Abstract
Background:
Ease of injection is important to patients. An autoinjector was developed to deliver exenatide, a glucagon-like peptide-1 receptor agonist for type 2 diabetes mellitus. For autoinjection, 0.06-mm exenatide-containing microspheres are suspended in medium-chain triglycerides. Herein, we report design verification and usability testing of the autoinjector for exenatide once-weekly suspension (QWS) delivery.
Methods:
Exenatide QWS in a single-chamber cartridge is self-injected subcutaneously with three main steps: mix, unlock, and inject. Design verification testing used validated testing methodology. A summative validation study with simulated-use scenarios evaluated unassisted performance on critical tasks (ease of use and the injection process).
Results:
The autoinjector met specified design requirements for dose accuracy and torque/force. Of 104 participants enrolled (73 lay users, 16 health care professionals, and 15 pharmacists), 90 independently referred to instructions for use during testing. Users successfully achieved critical tasks on first attempt 87-100% of the time. Approximately 78% of participants successfully completed the full injection scenario, including 72% of lay users reporting visual or dexterity impairments. Initial use errors on critical tasks included not mixing well (n = 12), not removing needle cap (n = 8), and not holding needle to the skin for complete injection (n = 5). Untrained injection-naïve and trained injection-experienced lay users made the fewest errors (7% and 3%, respectively). Trained and untrained participants took 2:33 and 5:03 minutes, respectively, to complete a weekly injection.
Conclusions:
Users with a range of injection experience can rapidly learn to administer exenatide QWS autoinjector correctly, thus minimizing patient effort to manage their diabetes with injectable therapy.
Keywords: exenatide, exenatide once-weekly suspension autoinjector, type 2 diabetes mellitus, usability, validation study
The need for consistent, frequent, substantial, and sometimes complex treatment regimens for type 2 diabetes mellitus (T2DM) poses a challenge for successful long-term patient management. Outcomes are worsened by lack of adherence and failure to intensify treatment.1 Among patients who receive injected glucose-lowering medications, both incorrect injection technique and incorrect or irregular dosing schedules are associated with higher glycated hemoglobin (HbA1c).2 While most diabetes education addresses medication dosing, many patients do not receive training on injection technique.3 Furthermore, fear of injection is associated with increased HbA1c and disease progression.4 Although true needle phobias are rare, injection anxiety is common and may be related to a fear of pain or bleeding, lack of confidence about proper technique, or concern that injection is needed because the disease has progressed.3-5 Thus, it is important that injections to treat T2DM be designed to minimize patient effort and thereby maximize the therapeutic benefits of receiving each injection.
Exenatide, a glucagon-like peptide-1 receptor agonist, has been shown to improve glycemic control and reduce body weight in patients with T2DM.6,7 The most commonly used form of exenatide, exenatide once weekly (QW), is dispersed into biodegradable poly(d,l-lactide-coglycolide) microspheres and has been administered using a single-dose tray or a single-dose dual-chamber pen.8,9 However, both devices require drug reconstitution and priming of the pen.10
To better support patient management of T2DM with exenatide, exenatide QW has been reformulated as a QW suspension (QWS) of 0.06-mm biodegradable microspheres containing exenatide 2.0 mg suspended in medium-chain triglycerides (Miglyol® 812).7 This exenatide QWS formulation can be delivered using an autoinjector device (exenatide QWS-AI). Compared with the exenatide QW single-dose tray or dual-chamber pen, exenatide QWS-AI requires fewer discrete steps (Table 1), eliminates the need for assembly, reconstitution, or dose selection, and contains a hidden needle. As such, exenatide QWS-AI may be an attractive alternative for patients seeking a simpler injection process with a hidden needle. This article describes the design verification and usability testing of the autoinjector device for delivery of exenatide QWS for the treatment of T2DM.
Table 1.
Number of Discrete Use Steps and Comparison of Major Steps Required for Administration of Exenatide Once Weekly as a Single-Dose Injection Tray, Dual-Chamber Pen, or Suspension for Autoinjection.
| Single-dose injection tray | Dual-chamber pen | Suspension for autoinjection | |
|---|---|---|---|
| Number of discrete use stepsa | 35 | 16 | 13 |
| Major stepsa | • Prepare vial • Connect vial to syringe • Mix • Fill syringe • Remove vial • Attach needle • Prime syringe/needle • Inject |
• Assemble device (attach needle to pen) • Mix • Inject |
• Mix • Unlock • Inject |
Summarized and described based on the instructions for use for each device.
Development Goals and Iterative Design Process for Exenatide Once-Weekly Suspension Autoinjector
Relative to the dual-chamber pen, the autoinjector was designed to simplify exenatide administration by presuspending exenatide microspheres housed in a single-chamber cartridge rather than storing microspheres and solution separately, as required for aqueous solutions. Additional goals included designing a device with comfortable-to-use attributes (a size and shape for easy transporting and handling with acceptable torque/force requirements); simple and intuitive administration; visualization of cartridge contents to provide feedback on mixing; facilitation of easy mixing and priming (purging air from the cartridge); safe and sterile needle containment; indication of complete dose delivery and that the device has been used; and reliable and accurate delivery of a fixed, single weekly dose (Table 2).
Table 2.
Key Design Goals of Exenatide Once-Weekly Suspension Autoinjector.
| Design goal | Strategies for and evidence of achievement |
|---|---|
| Simpler administration relative to the dual-chamber pen | • Device requires fewer overall steps to administer • Device does not require reconstitution or assembly |
| Comfortable to use (size and shape for convenient transporting and handling, acceptable torque/force requirements) | • Device has a square body with rounded edges that is ergonomic • Device is quiet (does not click at end of injection stroke) • Unlocking device and removing needle cap require only 0.0230-0.0250 Nm of torque • 23-gauge needle is the same size as the dual-chamber pen needle |
| Easy to use | • 78% of tested participants successfully completed an injection within the summative study • Most participants rated all tasks related to injection as “easy” or “very easy” |
| Cartridge contents visible to user | • Device includes a large viewing window |
| Fixed, single dose | • Device contains a single dose • Device locks after single use |
| Facilitation of mixing, purging of air from the cartridge, and complete dose delivery | • Large viewing window enables users to ensure suspension is properly mixed • Priming occurs automatically when needle cap is removed • Auditory signal indicates successful device unlocking and start of injection • Orange rod in viewing window indicates that the injection is complete |
| Safe and sterile containment of needle | • Needle is hidden by a needle shield and kept sterile until activation at time of injection • Needle shield automatically retracts during injection • Needle shield locks after use and cannot be overridden |
Three initial variations in the autoinjector design were evaluated before the final design was selected: front actuated with manual needle insertion, front actuated with automatic needle insertion, and button actuated with automatic needle insertion. Participants (N = 51) preferred the quietest device (front actuated with manual subcutaneous needle insertion) and reacted negatively to loud noises associated with designs having automatic subcutaneous needle insertion. A second set of testing determined that the device should have a square body with rounded edges and a cylindrical skin contact surface, be simple to unlock and uncap, and have a large viewing window, an orange cap, and an orange indicator for complete injection.
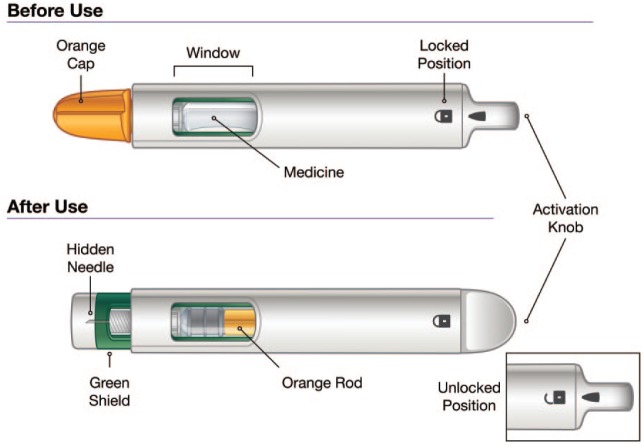
The resulting device (Figure 1) was believed to be comfortable, intuitive to use, and to have achieved its design goals (Table 2). After allowing the medication to come to room temperature, selecting an injection site (thigh, stomach, or back of upper arms), and hand washing, administration of exenatide using the autoinjector requires only three broad steps: mix (shake the autoinjector for 15 seconds), unlock (unlock device and then unscrew the cap), and inject (press against the skin and hold for 15 seconds).
Figure 1.
Exenatide once-weekly suspension autoinjector device before and after use.
Design Verification and Usability Testing
After selecting the design, rigorous testing was performed regarding design verification of the device and human interaction with the device design, carton, labels, and instructions for use (IFU). Five formative studies (N = 155) evaluated participants’ ability to use the autoinjector, IFU on the mixing step and injection hold time, package configuration, and overall effectiveness of the IFU. Two pilot summative studies (N = 61) further evaluated the autoinjector design and IFU. The IFU were developed in booklet format and targeted a grade 6-7 reading level, and the IFU, carton, and labels were iteratively revised throughout testing.
Subsequently, a final, summative validation study was conducted to evaluate the optimized safety and ease of use regarding the autoinjector design, IFU, and packaging. Herein, we report the results of design verification testing and the summative validation study.
Materials and Methods
Design Verification Testing
The key attributes of the autoinjector tested included dose accuracy, the torque required to unlock and uncap the autoinjector, and the force required to depress the needle shield and start the injection. The attributes were tested in accordance with international standards, including applicable sections of ISO 11608. Torque and force specifications were derived from anthropometric data of the intended user population. Each parameter was tested in a laboratory setting under standard atmospheric conditions, warm and cool temperatures, and after being subjected to cold storage, dry heat storage, and a free fall. For each condition, 60 devices were tested.
Usability Validation Study
The protocol for the final summative validation study was based on results from five formative and two pilot summative studies. The protocol and study materials were approved by Chesapeake Institutional Review Board. All participants provided written informed consent before beginning the study.
The validation study enrolled adult lay users (patients with T2DM or their caregivers [representative of the intended user population]), health care professionals (HCPs), and retail pharmacists with a mix of sexes, races/ethnicities, and (for lay users) dexterity and education levels. Lay users were aged 18-75 years (enrollment was intentionally skewed toward participants aged 55-75 years, who are more likely to have visual or dexterity limitations), were able to understand spoken/written English, and were either “injection naïve” (no experience injecting any type of medication) or “injection experienced” (had previously self-injected or injected another person with any medication). HCPs and pharmacists were enrolled if they currently prescribed or dispensed diabetes medications and had experience teaching patients how to inject diabetes medications.
Approximately one-half of lay users (no HCPs or pharmacists) were trained by a certified diabetes educator for up to 30 minutes 1-3 days before using exenatide QWS-AI. Individuals with and without training had access to information associated with the prescription (ie, carton, autoinjector, and IFU). To allow for learning decay, ⩾1 day was scheduled between the training and testing sessions.
Outcome Measures
This study evaluated performance on critical tasks, defined as segments of the overall injection process of which errors could result in negative clinical impact. The study also assessed difficulties in using the device, carton, or IFU and identified potential use errors. Examples of critical tasks included shaking the medication and confirming it was well mixed before injecting, removing the needle cap, injecting the dose, and holding the needle against the skin for the complete injection (see Table 3 for a complete list). Essential tasks, defined as tasks necessary to achieve the medical benefit of the autoinjector but not having the potential to cause direct harm, were also assessed. Examples of essential tasks included storing the autoinjector horizontally, unlocking the device, and allowing it to come to room temperature before use.
Table 3.
Performance on Critical Tasks (N = 104).
| Task | Success,a n | Success with difficulty,a n | Use error,a n | Success with or without difficulty,a % |
|---|---|---|---|---|
| Differentiation scenarios | ||||
| Visually distinguish and select correct device from among other injection devices (loose packaging)b | 79 | 3c | 7c | 92 |
| Visually distinguish and select correct device from among other injection devices | 100 | 1 | 3c | 97 |
| Injection scenarios | ||||
| Select the appropriate injection site | 103 | 1 | 0 | 100 |
| Mix the medication by shaking | 102 | 1 | 1 | 99 |
| Check consistency of mixture to determine if well mixed; shake more if not mixed | 103 | 0 | 1 | 99 |
| Remove needle cap | 65 | 31 | 8 | 92 |
| Complete injection of well-mixed productd | 89 | 0 | 13e | 87 |
| Inject the dose | 96 | 3 | 5f | 95 |
| Hold to skin for complete injection | 99 | 0 | 5 | 95 |
| Post-scenario questions about storage of autoinjector | ||||
| Properly store autoinjector | 104g | 0 | 0 | 100 |
| Use of device label or carton to answer comprehension questions | ||||
| Finding/understanding expiration date | 100g | 4c | 0 | 100 |
| Frequency of injection | 104g | 0 | 0 | 100 |
| Use of the instructions for use to answer comprehension questions | ||||
| Do not use expired autoinjectors | 96g | 8 | 0 | 100 |
| Inject on the skin | 104g | 0 | 0 | 100 |
| Confirm well-mixed medication through inspection of autoinjector window | 103g,h | 0 | 0 | 100 |
Participants were asked to perform only one injection attempt. Ratings are for this single, initial attempt.
Retail pharmacists did not complete this scenario because pharmacies stock full packaging carton; therefore, the denominator is 89 for this task.
Errors were due to study artifact; artifacts were defined as errors that were not attributed to the design interface but attributed to the study itself (ie, unable to simulate real-world conditions completely).
Two mixes could not be observed by the moderator because of premature dispensing of medication; therefore, the denominator is 102 for this task.
One use error was due to study artifact.
Three use errors were due to study artifact; two participants recapped but continued with the needle shield locked.
Correct response.
One participant was not asked this question.
All participants independently completed simulated-use scenarios moderated by qualified professionals, after which participants provided a subjective rating (5-point scale ranging from “very difficult” to “very easy”) regarding each task’s ease of use. Each participant was asked to administer a single injection but had the opportunity to self-correct and initiate additional injection attempts. To evaluate the safety and effectiveness of the autoinjector, moderators rated participant performance in the scenarios as a “success,” “success with difficulty” (ie, succeeding after self-correction or with confusion/frustration), or “use error.” A use error was recorded when a participant did not perform the task, performed the task incorrectly, or required moderator assistance. Participants were asked scenario-based questions to test their understanding of the autoinjector, injection process, and/or labeling, with responses recorded as “correct,” “acceptable,” or “incorrect.” After all scenarios and comprehension questions were complete, the moderator discussed any use errors or difficulties observed during testing with the participant.
Analysis
The analysis included a summary of the number of participants who successfully injected a dose and the overall number of difficulties and use errors. Successful injection was based on the number of participants who held the autoinjector to the skin for a complete injection, injected the dose, and completed the injection of a well-mixed product. Overall performance was examined by group to identify potential between-group differences.
Timing Study
Lay users were independently timed from investigator initiation of the simulated-use scenario to the completion of the injection with a stopwatch by two observers. Results were averaged across participants and observers. Timings included any preparation time, reading of the IFU, and delivery of dose.
Results
Design Verification Testing
The autoinjector successfully met the acceptance criteria for dose accuracy, torque to unlock and uncap, and force to depress the needle shield and start injection under all conditions tested. Test results under standard atmospheric conditions are reported in Table 4.
Table 4.
Design Verification Testing Under Standard Atmospheric Conditionsa (N = 60).
| System characteristic | Specification range | Minimum | Maximum | Mean ± SD | Result |
|---|---|---|---|---|---|
| Dose accuracy, mL | 0.8075-0.8925 | 0.8319 | 0.8482 | 0.8402 ± 0.0039 | Pass |
| Torque to unlock, Nm | 0.023-0.250 | 0.109 | 0.182 | 0.152 ± 0.018 | Pass |
| Torque to uncap, Nm | 0.023-0.250 | 0.117 | 0.199 | 0.145 ± 0.016 | Pass |
| Force to depress needle shield and start injection, N | 5.00-15.00 | 9.77 | 11.32 | 10.65 ± 0.37 | Pass |
Standard atmospheric conditions: temperature, 18-28°C; relative humidity, 25-75%.
SD, standard deviation.
Validation Study Participants
The usability validation study was conducted at three study centers in the United States during February and March 2016 and enrolled 104 participants, including 73 lay users, 16 HCPs, and 15 pharmacists (Table 5). Of 73 lay users, 32 (44%) reported impairments common to patients with T2DM, including visual (n = 15; eg, cataracts) and dexterity impairments (n = 17; eg, arthritis, neuropathy). A similar proportion of lay users received injection training (n = 39) versus no training (n = 34) and were injection experienced (n = 41) versus injection naïve (n = 32). HCPs and pharmacists did not receive training.
Table 5.
Participant Demographics.
| Demographics, n (%) | Lay users (n = 73) | Health care professionals (n = 16) | Retail pharmacists (n = 15) |
|---|---|---|---|
| Sex | |||
| Female | 34 (47) | 7 (44) | 6 (40) |
| Male | 39 (53) | 9 (56) | 9 (60) |
| Age, years | |||
| 18-54 | 18 (25) | NA | NA |
| 55-69 | 46 (63) | NA | NA |
| 70-75 | 9 (12) | NA | NA |
| Race/ethnicity | |||
| Caucasian | 41 (56) | 5 (31) | 7 (47) |
| Black | 11 (15)a | 2 (13) | 1 (7) |
| Hispanic | 13 (18)a | 5 (31) | 0 (0) |
| Asian | 3 (4) | 2 (13) | 1 (7) |
| Middle Eastern | 1 (1) | 0 (0) | 5 (33) |
| Other/unknown | 5 (7) | 2 (13) | 1 (7) |
| Education | |||
| High school graduate | 2 (3) | NA | NA |
| Some college | 21 (29) | NA | NA |
| College graduate | 36 (49) | NA | NA |
| Postgraduate | 9 (12) | NA | NA |
| Unknown | 5 (7) | NA | NA |
| Right-handed | 62 (85) | 15 (94) | 15 (100) |
| Colorblind | 2 (3) | 0 (0) | 0 (0) |
| Wear glasses | 57 (78) | 12 (75) | 3 (20) |
| Patient/caregiver status | |||
| Patients | 61 (84) | NA | NA |
| Caregivers | 12 (16) | NA | NA |
| Injection naïveb | 32 (44) | NA | NA |
| Administer injectionsb | NA | 12 (75) | 4 (27) |
One lay user was recorded as black race and Hispanic ethnicity.
Lay users were categorized as injection experienced or naïve. Health care professionals and pharmacists were categorized by whether they administered injections.
NA, not applicable.
Simulated-Use Scenarios
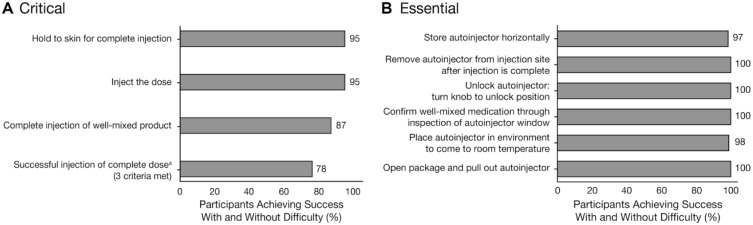
During the simulated-use scenarios, 87-100% of participants completed critical tasks successfully (with or without difficulty) on the first attempt (Table 3). Overall, 81 of 104 participants (78%) successfully completed an injection, defined as success on three tasks: holding the autoinjector to the skin for complete injection, injecting the dose, and completing the injection of a well-mixed product (Figure 2A). Successful injections were also completed by 23 of 32 lay users (72%) who reported visual or dexterity impairments. All essential tasks were successfully completed by ⩾97% of participants (Figure 2B).
Figure 2.
Participant success on key (A) critical and (B) essential tasks with and without difficulty.
aSuccessful injection of a complete dose required success on the three criteria shown: completing injection of a well-mixed product, injecting the dose, and holding the device to the skin for complete injection.
Eight of 19 participants (42%) who made errors and did not successfully complete an injection understood their errors and demonstrated learning. Participants who made use errors on critical tasks included 13 of 73 lay users (18%), five of 16 HCPs (31%), and five of 15 pharmacists (33%). On critical tasks, the most common use errors not due to study artifact were related to completing the injection of a well-mixed product (n = 12) and removing the needle cap (n = 8) (Table 3).
Overall, untrained injection-naïve and trained injection-experienced lay users had the highest success rate on critical tasks. Of the 29 use errors not due to study artifact, injection-naïve lay users (trained and untrained) had fewer use errors (n = 5) than injection-experienced lay users (n = 11). Although untrained injection-experienced lay users had the highest rate of use errors (n = 10/29 [34%]), they self-corrected in seven cases. Trained lay users (naïve and experienced) made fewer use errors (n = 4) than untrained lay users (n = 12). HCPs and retail pharmacists made six and seven use errors, respectively, but self-corrected or independently identified the error in three cases.
Timing Study
The mean time to prepare and inject a dose was 5:03 minutes for untrained lay users and 2:33 minutes for trained lay users based on timing of 68 of 73 lay users (five were excluded due to study artifact).
Ease-of-Use Ratings
Overall, 91% of responses to the subjective ease-of-use questions following the simulated-use scenarios were rated as “easy” or “very easy” (Figure 3). Fewer than 15 participants rated any task as “neither easy nor difficult”; none were rated as “very difficult.” “Difficult” ratings were generally consistent with the difficulties and use errors observed during the simulated-use scenarios.
Figure 3.
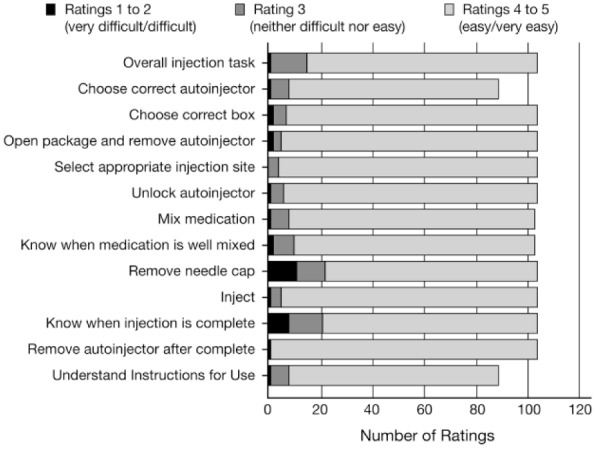
Participant ease-of-use ratings by task (N = 104). Participants were only asked about the tasks they completed. No participants rated any task as “very difficult.”
Evaluation of the Instructions for Use
Of the 104 study participants, 90 (n = 60/73 lay users, n = 16/16 HCPs, and n = 14/15 pharmacists) independently referred to the IFU at some point during the simulated-use scenario. Thirteen of 14 participants who did not refer to the IFU were lay users, all of whom had been trained to use the autoinjector before beginning the study and demonstrated safe and effective use of the autoinjector.
Discussion
Challenges to successful therapeutic management of T2DM include suboptimal adherence and irregular dosing schedules. Furthermore, patients who receive injectable medications are at risk for administration of a partial dose, which may inadvertently reduce treatment efficacy.2 When initiating treatment with an injectable medication, health care providers often have limited time with the patient and opt to focus on dosing, treatment adherence, and site rotation, rather than the specifics of proper injection technique.3 As such, administration of injectable medications should be simple, logical, and necessitate minimal training.
The autoinjector device utilized for exenatide QWS was designed to require no assembly and fewer preparatory steps than needed for syringes and pens (Table 1). The resulting autoinjector is ergonomically designed for comfortable use and contains a large viewing window to facilitate feedback on proper mixing. A needle shield reduces the risk of injury, keeps the needle sterile, and prevents reuse by locking in position after injection. As reported here, the autoinjector met its design performance goals related to dose accuracy and torque/force requirements.
In the final usability validation study reported here, critical tasks required for safe and effective dose administration were successfully completed by 87-100% of participants on the first attempt, with 78% successfully completing an injection and 72% of participants with visual or dexterity impairments successfully completing an injection. Importantly, when making an error, many participants were able to either self-correct and complete a successful injection or learn from their errors. This implies that the steps for injection are logical and that the device provides sufficient feedback for users to independently derive the correct procedure. Interestingly, critical errors occurred more frequently among HCPs and pharmacists than lay users. This may be a result of carryover effect, as medical professionals may have prior experience with other injectable medications and may have inappropriately applied those techniques to exenatide QWS-AI. To support proper use of exenatide QWS-AI, unique steps for administration are outlined in Table 6.
Table 6.
Unique Steps in the Instructions for Use of Exenatide Once-Weekly Suspension Autoinjector.
| Instruction | Rationale |
|---|---|
| Store the autoinjector flat | The medication will be easier to mix when the autoinjector has been stored flat because the microspheres will settle over a larger surface area |
| Remove the autoinjector from the refrigerator at least 15 minutes before use | The medication is easier to mix well at warmer temperatures |
| Shake the autoinjector hard for at least 15 seconds | Shaking is required to mix the suspension well |
| Shake the autoinjector until the medicine is well-mixed and cloudy | A cloudy appearance indicates thorough mixing as the microspheres are in suspension |
| When unlocking the autoinjector and unscrewing the orange cap, the needle and orange cap should be pointing up | The needle-up position allows the microspheres to stay within the syringe |
| Inject the medicine as soon as the mixing is complete; do not unlock the autoinjector until just before injection | Injecting the medication as soon as the mixing is complete ensures the microspheres remain in suspension |
| After pushing the autoinjector against the skin, keep holding it there for 15 seconds | Holding the autoinjector in place for 15 seconds allows sufficient time to deliver the required volume |
The IFU proved to be a helpful resource and was often referred to during the simulated-use scenarios. However, because exenatide QWS-AI does not require reconstitution, users may assume that minimal (or no) mixing of the drug is required. Indeed, errors related to mixing were among the more frequent use errors in this study. During the performative and formative studies, the IFU and labeling were revised to emphasize and clearly describe the mixing step. The packaging was also designed to encourage users to read the IFU, with the booklet placed at the top of the carton. Despite these design efforts, the risk remains that users will not fully read the IFU. Thus, it is particularly important that health care providers and pharmacists remind patients and their caregivers to refer to the IFU before and during injections. Health care providers may also ask patients to demonstrate use of the autoinjector to confirm accurate technique.
Exenatide QWS-AI has been clinically evaluated in two large, 28-week, phase 3, randomized, open-label, controlled studies of patients with T2DM.7,9 In the first study (DURATION-NEO-1), exenatide QWS-AI treatment resulted in a significantly greater reduction in HbA1c (least-squares mean change [standard error]: –1.39% [0.09%]) than exenatide twice-daily (BID) treatment (−1.02% [0.11%]; difference: −0.37% [0.13%]; P = .0072).9 Injection-site-related adverse events were more frequent with exenatide QWS-AI (26.6%) than with exenatide BID (4.1%); for exenatide QWS-AI, nodules were the most frequent injection-site-related adverse event and were generally mild, single events that occurred within the first 60 days of treatment. Compared with exenatide BID, exenatide QWS-AI treatment was associated with significantly greater improvements on the Diabetes Treatment Satisfaction Questionnaire overall score and subscores for treatment convenience, flexibility, satisfaction with current treatment, understanding of diabetes, continuing treatment, and perceived frequency of hyper- and hypoglycemia.11 In a second study (DURATION-NEO-2), exenatide QWS-AI resulted in a significantly greater reduction in HbA1c than both sitagliptin (least-squares mean difference: −0.38%; 95% confidence interval, −0.70% to −0.06%; P = .021) and placebo (−0.72%; −1.15% to −0.30%; P = .001).7 Only patients who received exenatide QWS-AI reported injection-site-related adverse events, most of which were nodules. In addition, patients who received exenatide QWS-AI scored higher on all four domains of the Diabetes Medication Satisfaction Tool (ease and convenience, lifestyle burden, well-being, and medical control) than patients who received sitagliptin or placebo. Together, these two studies demonstrate that exenatide QWS-AI is not only an effective and well-tolerated option for the treatment of T2DM, but that it may also improve patient satisfaction with regards to their treatment regimen.
In the present usability summative validation study, participants independently completed injections in a controlled environment and in the presence of a moderator. Although this provided consistent testing conditions, study artifacts, performance anxiety, or unfamiliar surroundings of the test environment may have affected the study outcomes. Since this study only evaluated a single injection, it is unclear how users will perform over time. Also, this study did not assess the effect of the hidden needle on injection anxiety. Despite these limitations, which are largely inherent to any human factors study, the results demonstrate that the autoinjector was an easily learned, rapid option for administration of exenatide QW.
Conclusion
Exenatide QWS-AI has been optimized to support users in safely, correctly, and rapidly administering an injection of exenatide suspension. Users overwhelmingly demonstrated successful injection technique and rated the autoinjector as “easy” or “very easy” to use. These findings are consistent with clinical trial data showing that patients consider the device more convenient than a BID injected medication or an oral tablet.7,11
Acknowledgments
The authors thank the participants and all investigators involved in this study. Elizabeth Strickland, PhD, of inScience Communications, Springer Healthcare (Philadelphia, PA, USA), and Mollie Marko, PhD, on behalf of inScience Communications, provided medical writing support funded by AstraZeneca. Agilis Consulting Group contributed to the human factors evaluation of the exenatide QWS-AI device and critically reviewed this manuscript.
Footnotes
Abbreviations: BID, twice daily; HbA1c, glycated hemoglobin; HCP, health care professional; IFU, instructions for use; NA, not applicable; QW, once weekly; QWS, once-weekly suspension; QWS-AI, once-weekly suspension autoinjector; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SL and JS were employees of AstraZeneca at the time this study was conducted. MN and JM are employees of AstraZeneca. CHW has received research support and served as a consultant, advisor, and/or speaker for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen, Novo Nordisk, and Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study and development of the manuscript were supported by AstraZeneca.
References
- 1. American Diabetes Association. Standard of medical care in diabetes: 2016. (1) Strategies for improving care. Diabetes Care. 2016;39(suppl 1):S6-S12. [DOI] [PubMed] [Google Scholar]
- 2. Trief PM, Cibula D, Rodriguez E, Akel B, Weinstock RS. Incorrect insulin administration: a problem that warrants attention. Clin Diabetes. 2016;34(1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(suppl 2):S3-S18. [DOI] [PubMed] [Google Scholar]
- 4. Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25(6):1413-1420. [DOI] [PubMed] [Google Scholar]
- 5. Funnel MM. Overcoming barriers to the initiation of insulin therapy. Clin Diabetes. 2007;25(1):36-38. [Google Scholar]
- 6. American Diabetes Association. Standard of medical care in diabetes: 2018. (8) Pharmacologic approaches to glycemic treatment. Diabetes Care. 2018;41(suppl 1):S73-S93. [DOI] [PubMed] [Google Scholar]
- 7. Gadde KM, Vetter ML, Iqbal N, Hardy E, Öhman P; DURATION-NEO-2 Study Investigators. Efficacy and safety of autoinjected exenatide once-weekly suspension versus sitagliptin or placebo with metformin in patients with type 2 diabetes: the DURATION-NEO-2 randomized clinical study. Diabetes Obes Metab. 2017;19(7):979-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol Ther. 2011;13(11):1145-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wysham CH, Rosenstock J, Vetter ML, Dong F, Öhman P, Iqbal N. Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20(1):165-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lorenzi G, Schreiner B, Osther J, Boardman M. Application of adult-learning principles to patient instructions: a usability study for an exenatide once-weekly injection device. Clin Diabetes. 2010;28(4):157-162. [Google Scholar]
- 11. Wysham CH, Vieke D, Vetter M, et al. Patient-reported treatment satisfaction with exenatide once weekly suspension for autoinjection (EQW-SAI) vs exenatide twice daily (EBID) in patients with inadequately controlled type 2 diabetes mellitus (T2DM) [abstract THR-647]. Paper presented at: Endocrine Society’s 97th Annual Meeting & Expo (ENDO); March 5-8, 2015; San Diego, CA. [Google Scholar]