Abstract
Background/purpose
Langerhans cells (LCs) are antigen-presenting cells. This study mainly evaluated the LC counts in 60 odontogenic keratocysts (OKCs).
Materials and methods
The CD1a-positive LC numbers in the lining epithelia and subepithelial connective tissues were counted at 60 OKC sites without inflammation, 39 OKC sites with mild/moderate inflammation, and 13 OKC sites with severe inflammation from 60 OKC specimens.
Results
The mean CD1a-positive LC counts in the lining epithelia and subepithelial connective tissues increased significantly from no inflammation (0.5 ± 0.4 and 0.2 ± 0.3 cell/high-power field or HPF, respectively) through mild/moderate inflammation (5.3 ± 2.5 and 2.5 ± 2.7 cells/HPF, respectively) to severe inflammation OKC sites (12.7 ± 5.6 and 9.3 ± 7.2 cells/HPF, respectively; all P-values < 0.001). OKC sites with inflammation had thicker lining epithelia than those without inflammation. Moreover, the mean CD1a-positive LC counts in the lining epithelia and subepithelial connective tissues of OKCs were significantly higher in the thicker lining epithelium (>100 μm) group (6.8 ± 5.1 and 3.7 ± 4.9 cells/HPF, respectively) than in the thinner lining epithelium (≦100 μm) group (1.0 ± 1.7 and 0.8 ± 2.5 cell/HPF, respectively; both P-values < 0.001).
Conclusion
There is a significant association of inflammation grade with the number of LCs in OKCs. The scarce LCs in the lining epithelia of OKCs without inflammation suggests the loss of immunosurveillance ability against the OKC lining epithelial cells; this can explain why OKCs have aggressive clinical behavior, a great growth potential, and a high recurrence rate.
Keywords: Langerhans cell, odontogenic keratocyst, CD1a, aggressive clinical behavior, high recurrence rate, immunosurveillance
Introduction
Odontogenic keratocyst (OKC) is a special type of odontogenic cyst that shows aggressive clinical behavior, a high recurrence rate, and an association with nevoid basal cell carcinoma syndrome (NBCCS).1, 2 Pathogenetic mechanisms that favor growth and expansion of OKCs include a high proliferation rate, overexpression of antiapoptotic protein Bcl-2, and expression of matrix metalloproteinases 2 and 9.2 Mutations in PTCH tumor suppressor gene have been found in 85% of NBCCS-associated OKCs and 30% of sporadic OKCs.1 If the PTCH gene is nonfunctional, no PTCH gene products are generated and this in turn results in overexpression of sonic hedgehog and/or smoothened proteins, leading to increased proliferation of OKC lining epithelial cells.2 Although the OKC has been called as keratocystic odontogenic tumor in the third edition of World Health Organization (WHO) Classification of Head and Neck Tumours published in 2005,3 the old name of OKC was reused recently in the fourth edition of the same book published in 2017 probably due to insufficient evidence to support a neoplastic origin of OKC.4
Langerhans cells (LC) are bone marrow-derived dendritic cells that reside within the suprabasal and spinous cell layers of oral stratified squamous epithelium. LCs work as antigen-presenting cells that phagocytose the antigens in the oral epithelium, migrate from the oral epithelium to lamina propria and further to the paracortical area of the draining lymph node where they process the antigenic proteins into antigenic peptides and then present the antigenic peptides to T cells. Consequently, T-cell-mediated effector mechanisms against the specific antigens are activated.5
Previous studies have shown the presence of LCs in the parakeratinized lining epithelia and subepithelial connective tissues of small serieses of OKCs.6, 7, 8, 9, 10, 11 The frequency of detection of LCs in the lining epithelia is greater in orthokeratinized odontogenic cysts than in OKCs.6, 7 Moreover, there are more LCs found in the thick hyperplastic lining epithelia overlying the fibrous connective tissue wall with inflammation than in the thin atrophic lining epithelia overlying the fibrous connective tissue wall without inflammation.9, 10 However, it was still not clear whether the LC counts in the lining epithelia and subepithelial connective tissues of OKCs were associated with the grade of inflammation in the subepithelial connective tissue, the thickness of the lining epithelium, and the clinical parameters of OKC.
In this study, we used the anti-CD1a immunostaining to assess the LCs in a relatively large series of 60 OKCs. The CD1a-positive LC counts in the lining epithelia and subepithelial connective tissues of OKC samples were calculated and compared between groups to see whether there might be an association of LC numbers with the grade of inflammation in the focal fibrous cystic wall site, the thickness of the lining epithelium, and the clinical parameters such as the patient age and gender as well as the location, size and recurrence of OKC.
Materials and methods
Patients and specimens
Formalin-fixed, paraffin-embedded specimens from 60 OKC patients (33 men and 27 women, mean age 34 ± 18 years, range 6–75 years) were included in this study. Two of the 60 OKC patients had the associated NBCCS. Diagnosis of OKC was based on histological examination of hematoxylin and eosin-stained tissue sections. The OKC was characterized as having parakeratinized stratified squamous lining epithelium of 6–8 cells in thickness overlying a thin layer of fibrous connective tissue wall without inflammation or with a focal mild, moderate or severe chronic inflammatory cell infiltrate. The surface parakeratin usually showed a wavy or corrugated appearance and the basal epithelial layer was composed of a palisaded layer of cuboidal or columnar epithelial cells. All patients received total surgical enucleation of their OKC lesions at the Department of Oral and Maxillofacial Surgery, National Taiwan University Hospital (NTUH), Taipei, Taiwan during the period from 2005 to 2011. Specimens were obtained from total surgical excision of the lesions. Of the 60 OKC lesions, 23 (38%) were located in the maxilla (3 in the anterior and 20 in the posterior region) and 37 (62%) in the mandible (5 in the anterior and 32 in the posterior region). The mean greatest dimension of the OKC measured from the panoramic radiographs was 3.4 ± 1.4 (range 1.0–7.1) cm. Of the 60 OKCs, 54 were primary and 6 were recurrent OKCs. This study has been reviewed and approved by the Institutional Review Board of NTUH.
Immunohistochemical staining for Langerhans cells
The immunohistochemistry for identification of LCs in various lesional tissues has been described previously.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Briefly, all the specimens for immunostaining were fixed in 10% neutral formalin, embedded in paraffin, and cut in serial sections of 4 μm. Immunohistochemical staining was performed using a super-sensitive polymer-horseradish peroxidase (HRP) technique. Tissues sections were first deparaffinized and rehydrated. Then, sections were heated in a plastic slide holder (Dako, Copenhagen, Denmark) containing 0.01 M citrate buffer in a microwave oven for 15 min to retrieve antigenicity, and then treated with 3% H2O2 in methanol for 10 min to quench endogenous peroxidase activity. After washing in 10 mM Tris-buffered saline (TBS), pH 7.6, sections were incubated with 10% normal goat serum (BioGenex, San Ramon, CA, USA) to block nonspecific binding. Sections were then incubated overnight at 4 °C with mouse anti-CD1a monoclonal antibody (Ab-5, Thermo Fisher Scientific, Runcorn, UK) at a dilution of 1:50. The BioGenex Super Sensitive TM detection systems® were used for detection of bound antibodies. After washing in TBS, sections were treated with super enhancer reagent for 10 min, and subsequently treated with the polymer-HRP reagent for another 10 min. The 0.02% diaminobenzidine hydrochloride (DAB, Zymed Laboratories, San Francisco, CA, USA) containing 0.03% H2O2 was used as chromogen to visualize the peroxidase activity. The preparations were lightly counterstained with hematoxylin, mounted with Permount, and examined by light microscopy. Human Langerhans cell histiocytosis gingival tissue sections that were previously shown to contain CD1a-positive LCs were used as positive controls. TBS instead of primary antibody was used as negative controls.
The LC in the lining epithelia and subepithelial fibrous connective tissues was counted positive when its cell body and at least one associated dendritic process showed CD1a-positive brown staining. The sections were initially scanned at the low power. For sections that showed heterogeneous patterns of positive stain, the predominant pattern was taken into account for scoring. At least three high-power (200×) fields (HPFs) of the epithelium or of the subepithelial connective tissue were chosen randomly, and the LC number in each HPF was counted. The LC count for each OKC site with mild, moderate, or severe inflammation was the mean of three or more LC counts per HPF. The lining epithelial thickness was measured from the surface of the parakeratinized stratified squamous epithelium to the basement membrane. The lining epithelial thickness for each OKC site with mild, moderate, or severe inflammation was also the mean of three or more epithelial thickness measurements. The inflammatory cell infiltrate in the subepithelial fibrous connective tissue was arbitrarily classified into three grades (mild, moderate and severe) according to the number of chronic inflammatory cells in the subepithelial connective tissue wall of the OKC. In general, chronic inflammatory cell infiltrate was found in the superficial, middle, and deep layers of the subepithelial fibrous connective tissue for those sites classified into mild, moderate, and severe inflammation OKC sites, respectively. Each of these assessments was independently carried out by two investigators. The LC counts with an interobserver variation of more than 10% and the disagreement in the grade of inflammation in the focal fibrous cystic wall of OKC were reassessed using a double-headed light microscope to achieve consensus. In this study, the interobserver reproducibility was approximately 94%.
Statistical analyses
The mean LC counts in the lining epithelia or in the subepithelial connective tissues of OKC samples were compared first among 3 groups by analysis of variance (ANOVA) and then between any 2 groups by Student's t-test. The mean CD1a-positive LC counts were compared with the previously-published mean S100-positive LC counts in each of three different groups of OKC with severe, mild/moderate, and no inflammation by Student's t-test.25 The correlation between LC counts in the lining epithelia or in the subepithelial connective tissues of OKC samples and clinicopathological parameters of OKC patients was analyzed by Student's t-test. A P-value of less than 0.05 was considered statistically significant.
Results
Langerhans cells in OKC samples
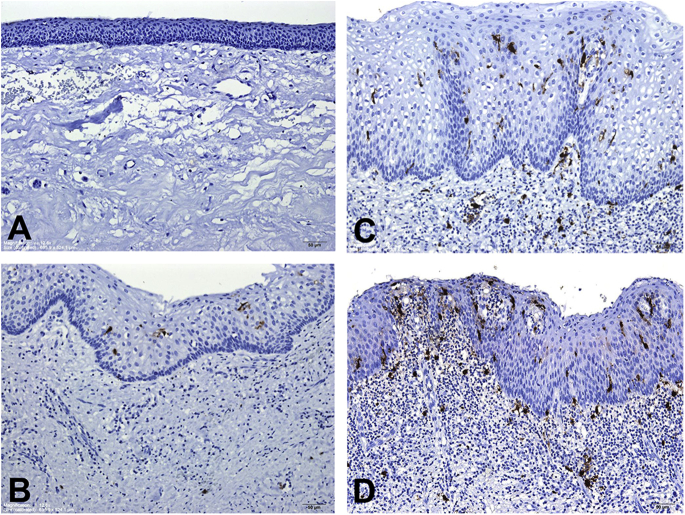
Representative anti-CD1a-immunostained microphotographs of LCs in the lining epithelia and subepithelial connective tissues of OKC specimens are shown in Fig. 1. When there was no inflammation in the fibrous cystic wall of OKC, dendritic LCs were not found or detected only occasionally in the lining epithelia and subepithelial connective tissues of OKC specimens (Fig. 1A). When there was a mild, moderate, or severe inflammatory cell infiltrate in the focal fibrous cystic wall of OKC, dendritic LCs could be found in the basal, suprabasal and spinous cell layers of the lining epithelia and subepithelial connective tissues of OKC specimens (Fig. 1B–D). Moreover, the number of LCs in the lining epithelia and subepithelial connective tissues of OKC specimens increased gradually from OKC sites with mild inflammation, to OKC sites with moderate inflammation, and further to OKC sites with severe inflammation (Fig. 1B–D).
Fig. 1.
Anti-CD1a-immunostained microphotographs of Langerhans cells (LCs) in odontogenic keratocyst (OKC) specimens. (A) An OKC without inflammation showing none of LCs in both the thin lining epithelium and subepithelial connective tissue. (B) An OKC with mild inflammation exhibiting few LCs in the lining epithelium. (C) An OKC with moderate inflammation demonstrating a few LCs in both the lining epithelium and the moderately-inflamed subepithelial connective tissue. (D) An OKC with severe inflammation showing more LCs in both the lining epithelium and the severely-inflamed subepithelial connective tissue. (Original magnification; A, B, C and D: 12.6×).
Langerhans cell counts in the lining epithelia and subepithelial connective tissues of OKC specimens
The number of LCs in the lining epithelia and subepithelial connective tissues of OKC specimens varied according to the grade of inflammation in focal fibrous cystic wall sites. Therefore, we observed the whole section of each OKC specimen to identify all focal fibrous cystic wall sites with different grades of inflammation. In the 60 OKC specimens, 60 sites without inflammation, 39 sites with mild/moderate inflammation, and 13 sites with severe inflammation could be detected. To avoid the bias, focal fibrous cystic wall sites with mild inflammation and those with moderate inflammation were integrated into one group of OKCs with mild/moderate inflammation. We found that the mean LC count in either the lining epithelium or in the subepithelial connective tissue was significantly higher in the severe inflammation or mild/moderate inflammation group (all P-values < 0.001) than in no inflammation group, and significantly higher in the severe inflammation group than in the mild/moderate inflammation group (P < 0.001) (Table 1). In addition, for both the mild/moderate inflammation and no inflammation groups, the mean LC count in the lining epithelium was greater than that in the subepithelial connective tissue (both P-values < 0.001) (Table 1).
Table 1.
Mean CD1a-positive Langerhans cell (LC) counts in the lining epithelia and subepithelial connective tissues of 60 odontogenic keratocysts according to different grades of inflammation in focal fibrous cystic wall sites.
| Inflammation in focal fibrous cystic wall site | Mean CD1a-positive LC count ± SD (cells/high-power field) |
||
|---|---|---|---|
| Lining epithelium | Subepithelial connective tissue | P-value (Student's t-test) | |
| Severe inflammation (n = 13) | 12.7 ± 5.6 | 9.3 ± 7.2 | 0.187 |
| aP-value | <0.001 | <0.001 | |
| bP-value | <0.001 | <0.001 | |
| Mild/moderate inflammation (n = 39) | 5.3 ± 2.5 | 2.5 ± 2.7 | <0.001 |
| aP-value | <0.001 | <0.001 | |
| No inflammation (n = 60) | 0.5 ± 0.4 | 0.2 ± 0.3 | <0.001 |
Comparison of mean CD1a-positive LC count between severe or mild/moderate inflammation and no inflammation groups by Student's t-test.
Comparison of mean CD1a-positive LC count between severe and mild/moderate inflammation groups by Student's t-test.
To find out whether there were differences in the LC counts in the lining epithelia and subepithelial connective tissues of OKCs stained with either anti-CD1a or anti-S100 antibody, we compared the mean CD1a-positive LC counts with the previously-published mean S100-positive LC counts in each of three different groups of OKC with severe, mild/moderate, and no inflammation in the focal fibrous cystic walls.25 In both the mild/moderate and no inflammation groups, the mean CD1a-positive LC counts in the subepithelial connective tissue (2.5 ± 2.7 and 0.2 ± 0.3 cells/HPF, respectively) were significantly lower than the mean S100-positive LC counts (5.0 ± 3.5 and 0.7 ± 0.6 cells/HPF, respectively) in the subepithelial connective tissue (both P-values < 0.001) (Table 2).
Table 2.
Comparisons of mean CD1a-positive Langerhans cell (LC) count with mean S100-positive LC count in the lining epithelium or in the subepithelial connective tissue of 60 odontogenic keratocysts.
| Inflammation in focal fibrous cystic wall site | Mean LC number ± SD (cells/high-power field) |
|
|---|---|---|
| Lining epithelium | Subepithelial connective tissue | |
| Severe inflammation (n = 13) | ||
| CD1a-positive | 12.7 ± 5.6 | 9.3 ± 7.2 |
| aS100-positive | 14.7 ± 5.3 | 13.3 ± 6.8 |
| bP-value | 0.354 | 0.156 |
| Mild/moderate inflammation (n = 39) | ||
| CD1a-positive | 5.3 ± 2.5 | 2.5 ± 2.7 |
| aS100-positive | 5.9 ± 2.7 | 5.0 ± 3.5 |
| bP-value | 0.314 | <0.001 |
| No inflammation (n = 60) | ||
| CD1a-positive | 0.5 ± 0.4 | 0.2 ± 0.3 |
| aS100-positive | 0.5 ± 0.4 | 0.7 ± 0.6 |
| bP-value | 0.92 | <0.001 |
S100-positive LCs in 60 OKC samples have been published previously.25
Comparison of the mean CD1a-positive LC count with the mean S100-positive LC count by Student's t-test.
The mean thickness of lining epithelium overlying the focal fibrous cystic wall with severe, mild/moderate, or no inflammation was 305 ± 161 μm, 140 ± 47 μm, or 60 ± 25 μm, respectively. When the 112 LC counts (13 in the severe inflammation group, 39 in the mild/moderate inflammation group, and 60 in the no inflammation group) were divided into two groups according to the thickness of lining epithelium, the mean LC count in the lining epithelia or in the subepithelial connective tissues of OKCs was significantly higher in the thicker lining epithelium (>100 μm) group than in the thinner lining epithelium (≦100 μm) group (both P-values < 0.001) (Table 3).
Table 3.
Comparison of the mean CD1a-positive LC counts in the lining epithelia or in the subepithelial connective tissues between the thinner lining epithelium (≦ 100 μm) group and the thicker lining epithelium (>100 μm) group.
| Thickness of lining epithelium | Mean CD1a-positive LC count ± SD (cells/high-power field) |
||
|---|---|---|---|
| Lining epithelium | Subepithelial connective tissue | P-value | |
| ≤100 μm (n = 50) | 1.0 ± 1.7 | 0.8 ± 2.5 | 0.516 |
| >100 μm (n = 62) | 6.8 ± 5.1 | 3.7 ± 4.9 | 0.414 |
| aP-value | <0.001 | <0.001 | |
Comparison of the mean CD1a-positive LC counts between two different groups by Student's t-test.
Correlation between Langerhans cell counts in OKC samples and clinical parameters of OKC patients
Correlations between the CD1a-positive LC counts in the lining epithelia or in the subepithelial connective tissues of OKC samples and clinical parameters of OKC patients are shown in Table 4. The 112 data of LC counts in the lining epithelia or in the subepithelial connective tissues of OKC samples were divided into three groups: severe, mild/moderate, and no inflammation groups. At the severe inflammation OKC sites, the mean LC count in the lining epithelium was greater in female OKC patients than in male OKC patients (P = 0.021). In addition, at the no inflammation OKC sites, the mean LC count in the subepithelial connective tissue was higher in the maxillary OKCs than in the mandibular OKCs. However, the LC counts in the lining epithelia or in the subepithelial connective tissues of OKC samples had no association with the age of OKC patients as well as the size and recurrence of OKCs.
Table 4.
Correlations between the CD1a-positive Langerhans cell (LC) counts in the lining epithelia or in the subepithelial connective tissues of 60 odontogenic keratocyst (OKC) samples and clinical parameters of 60 OKC patients.
| Parameter | Mean CD1a-positive LC count ± SD (cells/high-power field) |
|||||
|---|---|---|---|---|---|---|
| Lining epithelium |
Subepithelial connective tissue |
|||||
| Severe | Mild/moderate | None | Severe | Mild/moderate | None | |
| Gender | ||||||
| Male (n = 33) | 10.0 ± 4.6 (n = 8) | 4.8 ± 2.6 (n = 22) | 0.5 ± 0.4 (n = 33) | 10.7 ± 6.0 (n = 8) | 3.2 ± 2.8 (n = 22) | 0.2 ± 0.3 (n = 33) |
| Female (n = 27) | 17.0 ± 4.5 (n = 5) | 6.0 ± 2.2 (n = 17) | 0.5 ± 0.4 (n = 27) | 7.0 ± 9.1 (n = 5) | 1.7 ± 2.5 (n = 17) | 0.2 ± 0.3 (n = 27) |
| aP-value | 0.021 | 0.148 | 0.944 | 0.398 | 0.086 | 0.765 |
| Age (yr) | ||||||
| ≤ 25 (n = 25) | 13.4 ± 2.8 (n = 4) | 5.3 ± 2.7 (n = 14) | 0.5 ± 0.3 (n = 25) | 12.4 ± 8.3 (n = 4) | 3.3 ± 3.4 (n = 14) | 0.2 ± 0.3 (n = 25) |
| > 25 (n = 35) | 12.4 ± 6.6 (n = 9) | 5.4 ± 2.4 (n = 25) | 0.5 ± 0.4 (n = 35) | 7.8 ± 6.7 (n = 9) | 2.1 ± 2.2 (n = 25) | 0.2 ± 0.3 (n = 35) |
| aP-value | 0.776 | 0.873 | 0.723 | 0.314 | 0.225 | 0.852 |
| Location | ||||||
| Maxilla (n = 23) | 12.8 ± 5.0 (n = 7) | 5.5 ± 2.5 (n = 16) | 0.5 ± 0.4 (n = 23) | 12.2 ± 6.7 (n = 7) | 3.2 ± 3.5 (n = 16) | 0.3 ± 0.4 (n = 23) |
| Mandible (n = 37) | 12.6 ± 6.8 (n = 6) | 5.2 ± 2.5 (n = 23) | 0.5 ± 0.4 (n = 37) | 5.8 ± 6.7 (n = 6) | 2.1 ± 1.9 (n = 23) | 0.1 ± 0.2 (n = 37) |
| aP-value | 0.959 | 0.689 | 0.720 | 0.114 | 0.187 | 0.034 |
| Size (cm) | ||||||
| ≤ 3 cm (n = 28) | 12.0 ± 5.7 (n = 6) | 4.9 ± 2.9 (n = 17) | 0.6 ± 0.3 (n = 28) | 12.7 ± 7.4 (n = 6) | 3.3 ± 3.3 (n = 17) | 0.2 ± 0.3 (n = 28) |
| > 3 cm (n = 32) | 13.3 ± 5.9 (n = 7) | 5.7 ± 2.1 (n = 22) | 0.5 ± 0.4 (n = 32) | 6.3 ± 6.0 (n = 7) | 2.0 ± 2.1 (n = 22) | 0.2 ± 0.3 (n = 32) |
| aP-value | 0.713 | 0.339 | 0.326 | 0.109 | 0.119 | 0.910 |
| Recurrence | ||||||
| With (n = 6) | 15.0 ± 0.0 (n = 1) | 3.7 ± 2.6 (n = 4) | 0.5 ± 0.4 (n = 6) | 2.4 ± 0.0 (n = 1) | 2.4 ± 2.4 (n = 4) | 0.1 ± 0.2 (n = 6) |
| Without (n = 54) | 12.5 ± 5.8 (n = 12) | 5.4 ± 2.4 (n = 35) | 0.5 ± 0.4 (n = 54) | 9.7 ± 6.9 (n = 12) | 2.5 ± 2.8 (n = 35) | 0.2 ± 0.3 (n = 54) |
| aP-value | 0.688 | 0.184 | 0.907 | 0.334 | 0.966 | 0.653 |
Comparison of mean CD1a-positive LC counts between two different groups by Student's t-test.
Discussion
There were three major findings in this study. First, when there was no inflammation in OKC, the LC was rarely found in both the lining epithelium (mean, 0.5 ± 0.4 cell/HPF) and subepithelial connective tissue (mean, 0.2 ± 0.3 cell/HPF). The OKC is a developmental odontogenic cyst. Thus, if the OKC is intrabony and has no communication with the oral cavity, there is usually no inflammatory cell infiltrate detected in the fibrous cystic wall of OKC.1, 2, 3, 4 Moreover, the lining epithelia of OKCs show significantly greater expression of proliferating cell nuclear antigen and Ki-67, suggesting that the OKC lining epithelial cells have a high proliferation potential.1 In addition, overexpression of Bcl-2 protein and expression of matrix metalloproteinases 2 and 9 in OKCs also increase the growth and local invasive potential of OKCs.2 The above immunohistochemical findings can explain why OKCs have more aggressive clinical behavior than the other odontogenic cysts.1, 2 In the present study, the rarely finding of LCs in the lining epithelia of OKCs without inflammation further indicates the loss of immunosurveillance ability against the OKC lining epithelial cells in OKC patients; this can in turn explain why OKCs have aggressive clinical behavior, a great growth potential, and a high recurrence rate.
Second, the mean LC counts in the lining epithelia or in the subepithelial connective tissues increased significantly from no inflammation through mild/moderate inflammation to severe inflammation OKC sites. Third, OKC sites with severe inflammation had the greatest mean thickness of lining epithelium, followed by OKC sites with mild/moderate inflammation and OKC sites without inflammation. Moreover, the mean CD1a-positive LC count in the lining epithelia or in the subepithelial connective tissues of OKCs was significantly higher in the thicker lining epithelium (>100 μm) group than in the thinner lining epithelium (≦100 μm) group. When OKC is infected by oral bacteria, an acute and chronic inflammatory cell infiltrate can be observed in the fibrous cystic wall of OKC. The bone marrow-derived LCs may migrate out from the blood vessels along with the acute and chronic inflammatory cells into the connective tissue wall of OKC. This can initially explain why we can see LCs in the subepithelial connective tissues with inflammation and why more LCs can be found in OKC sites with severer inflammation. Both the acute and chronic inflammatory cells can secrete growth factors that stimulate the proliferation of OKC lining epithelial cells. Therefore, when the subepithelial connective tissue is infiltrated by many inflammatory cells, the overlying epithelial cells usually proliferate to form a thick lining epithelial layer. As stated before, the OKC lining epithelial cells have genetic characters to grow without control. During the fast epithelial cell proliferation process, some of the lining epithelial cells may undergo apoptosis and release epithelial antigens into the lining epithelial layer. Because LCs have epithelial tropism, they may be attracted into the lining epithelia to phagocytose the released epithelial antigens. These epithelial antigen-carried LCs may migrate out from the lining epithelia into the inflamed subepithelial connective tissues and finally reach to the regional lymph nodes where they process the antigenic proteins into antigenic peptides and subsequently present the antigenic peptides to T cells in the paracortical area of the lymph node. The epithelial tropism of LCs can explain why we can see LCs among lining epithelial cells, and the LC migration process using the inflamed subepithelial connective tissue as the midway stop can explain why we can see the LCs in OKC fibrous connective tissue walls with different grades of inflammation.
The LCs can be identified by either anti-CD1a or anti-S100 immunostaining. In general, anti-S100 immunostaining can detect a little bit more LCs in OKCs than anti-CD1a immunostaining (Table 2).25 The CD1a is a relatively specific marker of the LC but the sensitivity of anti-CD1a antibody is slightly lower than that of anti-S100 antibody. S100 protein is not only a marker of LC but also markers of melanocytes, Schwann cells, and myoepithelial cells.26 Because the melanocytes in the lining epithelia and Schwann cells in the subepithelial connective tissues of OKCs may also be immunostained by anti-S100 antibodies, this can also explain why anti-S100 immunostaining can detect a little bit more LCs in OKCs than anti-CD1a immunostaining. However, the Schwann cells are spindle-shaped cells without dendritic processes, thus they can be distinguished from LCs by careful examination.
Previous studies have also shown the presence of LCs in OKC samples. Piattelli et al.6 used anti-CD1a immunostaining to assess the LCs in 18 OKCs including 5 orthokeratotic and 13 parakeratotic OKCs. They discovered a greater frequency of detection of LCs in orthokeratotic OKCs (80%) than in parakeratotic OKCs (33%). They concluded that the LC number seems to be associated with the degree of differentiation of the lining epithelia of OKC.6 Hoshino et al.7 used anti-langerin immunostaining to evaluate the LCs in 11 orthokeratinized odontogenic cysts and 28 OKCs. They found LCs mainly in orthokeratinized odontogenic cysts and rarely in OKCs. In recent literatures, the orthokeratinized variant of OKC is recognized as another entity and is called as orthokeratinized odontogenic cyst, because orthokeratinized odontogenic cysts have a much lower recurrence rate (approximately 2%) than the typical OKCs that have a high recurrence rate of more than 30%.1, 2 Although this study did not examine the LCs in orthokeratinized odontogenic cysts, the findings of the above-mentioned two studies do show more LCs in orthokeratinized odontogenic cysts than in typical OKCs.6, 7
Mello et al.8 used anti-CD1a immunostaining to study the LC number in 18 OKCs. The CD1a-positive LCs are detected in 14 (78%) of 18 OKCs. The mean LC density for 18 OKCs is 1.38 ± 1.55 cells per mm2. Moreover, the LC density is slightly higher in NBCCS-associated OKCs (1.44 ± 1.45 cells per mm2) than in sporadic OKCs (1.37 ± 1.64 cells per mm2), but the difference is not significant. They concluded that the decrease in the number of LCs in OKCs suggests a reduced immunosurveillance ability against the lining epithelial cells and this in turn favors the local invasiveness of OKCs.8 Two NBCCS-associated OKCs were included in this study, but they also did not show a significantly higher LC count than the other 58 sporadic OKCs. A large series of NBCCS-associated OKCs are needed to study to further confirm whether there is a significantly higher LC density in NBCCS-associated OKCs than in sporadic OKCs.
Wu et al.9 used anti-S100 immunostaining to identify the LCs in the lining epithelium and fibrous connective tissue wall of one OKC. They found no LC in the parakeratinized lining epithelium overlying the uninflamed fibrous connective tissue wall of the OKC. However, some LCs can be discovered in both the lining epithelium and underlying inflamed fibrous connective tissue wall of the OKC. They concluded that the presence of LCs in the lining epithelium is associated with the inflammation in the underlying fibrous cystic wall. Their findings were comparable to the results of this study.
Meira et al.10 studied the LCs in 15 OKCs using the anti-CD1a immunostaining. LCs can be observed in all the 15 OKCs. However, more LCs are found in thick hyperplastic lining epithelia overlying the fibrous connective tissues with severe inflammation than in thin atrophic lining epithelia overlying the fibrous connective tissues without inflammation. They suggested that the lower number of LCs in the atrophic lining epithelia of OKCs may be due to decreased epithelial immunosurveillance and this may result in locally aggressive invasiveness.10 Murase et al.11 investigated the LCs in 11 OKCs and did not detect any significant number of S100-positive LCs in 11 OKCs that generally lack inflammatory responses. The results of the aforementioned two studies showed the close association of inflammation with the finding of LCs in OKCs; these findings were also very similar to the results of our study.
This study showed a significant and gradual elevation in LC count from OKC sites without inflammation to OKC sites with mild/moderate inflammation and further to OKC sites with severe inflammation, suggesting the significant association of high inflammation grade with the increased number of LCs in OKCs. At OKC sites without inflammation, the LC counts in the lining epithelia or in the subepithelial connective tissues of OKC samples had no correlations with the age and gender of OKC patients as well as the size and recurrence of OKCs. The detection of very few number of LCs in OKC without inflammation indicates the loss of immunosurveillance ability against the OKC lining epithelial cells in OKC patients. This specific finding can explain why OKCs have more aggressive clinical behavior, a greater growth potential, and a higher recurrence rate than the other odontogenic cysts.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Neville B.W., Damm D.D., Allen C.M., Chi A.C. Odontogenic cysts and tumors. In: Neville B.W., Damm D.D., Allen C.M., Chi A.C., editors. Oral and maxillofacial pathology. 4th ed. Elsevier; St. Louis: 2016. pp. 636–644. [Google Scholar]
- 2.Neville B.W., Damm D.D., Allen C.M., Bouquot J.E. Odontogenic cysts and tumors. In: Neville B.W., Damm D.D., Allen C.M., Bouquot J.E., editors. Oral and maxillofacial pathology. 3rd ed. Saunders Elsevier; St. Louis: 2009. pp. 683–691. [Google Scholar]
- 3.Philipsen H.P. Keratocystic odontogenic tumour. In: Barnes L., Eveson J.W., Reichart P., Sidransky D., editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. 3rd ed. IARC Press; Lyon: 2005. pp. 306–307. [Google Scholar]
- 4.Speight P., Devilliers P., Li T.J., Odell E.W., Wright J.M. Odontogenic keratocyst. In: El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J., editors. WHO classification of head and Neck tumours. 4th ed. IARC; Lyon: 2017. pp. 235–236. [Google Scholar]
- 5.Barrett A.W., Cruchley A.T., Williams D.M. Oral mucosal Langerhans' cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 6.Piattelli A., Rubini C., Iezzi G., Fioroni M. CD1a-positive cells in odontogenic cysts. J Endod. 2002;28:267–268. doi: 10.1097/00004770-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino M., Inoue H., Kikuchi K. Comparative study of cytokeratin and langerin expression in keratinized cystic lesions of the oral and maxillofacial regions. J Oral Sci. 2015;57:287–294. doi: 10.2334/josnusd.57.287. [DOI] [PubMed] [Google Scholar]
- 8.Mello L.A., Figueiredo A.L., Ramos E.A. CD1a-positive Langerhans cells and their relationship with E-cadherin in ameloblastomas and keratocystic odontogenic tumors. J Oral Pathol Med. 2013;42:454–461. doi: 10.1111/jop.12033. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y.C., Wang Y.P., Chang J.Y.F., Chiang C.P. Langerhans cells in lining epithelia of odontogenic cysts. J Formos Med Assoc. 2013;112:725–727. doi: 10.1016/j.jfma.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Meira T.M., Melo L.A., Gurgel C.A.S. Density of Langerhans cells in the keratocystic odontogenic tumor. L Bras Patol Med Lab. 2010;46:135–141. [Google Scholar]
- 11.Murase N., Tatemoto Y., Iwai Y., Okada Y., Mori M. Langerhans cells in odontogenic tumours and cysts as detected by S-100 protein immunohistochemistry. Basic Appl Histochem. 1990;34:135–141. [PubMed] [Google Scholar]
- 12.Cheng S.J., Wang Y.P., Chen H.M., Chiang C.P. Central granular cell odontogenic tumor of the mandible. J Formos Med Assoc. 2013;112:583–585. doi: 10.1016/j.jfma.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y.C., Wang Y.P., Chang J.Y.F., Chiang C.P. Langerhans cells in lining epithelia of epidermoid cysts. J Dent Sci. 2013;8:448–450. [Google Scholar]
- 14.Wu Y.C., Wang Y.P., Chang J.Y.F., Chen H.M., Sun A., Chiang C.P. Langerhans cells in odontogenic epithelia of odontogenic fibromas. J Formos Med Assoc. 2013;112:756–760. doi: 10.1016/j.jfma.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Chiang C.T., Hu K.Y., Tsai C.C. Central granular cell odontogenic tumor: the first reported case in oriental people and literature review. J Formos Med Assoc. 2014;113:321–325. doi: 10.1016/j.jfma.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.J., Wei L.Y., Wu Y.C., Chiang C.P. An early central granular cell odontogenic tumor arising from the dental follicle of an impacted mandibular third molar. J Formos Med Assoc. 2014;113:766–768. doi: 10.1016/j.jfma.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.C., Chang J.Y.F., Wang Y.P., Chiang C.P. Langerhans cells in keratoacanthoma. J Formos Med Assoc. 2015;114:475–476. doi: 10.1016/j.jfma.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Tseng C.H., Wang Y.P., Lee J.J., Chang J.Y.F. Noncalcifying variant of calcifying epithelial odontogenic tumor with Langerhans cells. J Formos Med Assoc. 2015;114:781–782. doi: 10.1016/j.jfma.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y.C., Wang Y.P., Liu Y.C., Chen H.M. Langerhans cells in lining epithelium of unicystic ameloblastoma. J Dent Sci. 2015;10:464–466. [Google Scholar]
- 20.Wu Y.H., Chang J.Y.F., Chang H.H., Chiang C.P. Langerhans cells in dermoid cyst lining epithelium. J Formos Med Assoc. 2016;115:57–58. doi: 10.1016/j.jfma.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Lin H.P., Kuo Y.S., Wu Y.C., Wang Y.P., Chang J.Y.F., Chiang C.P. Non-calcifying and Langerhans cell-rich variant of calcifying epithelial odontogenic tumor. J Dent Sci. 2016;11:117–122. doi: 10.1016/j.jds.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y.P., Chen I.C., Wu Y.H., Wu Y.C., Chen H.M., Chang J.Y.F. Langerhans cell counts in oral epithelial dysplasia and their correlations to clinicopathological parameters. J Formos Med Assoc. 2017 doi: 10.1016/j.jfma.2017.02.006. (in press) [DOI] [PubMed] [Google Scholar]
- 23.Wu Y.H., Chang J.Y.F., Wang Y.P., Chiang C.P. Langerhans cells in granular cell ameloblastoma. J Formos Med Assoc. 2017 doi: 10.1016/j.jfma.2017.02.007. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Wu Y.H., Chang J.Y.F., Wang Y.P., Chiang C.P. Langerhans cells in plexiform ameloblastoma. J Dent Sci. 2017;12:195–197. doi: 10.1016/j.jds.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C.H., Wu Y.C., Wu Y.H., Sun A., Cheng S.J., Chen H.M. Significant association of inflammation grade with the number of Langerhans cells in odontogenic keratocysts. J Formos Med Assoc. 2017 doi: 10.1016/j.jfma.2017.04.002. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Kahn H.J., Marks A., Thom H., Baumal R. Role of antibody to S100 protein in diagnostic pathology. Am J Clin Pathol. 1983;79:341–347. doi: 10.1093/ajcp/79.3.341. [DOI] [PubMed] [Google Scholar]