caregiver. Individuals with dystrophinopathy are increasingly voicing their desire to have their stories told from their own perspective as they approach adulthood while living with a chronic health condition and physical disability.48,49 To collect these perspectives, there is a need for both young-person-friendly and caregiver-free data collection methods to ensure an unbiased assessment. A recent article from MD STARnet utilized data from an Internet-based questionnaire that collected the perspectives of life transitions and health services among individuals aged 16 years and older.50 The response rate was low (25%), but of those who responded, gaps in services were consistent with those reported by the caregiver in this study. Another limitation is the geographical restriction of the MD STARnet sites and, as noted previously, caregivers responding to our survey were more likely to be non-Hispanic Whites and have higher education levels than all eligible MD STARnet caregivers combined. Thus, our survey findings might not represent the needs of all families identified by the MD STARnet or the wider population of families affected by a dystrophinopathy diagnosis across the United States. Further, surveys were collected over an extended period. Nonetheless, our findings are consistent with recent related publications suggesting current relevance. A final limitation is the inclusion of caregivers of DMD and BMD, which vary in clinical severity, and thus, may result in different disease management needs. However, our stratification by current ambulation status, which could be a more salient indicator of need, probably captured the association with disease severity that might emerge if the stratification was done by phenotype. Despite these limitations, our study has advantages over available clinic-based studies, including greater representativeness of all families affected by a dystrophinopathy diagnosis. Furthermore, our study included a broad range of needs across multiple domains and the lifespan allowing a more comprehensive assessment of areas needing additional resources and guidance from the clinical community.
In summary, the findings from our study are of value to providers, payers, and policymakers as they work together to meet the many needs identified by caregivers of individuals with dystrophinopathies. It is reassuring that families indicate their needs around medical care and disease-related information are generally being met. Additional resources and policies might focus on the unmet financial and psychosocial needs of this population, as recognized in the recently updated care considerations.38
Conclusion
Caregivers of patients diagnosed with a dystrophinopathy report diverse needs for the patient and the family in the management of the disease. Our study showed that needs associated with physical health and access to information were managed by most families. Less managed areas identified by our survey included funding for needs not covered by insurance and psychosocial support. We also demonstrated variability in needs and their management by ambulation status of the patient, household income, and caregiver race/ethnicity. Continued advocacy on behalf of patients with a dystrophinopathy to healthcare providers, public health practitioners, and policymakers should focus on addressing gaps in provision of needed financial assistance and psychosocial services.
Supplemental Material
Supplemental material, SupplementalFile1_Table_1 for Needs management in families affected by childhood-onset dystrophinopathies by Kristin M Conway, Katy Eichinger, Christina Trout, Paul A Romitti, Katherine D Mathews and Shree K Pandya in SAGE Open Medicine
Supplemental Material
Supplemental material, SupplementaryFile2_Needs_Frequencies for Needs management in families affected by childhood-onset dystrophinopathies by Kristin M Conway, Katy Eichinger, Christina Trout, Paul A Romitti, Katherine D Mathews and Shree K Pandya in SAGE Open Medicine
Supplemental Material
Supplemental material, SupplementaryFile3_Managed_Frequencies for Needs management in families affected by childhood-onset dystrophinopathies by Kristin M Conway, Katy Eichinger, Christina Trout, Paul A Romitti, Katherine D Mathews and Shree K Pandya in SAGE Open Medicine
Acknowledgments
We would like to acknowledge the MD STARnet Needs Assessment work group and thank the families who participated in the survey. All authors contributed to the design and conception of this study. K.M.C., K.E., and S.K.P. developed and implemented the data analysis plan and interpretation. C.T. contributed to the development of the questionnaire and data interpretation. K.D.M. contributed to case classification and data interpretation. P.A.R. contributed to study design, data collection, and data interpretation. All authors critically revised the article and gave final approval.
Footnotes
Declaration of conflicting interests: K.D.M. receives funding from National Institutes of Health (NIH) grant 2 U54 NS053672-11, the Friedreich’s Ataxia Research Alliance, the Centers for Disease Control and Prevention (DD000189), PTC Therapeutics Inc, Sarepta Therapeutics Inc, Pfizer Inc, FibroGen Inc, AMO, BMS, Santhera, Acceleron, Takeda, Reata, and Intalfarmaco. Previously, funding was received from GlaxoSmithKline, Eli Lilly and Company, Prosensa Therapeutics BV/BioMarin Pharmaceutical Inc, ViroPharma Inc, Marathon Pharmaceuticals LLC, aTyr Pharma Inc, and Horizon Pharma Ireland Ltd. K.D.M. is an advisory board member for Sarepta Therapeutics Inc and Santhera. K.D.M. is a scientific board member for the Muscular Dystrophy Association and the FSH Society. K.D.M. received no personal funding aside from travel expense reimbursement. The remaining authors (K.M.C., K.E., C.T., P.A.R., and S.K.P.) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Each site received institutional review board (IRB) approval for the caregiver survey (University of Arizona IRB-1, protocol 05-0426-01; Colorado Department of Public Health and Environment IRB, protocol 2006001; Georgia Department of Public Health IRB, protocol 090805; Centers for Disease Control and Prevention IRB-A, protocol 4792; University of Iowa IRB-1, protocol 200509724; New York State Department of Health, protocol 03-062).
Ethical publication statement: We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data collection for this publication was supported by the Cooperative Agreement numbers, DD000187, DD000189, DD000190, DD000191, and DD000392 funded by the Centers for Disease Control and Prevention. Writing of this publication was supported by Cooperative Agreement numbers, DD001119, DD001123, and DD001126.
Informed consent: The University of Arizona IRB-1, Georgia Department of Public Health IRB and Centers for Disease Control and Prevention IRB-A, University of Iowa IRB-1, and New York State Department of Health waived the requirement of signed consent and approved implied consent if the caregiver completed and returned the survey. Written consent was required by the Colorado Department of Public Health and Environment IRB for Colorado caregivers.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Kristin M Conway  https://orcid.org/0000-0001-8633-966X
https://orcid.org/0000-0001-8633-966X
Paul A Romitti  https://orcid.org/0000-0001-5393-9984
https://orcid.org/0000-0001-5393-9984
References
- 1. Emery AE. The muscular dystrophies. Lancet 2002; 359(9307): 687–695. [DOI] [PubMed] [Google Scholar]
- 2. Balaban B, Matthews DJ, Clayton GH, et al. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil 2005; 84(11): 843–850. [DOI] [PubMed] [Google Scholar]
- 3. Barber BJ, Andrews JG, Lu Z, et al. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy. J Pediatr 2013; 163(4): 1080–1084.e1. [DOI] [PubMed] [Google Scholar]
- 4. Biggar WD, Harris VA, Eliasoph L, et al. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord 2006; 16(4): 249–255. [DOI] [PubMed] [Google Scholar]
- 5. Houde S, Filiatrault M, Fournier A, et al. Deflazacort use in Duchenne muscular dystrophy: an 8-year follow-up. Pediatr Neurol 2008; 38(3): 200–206. [DOI] [PubMed] [Google Scholar]
- 6. King WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology 2007; 68(19): 1607–1613. [DOI] [PubMed] [Google Scholar]
- 7. Lebel DE, Corston JA, McAdam LC, et al. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. J Bone Joint Surg Am 2013; 95(12): 1057–1061. [DOI] [PubMed] [Google Scholar]
- 8. Manzur AY, Kuntzer T, Pike M, et al. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev 2008; 1: CD003725. [DOI] [PubMed] [Google Scholar]
- 9. Markham LW, Kinnett K, Wong BL, et al. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord 2008; 18(5): 365–370. [DOI] [PubMed] [Google Scholar]
- 10. Markham LW, Spicer RL, Khoury PR, et al. Steroid therapy and cardiac function in Duchenne muscular dystrophy. Pediatr Cardiol 2005; 26(6): 768–771. [DOI] [PubMed] [Google Scholar]
- 11. Matthews E, Brassington R, Kuntzer T, et al. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev 2016; 5: CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moxley RT, 3rd, Pandya S, Ciafaloni E, et al. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol 2010; 25(9): 1116–1129. [DOI] [PubMed] [Google Scholar]
- 13. Silversides CK, Webb GD, Harris VA, et al. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. Am J Cardiol 2003; 91(6): 769–772. [DOI] [PubMed] [Google Scholar]
- 14. Moxley RT, 3rd, Ashwal S, Pandya S, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2005; 64(1): 13–20. [DOI] [PubMed] [Google Scholar]
- 15. Henricson EK, Abresch RT, Cnaan A, et al. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve 2013; 48(1): 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gloss D, Moxley RT, 3rd, Ashwal S, et al. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016; 86(5): 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018; 17(4): 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 2018; 17(3): 251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schreiber-Katz O, Klug C, Thiele S, et al. Comparative cost of illness analysis and assessment of health care burden of Duchenne and Becker muscular dystrophies in Germany. Orphanet J Rare Dis 2014; 9: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryder S, Leadley RM, Armstrong N, et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis 2017; 12(1): 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahbek J, Steffensen BF, Bushby K, et al. 206th ENMC International Workshop: care for a novel group of patients—adults with Duchenne muscular dystrophy Naarden, The Netherlands, 23-25 May 2014. Neuromuscul Disord 2015; 25(9): 727–738. [DOI] [PubMed] [Google Scholar]
- 22. Anderson M, Elliott EJ, Zurynski YA. Australian families living with rare disease: experiences of diagnosis, health services use and needs for psychosocial support. Orphanet J Rare Dis 2013; 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kenneson A, Bobo JK. The effect of caregiving on women in families with Duchenne/Becker muscular dystrophy. Health Soc Care Community 2010; 18(5): 520–528. [DOI] [PubMed] [Google Scholar]
- 24. Ouyang L, Grosse SD, Kenneson A. Health care utilization and expenditures for children and young adults with muscular dystrophy in a privately insured population. J Child Neurol 2008; 23(8): 883–888. [DOI] [PubMed] [Google Scholar]
- 25. Nereo NE, Fee RJ, Hinton VJ. Parental stress in mothers of boys with Duchenne muscular dystrophy. J Pediatr Psychol 2003; 28(7): 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pangalila RF, van den Bos GA, Stam HJ, et al. Subjective caregiver burden of parents of adults with Duchenne muscular dystrophy. Disabil Rehabil 2012; 34(12): 988–996. [DOI] [PubMed] [Google Scholar]
- 27. Frishman N, Conway KC, Andrews J, et al. Perceived quality of life among caregivers of children with a childhood-onset dystrophinopathy: a double ABCX model of caregiver stressors and perceived resources. Health Qual Life Outcomes 2017; 15(1): 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landfeldt E, Lindgren P, Bell CF, et al. The burden of Duchenne muscular dystrophy: an international, cross-sectional study. Neurology 2014; 83(6): 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larkindale J, Yang W, Hogan PF, et al. Cost of illness for neuromuscular diseases in the United States. Muscle Nerve 2014; 49(3): 431–438. [DOI] [PubMed] [Google Scholar]
- 30. Magliano L, D’Angelo MG, Vita G, et al. Psychological and practical difficulties among parents and healthy siblings of children with Duchenne vs. Becker muscular dystrophy: an Italian comparative study. Acta Myol 2014; 33(3): 136–143. [PMC free article] [PubMed] [Google Scholar]
- 31. De Moura MC, Wutzki HC, Voos MC, et al. Is functional dependence of Duchenne muscular dystrophy patients determinant of the quality of life and burden of their caregivers. Arq Neuropsiquiatr 2015; 73(1): 52–57. [DOI] [PubMed] [Google Scholar]
- 32. Chen JY, Clark MJ. Family function in families of children with Duchenne muscular dystrophy. Fam Community Health 2007; 30(4): 296–304. [DOI] [PubMed] [Google Scholar]
- 33. Chen JY, Clark MJ. Family resources and parental health in families of children with Duchenne muscular dystrophy. J Nurs Res 2010; 18(4): 239–248. [DOI] [PubMed] [Google Scholar]
- 34. Miller LA, Romitti PA, Cunniff C, et al. The Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): surveillance methodology. Birth Defects Res A Clin Mol Teratol 2006; 76(11): 793–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathews KD, Cunniff C, Kantamneni JR, et al. Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): case definition in surveillance for childhood-onset Duchenne/Becker muscular dystrophy. J Child Neurol 2010; 25(9): 1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. The American Association for Public Opinion Research (AAPOR). Standard definitions: final disposition of case codes and outcome rates for surveys. 7th ed., https://www.aapor.org/Standards-Ethics/Standard-Definitions-(1).aspx (2011, accessed 20 November 2011).
- 37. Cavazza M, Kodra Y, Armeni P, et al. Social/economic costs and health-related quality of life in patients with Duchenne muscular dystrophy in Europe. Eur J Health Econ 2016; 17(Suppl. 1): 19–29. [DOI] [PubMed] [Google Scholar]
- 38. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol 2018; 17(5): 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Banihani R, Smile S, Yoon G, et al. Cognitive and neurobehavioral profile in boys with Duchenne muscular dystrophy. J Child Neurol 2015; 30(11): 1472–1482. [DOI] [PubMed] [Google Scholar]
- 40. Ricotti V, Mandy WP, Scoto M, et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol 2016; 58(1): 77–84. [DOI] [PubMed] [Google Scholar]
- 41. Caspers Conway K, Mathews KD, Paramsothy P, et al. Neurobehavioral concerns among males with dystrophinopathy using population-based surveillance data from the Muscular Dystrophy Surveillance, Tracking, and Research Network. J Dev Behav Pediatr 2015; 36(6): 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoge MA, Morris JA, Stuart GW, et al. A national action plan for workforce development in behavioral health. Psychiatr Serv 2009; 60(7): 883–887. [DOI] [PubMed] [Google Scholar]
- 43. Magliano L, Patalano M, Sagliocchi A, et al. Burden, professional support, and social network in families of children and young adults with muscular dystrophies. Muscle Nerve 2015; 52(1): 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Landfeldt E, Lindgren P, Bell CF, et al. Quantifying the burden of caregiving in Duchenne muscular dystrophy. J Neurol 2016; 263(5): 906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peay HL, Meiser B, Kinnett K, et al. Mothers’ psychological adaptation to Duchenne/Becker muscular dystrophy. Eur J Hum Genet 2016; 24(5): 633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol 2010; 9(2): 177–189. [DOI] [PubMed] [Google Scholar]
- 47. Bushby K, Finkel R, Birnkrant DJ, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010; 9(1): 77–93. [DOI] [PubMed] [Google Scholar]
- 48. Gibson BE, Mistry B, Smith B, et al. Becoming men: gender, disability, and transitioning to adulthood. Health 2014; 18(1): 95–114. [DOI] [PubMed] [Google Scholar]
- 49. Hamdani Y, Mistry B, Gibson BE. Transitioning to adulthood with a progressive condition: best practice assumptions and individual experiences of young men with Duchenne muscular dystrophy. Disabil Rehabil 2015; 37(13): 1144–1151. [DOI] [PubMed] [Google Scholar]
- 50. Paramsothy P, Herron AR, Lamb M, et al. Health care transition experiences of males with childhood-onset Duchenne and Becker muscular dystrophy: findings from the Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet) Health Care transitions and other life experiences survey. PLoS Curr. Epub ahead of print 21 August 2018. DOI: 10.1371/currents.md.7de8a1c6798d7a48d38ea09bd624e1cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SupplementalFile1_Table_1 for Needs management in families affected by childhood-onset dystrophinopathies by Kristin M Conway, Katy Eichinger, Christina Trout, Paul A Romitti, Katherine D Mathews and Shree K Pandya in SAGE Open Medicine
Supplemental material, SupplementaryFile2_Needs_Frequencies for Needs management in families affected by childhood-onset dystrophinopathies by Kristin M Conway, Katy Eichinger, Christina Trout, Paul A Romitti, Katherine D Mathews and Shree K Pandya in SAGE Open Medicine
Supplemental material, SupplementaryFile3_Managed_Frequencies for Needs management in families affected by childhood-onset dystrophinopathies by Kristin M Conway, Katy Eichinger, Christina Trout, Paul A Romitti, Katherine D Mathews and Shree K Pandya in SAGE Open Medicine


