Abstract
Background:
The perceived value and consistent use of continuous glucose monitoring (CGM) systems depends in part on their accuracy. We assessed the performance of a sixth-generation CGM system (Dexcom G6) in children and adolescents.
Methods:
Forty-nine participants (6-17 years of age, mean ± SD of 13.5 ± 3.3 years), all with type 1 diabetes, enrolled and data were available from 37. Each participant wore 1 sensor for up to 10 days and was asked to undergo an in-clinic visit lasting 6-12 hours for frequent blood glucose (BG) sample testing on one of the sensor wear days. Estimated glucose values (EGVs) from the G6 system were compared with venous BG values measured with a laboratory reference instrument (YSI).
Results:
The overall mean absolute relative difference (MARD) for 1387 EGV-YSI pairs was 7.7%, and the overall percentage of EGVs within 20% or 20 mg/dL of the YSI reference value (for YSI > or ⩽100 mg/dL, respectively, the “%20/20”) was 96.2%. The %20/20 was 92.1% on Day 1 and 91.0% on Day 10 of sensor wear. For EGVs <70 mg/dL, 92.6% of the YSI values were within 20 mg/dL and for EGVs >250 mg/dL, 100% of the YSI values were within 20%. Differences between EGVs and YSI values in over 99.9% of the pairs posed no or only slight clinical risk as evaluated by surveillance error grid analysis.
Conclusions:
The accuracy of the G6 CGM system in pediatrics may encourage consistent use of the system and contribute to improved glycemic outcomes in this population.
Keywords: glucose sensor performance, clinical accuracy, MARD, continuous glucose monitoring, advanced algorithm, factory-calibrated
Use of early real-time continuous glucose monitoring (rtCGM) systems was correlated with significantly reduced hemoglobin A1c (A1C), reduced time in hypoglycemia (<70 mg/dL), reduced time in hyperglycemia (>180 mg/dL), and increased time in range (70-180 mg/dL) for adults >25 years with type 1 diabetes (T1D).1 However, glycemic control was not significantly improved for CGM users ages 4-25 years; this has been attributed to poor consistency of sensor wear in this age group.1,2 The lack of sustained use and poor acceptance of early-generation CGM technologies correlated with perceived and actual sensor inaccuracies, frustration with nuisance alarms, body issues (size and appearance of sensor/transmitter), painful insertion, and variable and often inadequate insurance coverage.3-5 Optimal CGM performance is especially important in pediatric populations because they experience greater glycemic variability and wider glycemic excursions than adults with diabetes. 6
CGM adoption rates in pediatric patients with diabetes have increased dramatically over the last decade,7 likely due to improved system performance, and the introduction of smartphone companion apps for real-time sharing and remote monitoring. However, CGM users are still in the minority. Barriers to increased adoption may include the requirement for periodic fingerstick calibrations and short sensor wear times. Here we report on the accuracy of a sixth-generation, factory-calibrated rtCGM system (“G6”) that addresses some of the potential challenges to improved adoption in children and adolescents.
Methods
Methods used to assess the performance of the G6 system (Dexcom, Inc, San Diego, CA) have been published previously.8 Briefly, G6 was evaluated using retrospective data from a prospectively designed, multicenter study involving both adults (ages 18 and older) and pediatric participants (ages 6 to 17) with T1D or insulin-requiring T2D at 4 U.S. sites. Data presented here are from pediatric participants.
Study Procedures and Data Collection
All sensor insertions were performed at the clinic by participants and/or guardians using the automated sensor applicator. All participants used the G6 system for one 10-day wear period (up to 240 hours).
Pediatric participants returned to the clinic for one session on days 1, 4-5, 7, or 10 of system use. Participants ages 6-12 years returned for one 6-hour clinic session and participants ages 13-17 years returned for one 12-hour clinic session for comparison of CGM readings with arterialized venous glucose concentrations using a laboratory reference method (YSI). Participants had venous sampling once every 15 ± 5 minutes for the duration of each clinic session for reference glucose measurement. CGM data were masked during the clinic session. The study was reviewed by the FDA through the Investigational Device Exemption process and registered at ClinicalTrials.gov (NCT02880267).
Methods of Data Analysis
Analysis was performed on estimated glucose values (EGVs) derived from reprocessed raw sensor data, without incorporating information from fingerstick calibrations. YSI values were paired with temporally matched EGVs; matched pairs within the CGM reportable range of 40-400 mg/dL were evaluated. No matched pair data were excluded from analysis.
Accuracy metrics included the proportion of the EGVs within ± 20% of paired YSI values >100 mg/dL or ± 20 mg/dL of YSI values ⩽100 mg/dL (hereafter referred to as %20/20), as well the analogous %15/15 and %30/30. The overall mean absolute relative difference (MARD) was determined as the average absolute relative difference between paired EGVs and YSI values. Surveillance error grid analysis9 was used to quantify the clinical risks resulting from CGM inaccuracies.
Accuracy across glucose concentrations was evaluated for the following ranges: hypoglycemia (<70 mg/dL), euglycemia (70-180 mg/dL), Level 1 hyperglycemia (181-250 mg/dL), and Level 2 hyperglycemia (>250 mg/dL).10
Trend accuracy assessed the concurrence between rates of glucose change measured by CGM and YSI. Rate of change (RoC) was calculated by interpolating per-minute glucose concentrations for YSI and CGM data separately. Six YSI RoC categories ranging from rapidly decreasing (<–2 mg/(dL∙min)) to rapidly increasing (>2 mg/(dL∙min)) were established. The frequency of CGM-measured RoCs that agreed with YSI-measured RoCs was assessed. Point and percentage accuracy was also determined at different EGV RoCs. All analyses were performed using SAS® software, version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Study Population
Twenty-one participants ages 6-12 years and 28 participants ages 13-17 years enrolled. The mean (SD) age was 13.5 (3.3) years; 61% were female; mean (SD) diabetes duration was 6.0 (4.0) years; 100% had T1D; 84% were using insulin pumps; and mean (SD) A1C was 8.1 (1.1)%.
Twelve pediatric participants were excluded from the accuracy assessment: 6 withdrew from the study voluntarily after sensor insertion, and 6 had a sensor or adhesive failure before the clinic session. Thus, 37 pediatric participants contributed EGV-YSI matched pairs for the accuracy assessment.
Accuracy Overall, Across Days of Wear, and Across Glucose Ranges
There were 1387 EGVs that had a temporally matched YSI reading and were used for analysis. The %15/15 accuracy between EGV and YSI was 91.1%, the %20/20 was 96.2%, and the MARD was 7.7%. The %30/30 was 99.6%, indicating very few outliers (Table 1).
Table 1.
Point and Percentage Accuracy in Pediatric Participants Overall and by Clinic Session Day.
| Clinic session day | Matched pairs (n) | %15/15 (%) | %20/20 (%) | %30/30 (%) | %40/40 (%) | MAD (mg/dL) | MARD (%) |
|---|---|---|---|---|---|---|---|
| Overall | 1387 | 91.1 | 96.2 | 99.6 | 99.9 | 11.1 | 7.7 |
| Day 1 | 253 | 78.3 | 92.1 | 98.8 | 100.0 | 15.8 | 10.5 |
| Day 2 | 260 | 94.2 | 100.0 | 100.0 | 100.0 | 13.4 | 7.8 |
| Days 4-5 | 322 | 95.7 | 98.8 | 99.7 | 99.7 | 8.8 | 7.2 |
| Day 7 | 253 | 97.2 | 99.2 | 100.0 | 100.0 | 7.9 | 6.2 |
| Day 10 | 299 | 89.3 | 91.0 | 99.3 | 100.0 | 10.5 | 7.1 |
Accuracy was consistent throughout the 10-day wear period. The %15/15 and %20/20 on the first day of wear were 78.3% and 92.1%, respectively. The %15/15 and %20/20 on Day 10 of sensor wear were 89.3% and 91.0%, respectively (Table 1). MARD values on Day 1 and Day 10 were 10.5% and 7.1%, respectively (Table 1).
There was consistently high accuracy across glucose concentrations. The %20/20 accuracy of the G6 System was 92.6% in the hypoglycemic range, 96.6% in the euglycemic range, 95.1% in the Level 1 hyperglycemic range, and 100% in the Level 2 hyperglycemia range (Table 2). The corresponding %15/15 and %30/30 accuracies across glucose ranges, as well as MAD and MARD, are reported in Table 2.
Table 2.
Point and Percentage Accuracy by Glucose Concentration Range.
| CGM glucose range (mg/dL) | Matched pairs (N) | %15/15 | %20/20 | %30/30 | MAD (mg/dL) | MARD (%) |
|---|---|---|---|---|---|---|
| <70 | 81 | 81.5 | 92.6 | 96.3 | 9.1 | 13.3 |
| 70-180 | 1017 | 91.9 | 96.6 | 99.7 | 9.7 | 7.4 |
| 181-250 | 245 | 89.8 | 95.1 | 100.0 | 17.4 | 7.7 |
| >250 | 44 | 97.7 | 100.0 | 100.0 | 12.7 | 4.5 |
Trend Accuracy and Accuracy Across Rates of Change
When G6 EGVs were falling rapidly (<–2 mg/(dL∙min)), corresponding YSI values were falling 100% of the time; when EGVs were rising rapidly (>2 mg/(dL∙min)), corresponding YSI values were rising 98.6% of the time (Table 3). When EGVs were steady (⩾-1 mg/(dL∙min) to ⩽ 1 mg/(dL∙min)), YSI glucose was changing rapidly less than 0.5% of the time.
Table 3.
Trend Accuracy.
| YSI rate ranges (mg/dL/min) | |||||||
|---|---|---|---|---|---|---|---|
| CGM rate ranges (mg/(dL∙min)) N Row % |
<–2 | [–2,–1) | [–1,–0) | [0,1] | (1,2] | >2 | Number of paired CGM-YSI |
| <–2 | 11 (50.0%) | 6 (27.3%) | 5 (22.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 22 |
| [–2,–1) | 4 (2.9%) | 83 (60.6%) | 46 (33.6%) | 4 (2.9%) | 0 (0.0%) | 0 (0.0%) | 137 |
| [–1,0) | 2 (0.4%) | 37 (6.6%) | 432 (77.0%) | 88 (15.7%) | 2 (0.4%) | 0 (0.0%) | 561 |
| [0,1] | 0 (0.0%) | 1 (0.2%) | 112 (24.9%) | 292 (64.9%) | 42 (9.3%) | 3 (0.7%) | 450 |
| (1,2] | 0 (0.0%) | 0 (0.0%) | 4 (3.7%) | 46 (42.6%) | 48 (44.4%) | 10 (9.3%) | 108 |
| >2 | 0 (0.0%) | 0 (0.0%) | 1 (1.4%) | 5 (7.2%) | 17 (24.6%) | 46 (66.7%) | 69 |
| Total | 17 | 127 | 600 | 435 | 109 | 59 | 1347 |
Percentage and point accuracy was demonstrated during rapid rates of change (Table 4), with %20/20 accuracy no less than 81.8% and MARD of no more than 11.9% for any RoC category analyzed. Remarkably, when EGVs were rising rapidly (>2 mg/(dl∙min)), the %20/20 was 94.2% and the MARD was 7.7% (Table 4).
Table 4.
Point and Percentage Accuracy by CGM Rate of Change.
| CGM rate ranges (mg/(dL∙min)) | Matched pairs (n) | %20/20 (%) | MARD (%) |
|---|---|---|---|
| <–2 | 22 | 81.8 | 11.9 |
| [–2,–1) | 137 | 97.1 | 7.8 |
| [–1,0) | 561 | 96.3 | 7.8 |
| [0,1] | 450 | 96.9 | 7.3 |
| (1,2] | 108 | 96.3 | 7.8 |
| >2 | 69 | 94.2 | 7.7 |
Surveillance Error Grid Analysis
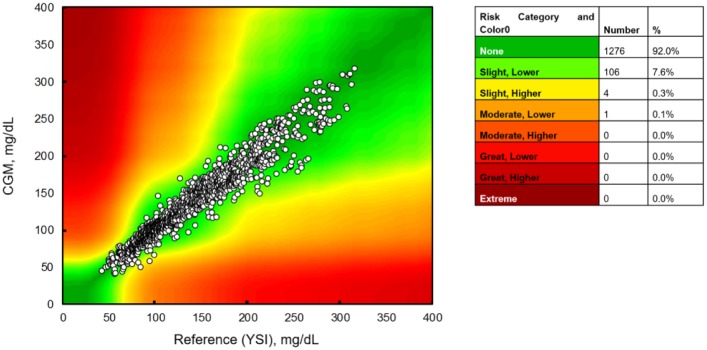
The discrepancies between the vast majority of EGV-YSI pairs posed no or only slight clinical risk when evaluated by surveillance error grid analysis (Figure 1). These plots demonstrate that 1276 (92.0%) pairs fell in the “No Risk” zone, 106 (7.6%) were in the “Slight, Lower” risk zone, 4 (0.3%) were in the “Slight, Higher” risk zone and only 1 (<0.1%) was in the “Moderate, Lower” risk zone (Figure 1).
Figure 1.
Surveillance error grid analysis of 1347 paired points.
Conclusion/Discussion
In the landmark 2008 JDRF CGM study,1 only 50% of 8- to 14-year-olds and only 30% of 15- to 24-year-olds wore CGM devices consistently for ⩾6 days a week. In contrast, 86% of adults ages 18+ years in the same study wore CGM ⩾6 days a week. A separate study of CGM adherence in children <4 years published in 201211 showed that only 45% of participants used CGM ⩾6 days a week. Poor durability among pediatric participants was associated with discomfort, too-frequent alarms, overwhelming amounts of information, and “glitches” found with early-generation CGM systems.3,5,11 However, the transition from an early- to a newer-generation CGM system (Dexcom SEVEN PLUS to Dexcom G4 Platinum) significantly improved adherence in pediatric patients.12
The new G6 system offers several new features, including elimination of calibration requirements, 10-day sensor wear time, resistance to acetaminophen interference,13 and simplified sensor insertion.14 G6 accuracy in pediatric participants is consistently high across wear sites,8 days of wear, glucose ranges, and rates of glucose change. Performance of G6 in pediatric participants eclipses that of G4 Platinum and G5 Mobile, especially with regard to overall accuracy.12 Together these attributes should provide pediatric users and their guardians with high device confidence and facilitate persistent use.
Outcome studies utilizing current-generation CGM technologies, including G6, in pediatric patients are pending. The Strategies to Enhance New CGM Use in Early Childhood (SENCE) trial (NCT02912728) will evaluate the impact of CGM alone and CGM combined with family behavioral training on CGM-derived glycemic metrics, A1C, and quality-of-life measures in young children (<8 years) with T1D. The CGM Intervention in Teens and Young Adults with type 1 Diabetes (CITY) trial (NCT03263494) seeks to determine the effect of CGM use on A1C, CGM-derived glycemic metrics, and quality-of-life measures in adolescents and young adults (14-<25 years) with suboptimally controlled diabetes (A1C 7.5-<11%). These studies will help to determine the extent to which improvements made in technology facilitate adherence and improve glycemic control in challenging, pediatric patient populations. Hopefully, these studies will also influence payer coverage policies to facilitate CGM use for all eligible patients.
Acknowledgments
The authors thank the subjects who participated in the study and the research staff at the investigational centers.
Footnotes
Abbreviations: ARD, absolute relative difference; CGM, continuous glucose monitoring; EGV, estimated glucose value; MARD, mean absolute relative difference; T1D, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors are employees of Dexcom, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from Dexcom, Inc.
ORCID iD: Sarah A. Puhr  https://orcid.org/0000-0003-1564-7563
https://orcid.org/0000-0003-1564-7563
References
- 1. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. [DOI] [PubMed] [Google Scholar]
- 2. Mauras N, Beck R, Xing D, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2012;35(2):204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tansey M, Laffel L, Cheng J, et al. Satisfaction with continuous glucose monitoring in adults and youths with type 1 diabetes. Diabet Med. 2011;28(9):1118-1122. [DOI] [PubMed] [Google Scholar]
- 4. Polonsky WH, Hessler D. Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol. 2015;9(2):339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5(4):860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diabetes Control and Complications Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177-188. [DOI] [PubMed] [Google Scholar]
- 7. Foster NC, Miller K, Dimeglio L, et al. Marked increases in CGM use has not prevented increases in HbA1c levels in participants in the T1D Exchange (T1DX) Clinic Network. Diabetes. 2018;67(S1):A451. [Google Scholar]
- 8. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated, real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klonoff DC, Lias C, Vigersky R, et al. The surveillance error grid. J Diabetes Sci Technol. 2014;8(4):658-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, the Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017;40(12):1622-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsalikian E, Fox L, Weinzimer S, et al. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes. 2012;13(4):301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther. 2016;18(suppl 2):S223-S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calhoun P, Johnson TK, Hughes J, Price D, Balo AK. Resistance to acetaminophen interference in a novel continuous glucose monitoring system. J Diabetes Sci Technol. 2018;12(2):393-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clukey R, North B, Parkin C. Utilization of human factors analysis throughout product development enhances usability of CGM sensor applicators. Diabetes. 2017;66(S1):A184. [Google Scholar]