Abstract
Background/purpose
Periodontitis is an inflammatory, destructive disease caused by periodontal bacteria, and its molecular mechanism remains unclear. The aims of this study are to evaluate the expressions of Wnt3a and Dkk1 in experimental periodontitis (EP) and preliminarily explore their roles in periodontal diseases.
Materials and methods
A total of 64 six-week-old male Sprague–Dawley rats were randomly divided into a normal group and an EP group. The EP group was prepared by using silk ligature combined with intraoral bacteria inoculation. To assess the periodontal inflammation and bone destruction extent, hematoxylin and eosin staining and tartrate-resistant acid phosphatase staining was performed 2 weeks, 4 weeks, and 6 weeks after the modeling, respectively, and immunohistochemistry and enzyme-linked immunosorbent assay were also performed to detect the changes of Wnt3a and Dkk1 in periodontal tissue and plasma.
Results
Wnt3a expression was significantly decreased in the EP group when compared with the normal group (P < 0.05). Meanwhile, Dkk1 expression was significantly increased in the EP group when compared with the normal group (P < 0.05).
Conclusion
The expression of Wnt3a and Dkk1 was well correlated with EP. It is suggested that Wnt3a and Dkk1 may be involved in periodontal diseases.
Keywords: Dkk1, osteoblasts, periodontitis, Wnt3a
Introduction
Periodontitis is a type of inflammatory, destructive disease caused by periodontal bacteria, and its molecular mechanism is unclear. Studies had shown that the major pathogen causing periodontitis is Porphyromonas gingivalis. The proinflammatory cytokines are produced by the host cells such as leukocytes and other cells after the challenge of bacteria, and then promote the release of chemokines and prostaglandin so as to cause bone damage;1 meanwhile, such proinflammatory cytokines could inhibit the proproliferation signals, such as mitogen-activated protein kinase2 and c-Jun N-terminal kinase,3 thus resulting in the growth inhibition of normal cells followed by bone destruction.
The Wnt signal is an important signaling pathway that extensively exists in invertebrates and vertebrates and could promote cell proliferation. Its key factor, Wnt3a, could bind LRP5 and β-catenin so as to form complexes and participate in a series of life processes such as embryonic development, organogenesis, and tumorigenesis. Studies had shown that Wnt3a has important regulatory roles in each differentiation period of osteoblasts. In C3G10T1/2 and ST2 cell lines, Wnt3a could induce the activity of alkaline phosphatase, and promote the differentiation of osteoblasts,4 which is an important molecular event in the early differentiation of osteoblasts. In the late differentiation stage of osteoblasts, Wnt3a could regulate the bone morphogenetic protein and osteoprotegerin (OPG) so as to mediate the differentiation of osteoblasts.5 Another key factor in the Wnt signal, Dkk1, is a type of glycoprotein with a molecular weight of 29 kDa, and it would be abnormally expressed in a variety of cancers, osteoporosis, or arthritis.6, 7, 8 As an antagonist of Wnt3a, Dkk1 could competitively bind LRP5 and β-catenin and form complexes, thus blocking the transfer of the Wnt3a signal. It was found that in mice with Dkk1, the overexpression of Dkk1 not only resulted in osteoporosis but also significantly reduced the number of mouse osteoblasts; by contrast, in mice with Dkk1 knocked out or silenced, their bone formation abilities were increased, and the bone mass was also increased.9, 10 Further studies showed that Dkk1 could inhibit the expression of Wnt signaling, thus decreasing the expression of OPG and resulting in bone loss.11
Considering the key roles of the Wnt signal on osteoblasts, it has become an important target for bone metabolic diseases. Accordingly, this study first established the experimental periodontitis (EP) model, and then explored the changes of the key factors of the Wnt signal in this model using immunohistochemistry and enzyme-linked immunosorbent assay (ELISA), aiming to provide the theoretical and experimental basis for the treatment of periodontitis.
Materials and methods
Reagents and instruments
P. gingivalis (American Type Culture Collection 33277) was provided by the School of Dentistry, Huaxi Medical University, Chengu, Sichuan, China. Hematoxylin and eosin (HE) were purchased from Beijing Solarbio Science & Technology Co., Ltd, and the tartrate-resistant acid phosphatase (T-ACP) staining kit was purchased from the Blood Institute, Chinese Academy of Medical Sciences. The primary and secondary antibodies of Wnt3a and Dkk1, as well as the ELISA assay kit, were provided by Sigma; the PXS-1040 stereomicroscope was purchased from Hangzhou Tuopu instrument Co., Ltd (Hangzhou, China).
Establishment of animal periodontitis model
A total of 64 male Sprague–Dawley rats, aged 6–8 weeks (220–240 g), were randomly divided into two groups: the normal group (group N, n = 32) and the periodontitis group (group P, n = 32); the rat EP model was prepared according to Huang et al12 and Branco-de-Almeida et al.13 After we ligatured the neck of the maxillary second molar tooth with silk, each rat was orally administered ampicillin (20 mg/d) with water so as to inhibit other bacteria that are nonbeneficial to the growth of P. gingivalis. Three days later, each rat was tube-fed 0.5 mL of 1.5 × 109 CFU/mL P. gingivalis (American Type Culture Collection 33277), and refed once every 48 hours for a total of three times.
After a successful modeling, the animals were sacrificed at 2 weeks, 4 weeks, or 6 weeks, and HE staining, T-ACP staining, immunohistochemistry, and ELISA were performed.
Specimen collection and processing
The blood was sampled from the heart after each rat was killed in the 2nd week, 4th week, or 6th week, followed by centrifugation to obtain the supernatant for the determination of Wnt3a and Dkk1. Each rat was decapitated, and then we rapidly separated the bilateral maxillary alveolar bone, which was then soaked in 4% paraformaldehyde for fixation, followed by dehydration in ethanol series, xylene replacement, and paraffin embedding. Paraffin sections (3 μm thick) were then continuously sliced in turn for the HE staining, T-ACP staining, and immunohistochemical staining of Wnt3a and Dkk1.
Detection of serum factors by ELISA
The changes in Wnt3a and Dkk1 were detected according to the kit instructions, and according to the concentrations and corresponding optical density (OD) values of the standards. The linear regression equation of the standard curve was then calculated so as to calculate the corresponding sample concentrations according to their own OD values.
HE staining
The slices were performed in conventional HE staining, and then the consecutive slices with complete dental crown and root system of adjacent teeth were microscopically observed—particularly, the pathological changes of periodontal tissue between the first and second molars, as well as those between the second and third molars.
T-ACP staining
T-ACP straining was performed according to the kit instructions, and the image analysis system was then used to observe the number of osteoclasts on a third of the alveolar surface of the proximal, middle, and distal crest of the second molar, and the number of osteoclasts within one unit length (1 mm) was then calculated.
Immunohistochemical staining of Wnt3a and Dkk1
The slices were performed using routine immunohistochemical staining, and then we observed the positive expressions of Wnt3a and Dkk1 in the bone cells on the third alveolar crest in the inflammatory area of the second molar. The positive cell was defined to appear as precipitate of brown particles in the bone cell cytoplasm. The slices were placed under a Leica DM4000B high-power (×400) microscope (Hangzhou Top instrument Co, Ltd., Hangzhou, China) to accurately position the target view field. The numerical cell images were then extracted by the imaging system, and then we input the data in the HPIA-1000 high-resolution color pathological image analysis system for the processing. Each specimen was randomly selected from three to five nonoverlapping fields of vision, and we determined the average OD value of the immunopositive particles, and calculated the mean. The expressions of Wnt3a and Dkk1 were then semiquantitatively analyzed.
Statistical analysis
SPSS17.0 statistical package (SPSS Inc., Chicago, IL, USA) was used for the analysis and processing of the experimental data. For the data of each index, we first performed the normality test and the homogeneity of variance test; those that met the normal distribution and homogeneity of variance were then subjected to Least significant difference test (one-way analysis of variance), and paired t test was applied to compare their differences, with P < 0.05 considered statistically significant.
Ethics statement
This study was approved by the laboratory animal ethics committee of Kunming Medical University.
Results
Changes in serum Wnt3a level
Four weeks after the modeling, the serum Wnt3a level in group P was significantly lower than that in group N; the difference was statistically significant and showed a time-dependent trend. Moreover, in the 6th week of modeling, the serum Wnt3a level was reduced by approximately 67.3% (Table 1).
Table 1.
Serum Wnt3a level in rat EP model (n = 8, ± SD).
| Time (wk) | SerumWnt3a (mg/L) |
|
|---|---|---|
| N | P | |
| 2 | 1.233 ± 0.032 | 1.057 ± 0.023 |
| 4 | 1.457 ± 0.024 | 0.843 ± 0.057*,** |
| 6 | 1.623 ± 0.052 | 0.531 ± 0.054*,** |
*Compared with the data in the 2nd week of modeling, significantly different, P < 0.05.
**Compared with group N, significantly different, P < 0.05.
EP = experimental periodontitis; SD = standard deviation.
Changes in serum Dkk1 level
Four weeks after the modeling, the serum Dkk1 level in group P was significantly higher than that in group N; the difference was statistically significant, and showed a time-dependent trend. Moreover, in the 6th week of modeling, the serum Dkk1 level was increased by approximately 57.4% (Table 2).
Table 2.
Serum Dkk1 level in rat EP model (n = 8, ± SD).
| Time (wk) | Serum Dkk1 (mg/L) |
|
|---|---|---|
| N | P | |
| 2 | 3.212 ± 1.124 | 3.908 ± 0.923 |
| 4 | 3.021 ± 1.063 | 4.847 ± 1.454*,** |
| 6 | 3.532 ± 1.335 | 5.561 ± 1.857*,** |
*Compared with the data in the 2nd week of modeling, significantly different, P < 0.05.
**Compared with group N, significantly different, P < 0.05.
EP = experimental periodontitis; SD = standard deviation.
HE staining
Based on the results of HE staining, group N showed complete alveolar structure, and no alveolar bone destruction was found; only a few inflammatory cells could be seen. Two weeks after the modeling, the alveolar structure was damaged, and the number of inflammatory cells was increased. Four weeks after the modeling, the alveolar structure showed obvious damage; some parts exhibited bone loss, and the number of inflammatory cells was increased significantly. Six weeks after the modeling, the alveolar structure showed severe damage, together with severe bone loss and a large number of inflammatory cells (Figure 1).
Figure 1.
Histopathological observation of the periodontal tissue of second molar (×100). (A–D) Conditions in group N and those in group P in the 2nd week, 4th week, and 6th week after the modeling. ab = alveolar bone; c = cementum; ct = connective tissue; d = dentin.
Observation of alveolar osteoclasts by T-ACP staining
Two weeks after the modeling, the alveolar osteoclasts level was significantly increased and exhibited statistical significance than that in group N; the alveolar osteoclast level was significantly increased in a time-dependent manner (Table 3, Figure 2).
Table 3.
Number of osteoclasts in the alveolar crest per unit length (n = 8, ± SD).
| Time (wk) | Number of osteoclasts (cells/mm) |
|
|---|---|---|
| N | P | |
| 2 | 2.23 ± 0.38 | 5.56 ± 0.51* |
| 4 | 2.34 ± 0.40 | 7.72 ± 0.57* |
| 6 | 2.26 ± 0.32 | 9.35 ± 0.52*,** |
*Compared with group N, significantly different, P < 0.05.
**Compared with the data in the 2nd week of modeling, significantly different, P < 0.05.
SD = standard deviation.
Figure 2.
Observation of osteoclasts in alveolar crest (×100). (A–D) Conditions in group N and those in group P in the 2nd week, 4th week, and 6th week after the modeling. Green arrows indicate positive osteoclasts. ab = alveolar bone; c = cementum; ct = connective tissue; d = dentin.
Changes of Wnt3a in the alveolar crest
Immunohistochemistry showed that the cytoplasm in group N exhibited numerous brown positive cells, but that in group P showed a time-dependent decrease, and the difference was statistically significant. In the 6th week of modeling, the number of positive cells was reduced by about 45.6% (Table 4, Figure 3).
Table 4.
Optical density of Wnt3a in the bone cells in the third inflammatory area of alveolar crest (n = 8, ± SD).
| Time (wk) | Optical density of positive cells |
|
|---|---|---|
| N | P | |
| 2 | 0.54 ± 0.03 | 0.57 ± 0.03 |
| 4 | 0.53 ± 0.04 | 0.43 ± 0.07*,** |
| 6 | 0.57 ± 0.02 | 0.31 ± 0.01*,** |
*Intragroup comparison at different time points with the data in the 2nd week of modeling, significantly different, P < 0.05.
** Intragroup comparison with group N, significantly different, P < 0.05.
SD = standard deviation.
Figure 3.
Immunohistochemical staining of Wnt3a (×100). (A–D) Conditions in group N and those in group P in the 2nd week, 4th week, and 6th week after the modeling. The arrow indicates the Wnt3a-positive bone cells, with cytoplasm stained tan and nuclei stained blue.
Changes of Dkk1 in the alveolar crest
Immunohistochemistry showed that the cytoplasm in group N exhibited numerous brown positive cells, and that in group P showed a time-dependent increasing trend, and the difference was statistically significant. In the 6th week of modeling, the number of positive cells was increased by about 45.6% (Table 5, Figure 4).
Table 5.
Optical density of Dkk1 in the bone cells in the third inflammatory area of alveolar crest ( ± SD).
| Time (wk) | Optical density of positive cells |
|
|---|---|---|
| N | P | |
| 2 | 0.10 ± 0.01 | 0.13 ± 0.03* |
| 4 | 0.11 ± 0.03 | 0.18 ± 0.04*,** |
| 6 | 0.13 ± 0.02 | 0.21 ± 0.07*,** |
*Compared with group N, significantly different, P < 0.05.
**Compared with the data in the 2nd week of modeling, significantly different, P < 0.05.
SD = standard deviation.
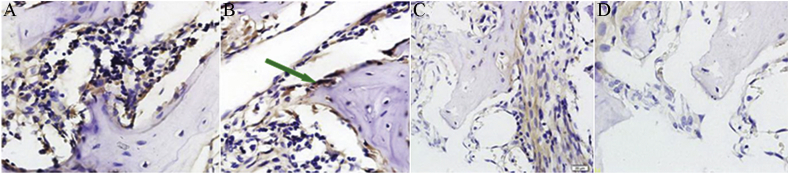
Figure 4.
Immunohistochemical staining of Dkk1 (×100). (A–D) Conditions in group N and those in group P in the 2nd week, 4th week, and 6th week after the modeling; the arrow indicates the Dkk1-positive bone cells, with cytoplasm stained tan and nuclei stained blue.
Discussion
Periodontitis is a class of inflammatory destructive diseases caused by osteoblast-induced osteogenesis reduction and/or osteoclast-induced increase in osteoclasia. During bone remodeling processes, osteoblasts could be migrated to the absorption site, and then secrete bone matrix to form new bones. Furthermore, osteoblasts could regulate the differentiation of osteoclasts via the receptor activator of nuclear factor kappa-B ligand/OPG system, thus affecting the changes of bone mass; therefore, osteoblasts play important roles in the bone remodeling process.
As they perform their functions, osteoblasts would be strictly regulated by multiple signaling pathways, such as the mitogen-activated protein kinase signal, the OPG_receptor activator of nuclear factor kappa-B ligand_receptor activator of nuclear factor kappa-B signal, or the Wnt signal.14, 15 The Wnt signaling pathway is a key factor in regulating the embryonic development and self-renewal and differentiation of stem cells, and is considered one of the most important signaling pathways in organisms, playing significant regulatory roles in all the differentiation aspects of osteoblasts. Studies have found that as endogenous ligands, Wnt7b and Wnt11 regulated the differentiation of osteoblasts,16 and rats with Wnt5a and Wnt3a knockout showed reduced bone mass; on the contrary, rats with high Dkk1 expression exhibited reduced osteogenesis and bone mass, which might be a result of its impacts toward the end-stage differentiation of osteoblasts.17 In rats with Dkk1 knockout, osteogenesis was found to be increased, and the formation of osteophyte was also found.16, 18 Recently, studies have elucidated that the messenger RNA and protein levels of Dkk1 were increased in chronic periodontitis compared to the nonperiodontitis group.19 The studies cited above showed that the Wnt proteins and the antagonizing factor Dkk1 played important roles in bone remodeling. This study detected the expressions of Wnt3a and Dkk1 by immunohistochemistry and ELISA, and found that Wnt3a was significantly decreased in rats with periodontitis in a time-dependent manner, whereas Dkk1 was significantly increased in a time-dependent manner, indicating that in periodontitis mainly characterized by inflammations, Wnt3a and Dkk1 might play important roles.
The Wnt signaling pathway regulates a variety of biological phenomena such as development, self-renewal of stem cells, metabolism, or cancer in almost all animals. Recent studies have found that the Wnt signaling pathway could regulate the proliferation, differentiation, and osteogenesis of osteoblasts, so it has become the therapeutic target of some bone loss diseases, such as osteoporosis.20, 21 The Wnt signal could bind its ligands such as LRP5 and Frizzled receptor, and then activate the classic Wnt signaling pathway so as to mediate the ubiquitination of intracytoplasmic proteins, which would then result in the cytoplasmic accumulation and nuclear translocation of β-catenin, and then exert the related biological effects. Moreover, the Wnt pathway could activate the associated transcription factors, such as Runx2 or Msx2, and then promote the osteogenic differentiation of periodontal ligament fibroblasts.22
In summary, our study showed that the key factors Wnt3a and Dkk1 were abnormally expressed in EP, so regulating the expressions of the key factors in the Wnt signal might be a promising direction for the treatment of periodontitis. However, many factors in the microenvironment of periodontal diseases would coregulate the occurrence of alveolar bone loss, so further studies are necessary to confirm the specific roles and mechanisms of the Wnt signal in periodontitis.
Conflicts of interest
All of the authors declare that they have no conflicts of interest regarding this paper.
Acknowledgments
The current study was supported in part by the National Natural Science Foundation of China (grant number 81060086) and a grant from the Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (number 2010CD215 and 2012FB008).
References
- 1.Assuma R., Oates T., Cochran D., Amar S., Graves D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. [PubMed] [Google Scholar]
- 2.Boyle D.L., Hammaker D., Edgar M. Differential roles of MAPK kinases MKK3 and MKK6 in osteoclastogenesis and bone loss. PLoS One. 2014;9:e84818. doi: 10.1371/journal.pone.0084818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamanaka Y., Clohisy J.C., Ito H., Matsuno T., Abu-Amer Y. Blockade of JNK and NFAT pathways attenuate orthopedic particle-stimulated osteoclastogenesis of human osteoclast precursors and murine calvarial osteolysis. J Orthop Res. 2013;31:67–72. doi: 10.1002/jor.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawadi G., Vayssière B., Dunn F., Baron R., Roman-Roman S. BMP-2 controls alkaline Phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 5.Lu W., Kim K.A., Liu J. R-spondin1 synergizes with Wnt3A in inducing osteoblast differentiation and osteoprotegerin expression. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Walsh N.C., Gravallese E.M. Bone remodeling in rheumatic disease: a question of balance. Immunol Rev. 2010;233:301–312. doi: 10.1111/j.0105-2896.2009.00857.x. [DOI] [PubMed] [Google Scholar]
- 7.Menezes M.E., Devine D.J., Shevde L.A., Samant R.S. Dickkopf1: a tumor suppressor or metastasis promoter? Int J Cancer. 2012;130:1477–1483. doi: 10.1002/ijc.26449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monroe D.G., McGee-Lawrence M.E., Oursler M.J., Westendorf J.J. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morvan F. Deletion of a single allele of the DKK-1 gene leads to an increase in bone formation and bone mass. Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- 10.Gavriatopoulou M., Dimopoulos M.A., Christoulas D. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets. 2009;13:839–848. doi: 10.1517/14728220903025770. [DOI] [PubMed] [Google Scholar]
- 11.Fujita K., Janz S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J., Wu C., Tian B., Zhou X., Ma N., Qian Y. Myricetin prevents alveolar bone loss in an experimental ovariectomized mouse model of periodontitis. Int J Mol Sci. 2016;17:422. doi: 10.3390/ijms17030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branco-de-Almeida L.S., Franco G.C., Castro M.L. Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J Periodontol. 2012;83:664–671. doi: 10.1902/jop.2011.110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen G., Deng C., Li Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce B.F., Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yavropoulou M.P., Yovos J.G. The role of the Wnt signaling pathway in osteoblast commitment and differentiation. Hormones (Athens) 2007;6:279–294. doi: 10.14310/horm.2002.1111024. [DOI] [PubMed] [Google Scholar]
- 17.Qiang Y.W., Barlogie B., Rudikoff S., Shaughnessy J.D., Jr. Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42:669–680. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Westendorf J.J., Kahler R.A., Schroeder T.M. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- 20.Napimoga M.H., Nametala C., Silva F.L. Involvement of the Wnt-β-catenin signalling antagonists, sclerostin and dickkopf-related protein 1, in chronic periodontitis. Journal of clinical periodontology. 2014;41:550–557. doi: 10.1111/jcpe.12245. [DOI] [PubMed] [Google Scholar]
- 21.Rossini M., Gatti D., Adami S. Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int. 2013;93:121–132. doi: 10.1007/s00223-013-9749-z. [DOI] [PubMed] [Google Scholar]
- 22.Heo J.S., Lee S.Y., Lee J.C. Wnt/β-catenin signaling enhances osteoblastogenic differentiation from human periodontal ligament fibroblasts. Mol Cells. 2010;30:449–454. doi: 10.1007/s10059-010-0139-3. [DOI] [PubMed] [Google Scholar]