Although appropriate glycemic control without hypoglycemia is required during the treatment of diabetes, the presence of advanced diabetic kidney disease (DKD) accompanied by renal insufficiency (RI) interrupts the usual intervention.1 The impairment of renal gluconeogenesis and insulin clearance in DKD may render patients susceptible to hypoglycemia. At the same time, most oral antidiabetic drugs (OADs) (including sulfonylureas) are contraindicated or restricted, since advanced DKD brings about profound pharmacokinetic abnormalities.2 The lack of accurate glycemic indices such as HbA1c and glycated albumin (GA) is also problematic.3 Both indices only represent blood glucose (BG) level averages during a certain period of time, and real-time BG fluctuations (including the swings between marked hyperglycemia and severe hypoglycemia) cannot be estimated. Especially, patients undergoing hemodialysis (HD) face glycemic disarrays induced by the HD session (see Figure 1, upper panel).4 HD sessions are usually started after meals, during the postprandial hyperglycemic state. Since the dialysate contains glucose (100-150 mg/dL in concentration in Japan), the HD session is supposed to reduce BG levels in a rapid pace. In contrast, plasma insulin is removed during the HD session, and this HD-induced insulin clearance comes from its adsorption to the dialyzer membrane. Simultaneously, the HD lowered BG levels may stimulate secretion of counterregulatory hormones such as glucagon, that may elevate the BG levels. According to these conditions, both HD-induced hypoglycemia and HD-associated hyperglycemia can occur during and after HD sessions.4 Since HD-induced BG fluctuations depend completely on the intensities of several factors, it is almost impossible to predict BG changes in patients undergoing HD. Against this background, the emergence of continuous glucose monitoring (CGM) systems has revealed detailed BG fluctuations, which cannot be evaluated by conventional self-monitoring of BG. Our case was a 68-year-old Japanese man undergoing HD for 18 years with 41 years of diabetes duration. He was treated with a premixed insulin analog (biphasic insulin aspart 30) twice a day (16 units before breakfast and 16 units before dinner). His HbA1c and GA levels were 6.0% and 19.5%, respectively. The CGM performed (see Figure 1, lower panel) showed relatively flat 24-hour BG levels on day 1 (non-HD day). In contrast, an acute BG level decline occurred during the HD session and a subsequent severe BG level elevation was observed after the HD on day 2 (HD day) (see Figure 1, lower panel). These phenomena might be comparable to the HD-induced hypoglycemia and the HD-associated hyperglycemia. In addition to individual indications, CGM is also a powerful tool to visualize BG fluctuations caused by various medications. The dipeptidyl peptidase-4 (DPP-4) inhibitors (OADs) are expected to be useful in those patients with type 2 diabetes because of its glucose-dependent action5; however, little is known about their effects on BG fluctuations in patients undergoing HD. We have shown by CGM that teneligliptin monotherapy, one DPP-4 inhibitor, significantly improves the glycemic control.6 Although the HD session was of concern due to the possible BG fluctuations, the monitor did not record severe hypoglycemic events. These findings suggest that teneligliptin monotherapy can automatically control BG levels to some degree, even under the difficult conditions of unpredictable BG fluctuations on HD session days.6 The popularization of CGM will lead to high-quality BG control and subsequent better outcomes in patients with advanced DKD.
Figure 1.
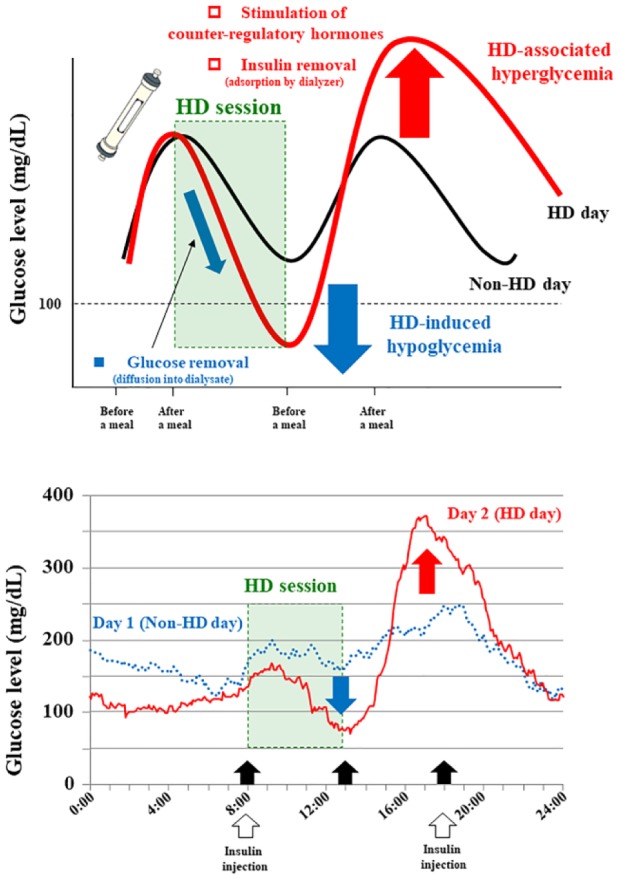
Hemodialysis (HD)-induced hypoglycemia and HD-associated hyperglycemia in patients with diabetes.
Footnotes
Abbreviations: BG, blood glucose; CGM, continuous glucose monitoring; DKD, diabetic kidney disease; DPP-4, dipeptidyl peptidase-4; GA, glycated albumin; OAD, oral antidiabetic drug; RI, renal insufficiency.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KM received lecture fees from Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo, Ono Pharmaceutical, Eli Lilly Japan K.K., MSD K.K., Nippon Boehringer Ingelheim. ME received lecture fees from Sanofi, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo, Takeda Pharmaceutical Company, Eli Lilly Japan K.K., Sumitomo Dainippon Pharma, Ono Pharmaceutical. MA chairs courses endowed by Chugai Pharmaceutical, Nikkiso, NIPRO Corporation, Ono Pharmaceutical, Otsuka Pharmaceutical, Terumo Corporation, and Toray Medical. MI received lecture fees from Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo, Astellas Pharma, Asahi Kasei Pharm Corporation, Kyowa Hakko Kirin, Chugai Pharmaceutical, Teijin Pharma, Takeda Pharmaceutical, Eli Lilly Japan K.K., MSD K.K., Bayer in Japan, Taisho Toyama Pharmaceutical Company, Pfizer, Ono Pharmaceutical. KM, ME, and MI received unrestricted research grants from MSD K.K., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo, Astellas Pharma, Asahi Kasei Pharm Corporation, Kyowa Hakko Kirin, Chugai Pharmaceutical, Teijin Pharma, Takeda Pharmaceutical Company, Ono Pharmaceutical, Eli Lilly Japan K.K., Nippon Boehringer Ingelheim, Sumitomo Dainippon Pharma, Bayer in Japan, Eisai, Kowa Pharmaceutical Company, Taisho Toyama Pharmaceutical Company, Kissei Pharmaceutical Company, Torii Pharmaceutical Company, Novartis Pharma K.K., Yoshindo.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end-stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12:57-69. [DOI] [PubMed] [Google Scholar]
- 3. Inaba M, Okuno S, Kumeda Y, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: effect of anemia and erythropoietin injection. J Am Soc Nephrol. 2007;18:896-903. [DOI] [PubMed] [Google Scholar]
- 4. Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11:302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mori K, Emoto M, Shoji T, Inaba M. Linagliptin monotherapy compared with voglibose monotherapy in patients with type 2 diabetes undergoing hemodialysis: a 12-week randomized trial. BMJ Open Diabetes Res Care. 2016;4:e000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wada N, Mori K, Nakagawa C, et al. Improved glycemic control with teneligliptin in patients with type 2 diabetes mellitus on hemodialysis: Evaluation by continuous glucose monitoring. J Diabetes Complications. 2015;29:1310-1313. [DOI] [PubMed] [Google Scholar]


