Abstract
Background/purpose
The two direct dental restorative materials most commonly used today are silver-mercury amalgam and resin-based composites. The purpose of this study was to examine the effects of these two restorative materials and substances released by these into the oral environment on lipid peroxidation and DNA oxidation after entering the blood circulation.
Materials and methods
Blood samples from 41 patients were collected before and 24 hours after the application of these restorative materials. The 8-hydroxydeoxyguanosine/deoxyguanosine ratio in these samples was measured to determine oxidative DNA damage, and malondialdehyde levels were measured to define lipid peroxidation. The paired samples t test and Pearson correlation analysis were used for the analysis of variables (α = 0.05).
Results
While no statistically significant difference was observed after amalgam filling application in terms of DNA oxidation, a significant difference was observed after composite application (P < 0.05). Furthermore, a significant increase was determined in malondialdehyde levels of two materials (P < 0.05). In both amalgam and composite applications, a significant difference was observed before and after application in terms of released substances (mercury and unpolymerized monomer, respectively, P < 0.001).
Conclusion
Mercury increased lipid peroxidation and Bis-GMA and TEGDMA dental resins increased both lipid peroxidation and DNA oxidation markers.
Keywords: 8-hydroxydeoxyguanosine, amalgam, composite resins, malondialdehyde, oxidative stress
Introduction
The two direct dental restorative materials most commonly used today are silver-mercury amalgam and resin-based composites. Amalgam, introduced more than 150 years ago, is the most frequently used material as a tooth filling restoration. Despite the availability of new materials, amalgam remains a popular restorative owing to its wide potential applications, ease of manipulation, adequate mechanical properties, and relatively low cost.1 The use of resin-based restorative materials in dentistry has grown due to better appearance and adhesion to enamel and dentine as well as concerns regarding the deleterious impact of mercury (Hg) from amalgam.2 Hg is a major component (50% by weight) of dental amalgam. Several studies have demonstrated the ability of Hg, similar to that of other metal ions, to interact with soluble and protein bound –SH groups to produce reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide, and hydroxyl radical, and to induce oxidative injury in tissues through various mechanisms, such as lipid peroxidation and DNA damage.1, 3
Previous studies have demonstrated that unpolymerized (co)monomers can be released from resin composites into the oral cavity and/or diffuse through the dentin into the pulp space.4 The most frequently used monomers are bisphenol A-glycidyl methacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA), urethane dimethacrylate (UDMA), and hydroxyethylmethacrylate (HEMA).5 These methacrylate monomers, polymerized through radical chain polymerization, involve major clinical disadvantages, such as polymerization shrinkage of the composites, leading to microleakage phenomena in the tooth–material interface as well as adverse effects caused by substances released from the resinous matrix due to incomplete polymerization or resin degradation.6, 7, 8, 9 As discussed elsewhere, elution of substances from resin composites is usually completed within a few hours or days after initial polymerization. However, leachable substances may also be generated by erosion and degradation over time.9, 10, 11, 12, 13
ROS form as a natural byproduct of the normal metabolism of oxygen, such as electron transport in mitochondria. However, during times of environmental stress (ultraviolet [UV] or heat exposure), ROS levels can rise dramatically. Abnormally high levels of ROS can damage cellular proteins, nucleic acids, and membrane lipids.14 ROS cause strand breaks in DNA, base modifications, and oxidative DNA damage. Oxidative DNA damage is a continual process in vivo, meaning that DNA repair enzymes are constantly required. Studies have also suggested that cumulative lifetime exposure to oxidative DNA may be implicated in the development of cancer. Therefore, oxidative DNA damage should be measured as accurately and quickly as possible. Such damage is generally assessed by measuring 8-hydroxydeoxyguanosine (8-OHdG). The advantage of this assay is that 8-OHdG is a major product of DNA damage and is triggered by attack by numerous ROS.15 ROS also affect lipids in the process known as lipid peroxidation. Lipid peroxides, a well-established mechanism of cellular injury in humans, are used as an indicator of oxidative damage in cells and tissues.16 Lipid peroxides are unstable and decompose to form a complex series of compounds, including reactive carbonyl compounds of which malondialdehyde (MDA) is the most abundant. Therefore, MDA levels are widely used as an indicator of lipid peroxides.17
We focused on TEGDMA and Bis-GMA monomers because recent studies have shown that TEGDMA induces mitochondrial damage and oxidative stress and that Bis-GMA induces DNA double-strand breaks.18, 19 TEGDMA also affects lipid turnover and the metabolic states of the cell.20 To date, studies regarding the effect of these monomers on oxidative damage are insufficient. Therefore, the purpose of this study was to examine the effects of two restorative materials (composite and amalgam) and substances released by these into the oral environment on lipid peroxidation and DNA oxidation after entering the blood circulation.
Materials and methods
The approval for this study was obtained from the Faculty of Dentistry, Atatürk University Review Board and conducted in accordance with the Declaration of Helsinki. From the patients referred to the University, Faculty of Dentistry, 41 young healthy donors (age, 17–23 years) were selected. Written informed consent was obtained from all participants to participate in the study. They had no systemic disorders, such as diabetes mellitus, thyroid function disorders, hepatic or renal dysfunction. To identify local and systemic factors that might affect body Hg concentrations and antioxidant levels, individuals meeting any of the following criteria were excluded from the study: smoking or alcohol habits, occupational exposure to Hg. As per the manufacturer's instructions, 19 amalgam and 22 composite filling were applied to participants. The masses of amalgam and composite fillings were measured as milligram.21
The composite material used for dental restorations was Filtek Z250 (3M ESPE Dental Products, St Paul, MN, USA) color A2, lot number N152614. The composition of the composite resin, as given by the manufacturer, was: TEGDMA 1–5%, Bis-GMA 1–5%, Bis-EMA 5–10%, and UDMA 5–10%. The adhesive Single Bond (3M ESPE Dental Products, St Paul, MN, USA) was used as a bonding agent. The etchant was 38% phosphoric acid (Scotchbond Etchant gel; 3M ESPE). In addition, the amalgam used for dental restorations was Cavex Avalloy (Cavex Co., Holland), lot number 130523. The composition of the amalgam, as given by the manufacturer, was: Ag 45.0%, Sn 30.5%, Cu 24.0%, Zn 0.5%.
Blood samples were collected, using ethylene diamine tetraacetic acid (EDTA) as anticoagulant, from all participants before filling application and 24 hours following completion of the application. Samples were centrifuged at 3500 rpm for 10 minutes at 4°C. The yellow plasma layer was stored at –80°C until MDA analysis, and cells were stored at –80°C for 8-OHdG/dG analysis.
Hg, TEGDMA, and Bis-GMA analysis
Levels of Hg in serum were determined using a Perkin Elmer A-Analyst 800 (Shelton, USA) atomic absorption spectrometer and expressed as μg/L.
TEGDMA and Bis-GMA analyses were performed using the method described by Pelka et al,22 with some modifications. High-pressure liquid chromatography (HPLC) method was used to detect Bis-GMA and TEGDMA. HPLC was performed using Agilent 1100 modular systems (including a gradient pump, auto sampler, column oven, and UV detector). The UV detector was set at 205 nm. Mobile phase A was a mixture of 90% water, 9.8% acetonitrile, and 2% tetrahydrofuran with 25mM/l KH2PO4 adjusted pH = 3. Mobile phase B was a water–acetonitrile mixture at a ratio of 10:90% (vol/vol). All reagents were obtained as HPLC grade. RP-C18 column (250 mm × 4.6 mm × 5.0 μm particle size, Phenomenex, CA, USA) was used for chromatographic separation. The flow rate was constant at 1 mL/minute. HPLC equipment gave us the concentrations of the samples according to the peak areas in parallel with the retention time of the monomers in the column, and the amount of monomers (Bis-GMA and TEGDMA) in serum was calculated directly from the standard calibration curve. Levels of TEGDMA and Bis-GMA were expressed as μM.
Isolation and hydrolyzation of DNA
DNA isolation from blood was performed following the method described by Miller et al23 and Khrosrow and Godwin24 with some modifications. Next, 2 mL blood with EDTA was mixed with 3mL of erythrocyte lysis buffer. Incubation for 10 minutes in ice was followed by centrifugation (10 min at 3500 rpm). The supernatant was decanted, and the pellet was thoroughly resuspended in sodium dodecyl sulfate (10%, v/v), proteinase K (20 mg/mL), and 1.9 mL leukocyte lysis buffer. The mixture was incubated at 65°C for 1 hour and then mixed with 0.8 mL of 9.5M ammonium acetate. After centrifugation at 3500 rpm for 25 minutes, the clear supernatant (2 mL) was transferred to a new sterile tube, and DNA was precipitated by the addition of 4 mL of ice-cold absolute ethanol. DNA samples were dissolved in Tris EDTA buffer (10mM, pH = 7.4) and were hydrolyzed according to the method described by Kaur and Halliwell.15
Analysis of 8-OHdG and dG using HPLC
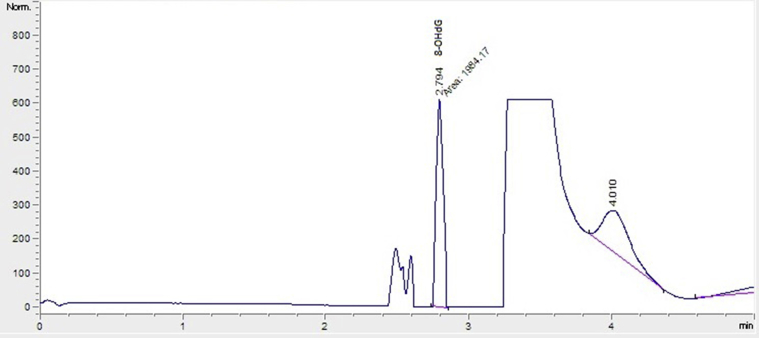
8-OHdG and dG levels in the hydrolyzed DNA samples were measured using HPLC with electrochemical (HPLC-ECD) and variable wavelength detector (HPLC-UV) systems, as previously described.25 Before analysis by HPLC, the hydrolyzed DNA samples were redissolved in the HPLC eluent (final volume 1 mL). Final hydrolysate (20 μL) was analyzed using HPLC-ECD (HP, Agilent 1100 modular systems with HP 1049A ECD detector, Germany): column, reverse phase-C18 (RP-C18) analytical column (250 mm × 4.6 mm × 4.0 μm, Phenomenex, CA, USA). The mobile phase consisted of 0.05M potassium phosphate buffer (pH 5.5) containing acetonitrile (97:3, v/v) with a flow rate of 1 mL/minute. The dG concentration was monitored based on absorbance (245 nm) and 8-OHdG was based on the electrochemical reading (600 mV). Levels of dG and 8-OHdG were quantified using the standards of dG and 8-OHdG from Sigma; the level of 8-OHdG level was expressed as the number of 8-OHdG molecules per 106 dG.26 Chromatogram of the standard monomer sample of 8-OHdG is shown in Figure 1.
Figure 1.
Chromatogram of the standard monomer sample of 8-OHdG. 8-OHdG = 8-hydroxydeoxyguanosine.
Analysis of plasma for MDA using HPLC
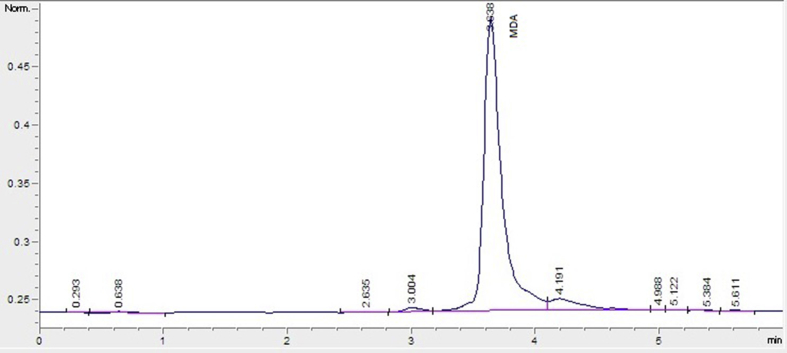
MDA concentrations in blood plasma samples were measured using HPLC with fluorescent detection (HPLC-FLD), as previously described.27 Briefly, 50 μL of plasma sample was mixed with 0.44M H3PO4 and 42mM thiobarbituric acid (TBA) and incubated for 30 minutes in a boiling water bath. After rapid cooling on ice, an equal volume of alkaline methanol was added to the sample, which was vigorously shaken and centrifuged (3000 rpm for 3 minutes), and the aqueous layer was removed. Then, 20 μL supernatant was analyzed using HPLC (HP, Agilent 1100 modular systems with FLD detector, Germany): column, RP-C18 (5 μm, 4.6 × 150 mm, Eclipse VDB- C18. Agilent); elution, methanol (40:60, v/v) containing 50mM KH2PO4 buffer (pH = 6.8); flow rate, 0.8 mL/minute. Fluorometric detection was performed with excitation at 527 nm and emission at 551 nm. The peak of the MDA-TBA adduct was calibrated as a 1,1,3,3- tetraethoxypropane standard solution using the same process as with the plasma sample. Chromatogram of the standard monomer sample of MDA is shown in Figure 2.
Figure 2.
Chromatogram of the standard monomer sample of malondialdehyde.
Statistical analyses were performed using SPSS for Windows (version 16.0, SPSS Inc., Chicago, IL, USA). Statistical significance was calculated using the paired samples t test. All results were expressed as mean values with standard deviation (mean ± SD). Correlation analysis (Pearson correlation analysis) was also used to examine association between features. The critical value (α) was set at 0.05.
Results
While no statistical significance was observed before and after amalgam filling application in terms of DNA oxidation (1.26 ± 0.16 and 1.38 ± 0.46, respectively), MDA concentrations differed significantly (5.64 ± 2.53μM and 9.78 ± 3.50μM, respectively, P < 0.001; Table 1). A significant difference was observed before and after composite application in terms of DNA oxidation (1.28 ± 0.23 and 1.46 ± 0.31, respectively, P < 0.05). A statistically significant increase was also determined in MDA concentrations (6.59 ± 3.37μM and 7.94 ± 5.24 μM, respectively; Table 1).
Table 1.
Mean ± SD values of MDA and 8-OHdG/106 dG quantified in samples baseline and 24 hours after application.
| 8-OHdG/106 dG | MDA (μM) | ||
|---|---|---|---|
| Amalgam (n = 19) | Before application | 1.26 (0.16) | 5.64 (2.53) |
| After application | 1.38 (0.46) | 9.78 (3.50)** | |
| Composite (n = 22) | Before application | 1.28 (0.23) | 6.59 (3.37) |
| After application | 1.46 (0.31)* | 7.94 (5.24)* |
*P < 0.05, **P < 0.001.
dG = deoxyguanosine; MDA = malondialdehyde; 8-OHdG = 8-hydroxydeoxyguanosine; SD = standard deviation.
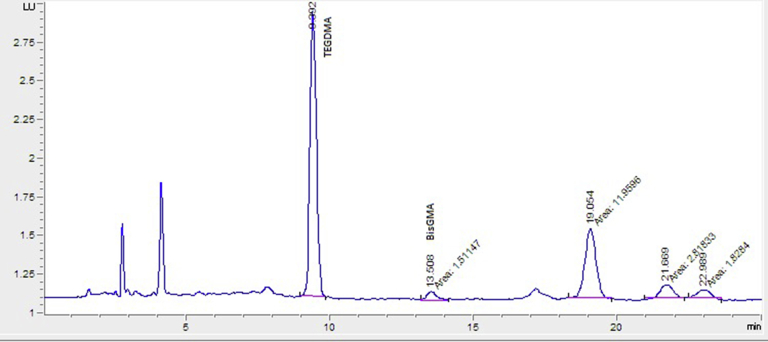
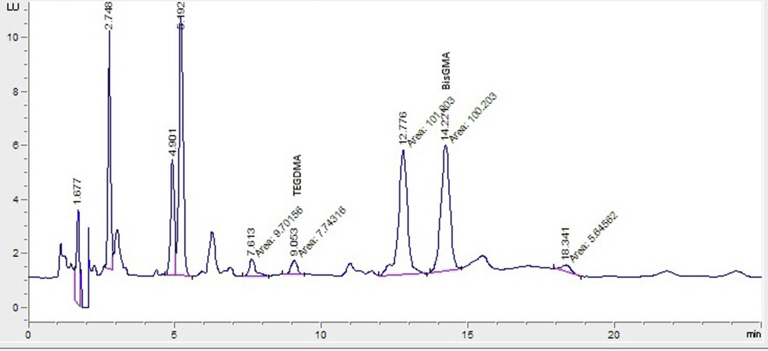
The retention times of TEGDMA and Bis-GMA monomer standard substances were designated as 9.392 minutes and 13.508 minutes, respectively (Figure 3). The chromatogram of monomers obtained from a patient was shown in Figure 4. In this study, the Bis-GMA and TEGDMA released into serum were determined after composite application as 1.93 ± 0.56 μM and 2.74 ± 0.81 μM, respectively. In addition, a significant difference was observed before and after amalgam application in terms of Hg release (1.77 ± 0.83 μg/L and 5.21 ± 2.42 μg/L, respectively, P < 0.001; Table 2).
Figure 3.
Chromatogram of the standard monomer sample of TEGDMA and Bis-GMA. Bis-GMA = bisphenol A-glycidyl methacrylate; TEGDMA = triethylene glycol dimethacrylate.
Figure 4.
Chromatogram of the obtained sample from the patient 1 day after treatment for TEGDMA and Bis-GMA. Bis-GMA = bisphenol A-glycidyl methacrylate; TEGDMA = triethylene glycol dimethacrylate.
Table 2.
Mean ± SD values of Hg, Bis-GMA, and TEGDMA quantified in samples baseline and 24 hours after application.
| Hg (μg/L) | BisGMA (μM) | TEGDMA (μM) | ||
|---|---|---|---|---|
| Amalgam (n = 19) | Before application | 1.77 (0.83) | ND | ND |
| After application | 5.21 (2.42)* | ND | ND | |
| Composite (n = 22) | Before application | 1.28 (0.36) | ND | ND |
| After application | 1.54 (0.60) | 1.93 (0.56) | 2.74 (0.81) |
*P < 0.001
Bis-GMA = bisphenol A-glycidyl methacrylate; Hg = mercury; ND = none detected; SD = standard deviation; TEGDMA = triethylene glycol dimethacrylate.
In both amalgam and composite applications, a significant difference was observed before and after application in terms of released substances (Hg and unpolymerized monomer, respectively, P < 0.001; Table 2). Amalgam and composite filling masses used in this study were found as 0.11 ± 0.10 g and 0.08 ± 0.05 g, respectively. No correlation was determined between amalgam filling mass and released Hg or between composite filling masses and amounts of released monomers (P > 0.05).
Discussion
Hg (released from amalgam fillings) and/or (co)monomers (released from composites) have been shown to be capable of entering the organism via oral and/or other tissues. They may also cause some adverse effects.8 Dental amalgam is the main source of elemental Hg in the general population.28 However, Hg does not accumulate irreversibly in human tissues. The average half-life of Hg is 55 days for transport through the body from entry to excretion. Thus, it can be said that Hg which had entered the body many years ago cannot exist in the body now.29 In agreement with some previous studies, we found no correlation between amalgam filling mass and released Hg.30, 31
Exposure to Hg in individuals with amalgam restoration occurs during the placement or removal of dental restorations. Once the reaction is complete, less Hg is released, the amount concerned being considerably below the current health standard.29 Following an assessment of numerous longitudinal studies involving blood samples in order to calculate Hg in the long term, the German MAK-Commission (whose remit was to establish occupational exposure thresholds without any health risks) reported that even urinary Hg levels of 100 μg/L or more resulting from many years of exposure produce no objective adverse effects. Urinary Hg levels were equivalent to those in blood at approximately 23 μg/L.32 Therefore, the BAT value (highest permissible concentrations of hazardous compounds or metabolites thereof in body fluids) was set at 100 μg/L of urine or 25 μg/L of blood. This is regarded as a no-adverse-effect-concentration for Hg in humans. Hg concentrations in the whole blood of individuals with or without amalgam fillings are usually below 5 μg/L blood.33, 34 Reference values for total Hg concentrations in biological media for the general population are set at whole blood 1–8 μg/L.35 Hg levels from amalgam fillings in our study were similar to these values. Dental amalgam restorations may increase these levels slightly, but this is of no practical or clinical significance.
MDA levels in this study were significantly higher in the amalgam filling group than baseline (P < 0.001). We concluded that amalgam filling increases lipid peroxidation although Hg levels from amalgam are not critical levels. Although Al-Saleh et al36 reported that amalgam which includes Hg increases oxidative damage, no difference was determined in MDA concentrations. They analyzed MDA in urine and described the result as proportional to creatinine levels.36 This may be due to creatinine levels being higher in the amalgam group than in the other group. Comparison of pre- and post-filling Hg levels revealed a significant increase in Hg levels; however, no correlation was determined between Hg levels and MDA concentration.
The 8-OHdG/106dG ratio in the amalgam group in the present study increased after application but not significantly. This suggests that the Hg released from the amalgam filling does not increase oxidative damage in DNA. Al-Saleh et al36 reported significantly lower 8-OHdG concentrations in the amalgam filling group than in children not receiving amalgam filling. This may be because those authors investigated 8-OHdG concentrations using the enzyme-linked immunosorbent assay (ELISA) method. In contrast, we investigated 8-OHdG and dG concentrations using the HPLC method, which is much more sensitive than ELISA.37
Resin monomers are usually used in composite fillings, the most common being TEGDMA, Bis-GMA, HEMA, and UDMA.5, 38 Studies have shown that TEGDMA depletes glutathione, which plays a more significant role than the oxidant-antioxidant balance.39, 40, 41 In our previous study,42 we examined oxidative damage in rabbit connective tissues and erythrocytes caused by resin filling materials. We determined that antioxidant enzyme activities such as superoxide dismutase decreased and MDA concentrations increased on the 7th day following application. In the present study, Bis-GMA and TEGDMA monomers also increased MDA concentrations, and the difference was statistically significant (P < 0.05). The results of these two studies show that composite filling also increases oxidative stress. In our other study,21 monomers released into saliva after the application of composite filling material were measured at various time intervals, and salivary MDA and some antioxidant enzyme levels were also assessed. MDA levels at all time intervals were significantly higher (P < 0.05) than baseline levels. The results from these studies are in accordance with the present study. Additionally, another study by Daokar et al43 found that the salivary levels of MDA increased significantly after amalgam for up to 2 weeks; however, no significant changes in salivary MDA was observed after composite and glass ionomer cement restoration. Therefore, the oxidative stress markers after short- and long-term exposure to restorative materials remain controversial.
In the present study, the 8-OHdG/106 dG ratio significantly increased postapplication in the composite filling group compared with the amalgam filling group (P < 0.05). Some previous research is compatible with ours, but the oxidative damage caused by resin monomers in DNA was evaluated using the single cell microgel electrophoresis (comet) assay method in those studies.44, 45 We determined 8-OHdG/106dG levels to reveal the oxidative damage caused by Bis-GMA and TEGDMA in DNA. This is used as an indicator of 8-OHdG genotoxicity.46 The increase in the 8-OHdG/106dG ratio postapplication in the composite filling group suggests that the monomers in the composite filling increased DNA oxidation. Our results are significant in that oxidization and transformation of the bases in DNA can lead to advanced complications. Previous results show that Bis-GMA inhibits DNA synthesis.44, 47 TEGDMA has also been reported to have a dose-dependent mutagenic effect on mammalian cell cultures.48 Previous studies suggest that resin monomers damage DNA, and our results support this thesis. Most studies have been performed under in vitro conditions. In their study on cell culture, Lefeuvre et al18 reported that TEGDMA was an important target for mitochondria in the cell and was a source of oxidative damage. Our examination of monomer concentrations using HPLC revealed significantly higher released TEGDMA concentrations than Bis-GMA concentrations (P < 0.001).
A significant (P < 0.05) increase in (co)monomers and Hg toxicity was determined in the order HEMA < TEGDMA < UDMA < Bis-GMA. HEMA and TEGDMA were up to 133-fold less toxic than Bis-GMA. The greater cytotoxicity of Bis-GMA than other (co)monomers may depend on its higher liposolubility than, for example, HEMA (the octanol/water partition of Bis-GMA is approximately 10 times higher than that of HEMA).49 The increased (co)monomer toxicity in the order HEMA < TEGDMA < UDMA < Bis-GMA may also be attributed to the increased molecular weights of these (co)monomers (HEMA = 130 Da, TEGDMA = 286 Da, UDMA = 471 Da, and Bis-GMA = 512 Da). The higher the molecular weight, the greater the cytotoxicity.50 TEGDMA emerged as cytotoxic and “apoptotic” in a dose- and time-dependent manner. TEGDMA at 5mM and 7.5mM inhibited proliferation and caused apoptosis, whereas no apoptosis or necrosis was observed at 1mM or 2.5mM. Bis-GMA also induced a rapid and intense decline of the glutathione pool of human gingival fibroblasts combined with the induction of apoptosis at much lower concentrations (>0.1mM) compared with TEGDMA (>5mM).51 However, TEGDMA and Bis-GMA levels in the present study were much lower than these levels (2.74 ± 0.81 μM and 1.93 ± 0.56 μM, respectively).
The intracellular levels of ROS (H2O2, superoxide anion, and OH radical) increased after exposure to HEMA, TEGDMA, or composite resin eluates.9 The prevailing role of radical species in genotoxicity elicited by amalgams and methacrylic compounds has been confirmed using ROS scavengers, such as N-acetylcysteine, ascorbate, and vitamin E, which are able to inhibit the biological effects of these compounds. Composites giving rise to the DNA damage observed may perhaps be attributed to deterioration of the cellular pro- and anti-oxidant redox balance.1 In vitro results do not always reflect the in vivo situation despite being carried out under strictly controlled conditions.52
Within the limitations of this study, amalgam and composite filling materials may both cause oxidative stress. In addition, Hg increased lipid peroxidation and Bis-GMA and TEGDMA increased both lipid peroxidation and DNA oxidation markers.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This study was presented at the FDI World Dental Federation Annual World Dental Congress- August 28/31, 2013 Istanbul, Turkey.
References
- 1.Pietro A.D., Visalli G., Maestra S.L. Biomonitoring of DNA damage in peripheral blood lymphocytes of subjects with dental restorative fillings. Mutat Res. 2008;650:115–122. doi: 10.1016/j.mrgentox.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Moharamzadeh K., Brook I.M., Van Noort R. Biocompatibility of resin-based dental materials. Materials. 2009;2:514–548. [Google Scholar]
- 3.Stohs S.J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 4.Noda M., Wataha J.C., Kaga M., Lockwood P.E., Volkmann K.R., Sano H. Components of dentinal adhesives modulate heat shock protein 72 expression in heat-stressed THP-1 human monocytes at sublethetal concentrations. J Dent Res. 2002;81:265–269. doi: 10.1177/154405910208100408. [DOI] [PubMed] [Google Scholar]
- 5.Geurtsen W. Substances released from dental resin composites and glass ionomer cements. Eur J Oral Sci. 1998;106:687–695. doi: 10.1046/j.0909-8836.1998.eos10602ii04.x. [DOI] [PubMed] [Google Scholar]
- 6.Silicas N., Eliades G., Watts D.C. Light intensity effects on resin composite degree of conversion and shrinkage strain. Dent Mater. 2000;16:292–296. doi: 10.1016/s0109-5641(00)00020-8. [DOI] [PubMed] [Google Scholar]
- 7.Braga R.R., Ballester R.Y., Ferracane J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21:962–970. doi: 10.1016/j.dental.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Reichl F.X., Esters M., Simon S. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch Toxicol. 2006;80:370–377. doi: 10.1007/s00204-005-0044-2. [DOI] [PubMed] [Google Scholar]
- 9.Bakopoulou A., Papadopoulos T., Garefis P. Molecular toxicology of substances released from resin–based dental restorative materials. Int J Mol Sci. 2009;10:3861–3899. doi: 10.3390/ijms10093861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferracane J.L. Elution of leachable components from composites. J Oral Rehabil. 1994;21:441–452. doi: 10.1111/j.1365-2842.1994.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 11.Santerre J.P., Shajii L., Leung B.W. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–151. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 12.Hume W.R., Gerzina T.M. Bioavailability of components or resin-based materials which are applied to teeth. Crit Rev Oral Biol Med. 1996;7:172–179. doi: 10.1177/10454411960070020501. [DOI] [PubMed] [Google Scholar]
- 13.Eliades G., Eliades T., Vavuranakis M. Quintessence Publishing Co Inc.; Chicago, USA: 2003. General Aspects of Biomaterial Surface Alterations Following Exposure to Biologic Fluids; pp. 3–20. [Google Scholar]
- 14.McCord J.M., Fridovich I. The biology and pathology of oxygen radicals. Annals Intern Med. 1978;89:122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- 15.Kaur H., Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J. 1996;318:21–23. doi: 10.1042/bj3180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irshad M., Chaudhuri P.S. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–1239. [PubMed] [Google Scholar]
- 17.Lykkesfeldt J. Malondialdehyde as biomarker of oxidative damage to lipids caused by smoking. Clin Chim Acta. 2007;380:50–58. doi: 10.1016/j.cca.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Lefeuvre M., Amjaad W., Goldberg M., Stanislawski L. TEGDMA induces mitochondrial damage and oxidative stress in human gingival fibroblasts. Biomaterials. 2005;26:5130–5137. doi: 10.1016/j.biomaterials.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Blasiak J., Synowiec E., Tarnawska J., Czarny P., Poplawski T., Reiter R.J. Dental methacrylates may exert genotoxic effects via the oxidative induction of DNA double strand breaks and the inhibition of their repair. Mol Biol Rep. 2012;39:7487–7496. doi: 10.1007/s11033-012-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelmann J., Leyhausen G., Leibfritz D., Geurtsen W. Metabolic effects of dental resin components in vitro detected by NMR spectroscopy. J Dent Res. 2001;80:869–875. doi: 10.1177/00220345010800030501. [DOI] [PubMed] [Google Scholar]
- 21.Gul P., Akgul N.A., Alp H.H., Kiziltunc A. Effects of composite restorations on oxidative stress in saliva: an in vivo study. J Dent Sci. 2015;10:394–400. [Google Scholar]
- 22.Pelka M., Distler W., Petschelt A. Elution parameters and HPLC-detection of single components from resin composite. Clin Oral Invest. 1999;3:194–200. doi: 10.1007/s007840050101. [DOI] [PubMed] [Google Scholar]
- 23.Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khrosrow A., Godwin O. Rapid purification of human DNA from whole blood for potential application in clinical chemistry laboratories. Clin Chem. 1990;36:261–264. [PubMed] [Google Scholar]
- 25.Shigenaga M.K., Aboujaoude E.N., Chen Q., Ames B.N. Assays of oxidative DNA damage biomarkers 8-oxo-2′-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- 26.Kaya Y., Çebi A., Söylemez N., Demir H., Alp H.H., Bakan E. Correlations between oxidative DNA damage, oxidative stress and coen-zyme Q10 in patients with coronary artery disease. Int J Med Sci. 2012;9:621–626. doi: 10.7150/ijms.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoschsorur G.A., Winklhofer-Roob B.M., Rabl H., Auer T., Peng Z., Schaur R.J. Evaluation of a sensitive HPLC method for the determination of malondialdehyde, and application of the method to different biological materials. Chromatographia. 2000;52:181–184. [Google Scholar]
- 28.Brownawell A.M., Berent S., Brent R.L. The potential adverse health effects of dental amalgam. Toxicol Rev. 2005;24:1–10. doi: 10.2165/00139709-200524010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Roberson T.M., Heymann H.O., Swift E.J. Mosby Inc; Missouri: 2006. Sturdevant's Art and Science of Operative Dentistry. [Google Scholar]
- 30.Molin M., Bergman B., Marklund S., Schütz A., Skerfving S. The influence of dental amalgam placement on mercury, selenium and glutathione peroxidase in man. Acta Odontol Scand. 1990;48:287–295. doi: 10.3109/00016359009005887. [DOI] [PubMed] [Google Scholar]
- 31.Berglund A., Molin M. Mercury vapor release from dentalamalgams in patients with symptoms allegedly caused by amalgam fillings. Eur J Oral Sci. 1996;104:56–63. doi: 10.1111/j.1600-0722.1996.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 32.MAK Kommission der Deutschen Forschungsgemeinschaft (DFG) Mercury and inorganic mercury compounds. In: Greim H., editor. Occupational Toxicants – Critical Data Evaluation for MAK Values and Classification of Carcinogens by the Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area. Vol. 15. Wiley-VCH; München: 1999. pp. 81–122. [Google Scholar]
- 33.ATSDR (Agency for Toxic Substances Disease Registry). Toxicological profile for mercury. Update. Atlanta-GA, Retrieved online November 1, 2008 from. 1999. URL: http://www.atsdr.cdc.gov/toxprofiles/tp46.html.
- 34.BAT Kommission der Deutschen Forschungsgemeinschaft (DFG) Mercury, metallic mercury and inorganic mercury compounds. In: Triebig G., Schaller K.-H., editors. Analyses of Hazardous Substances in Biological Material. Wiley-VCH; München: 1997. pp. 123–142. [Google Scholar]
- 35.WHO . World Health Organization; Geneva: 1990. Environmental Health Criteria 101: Methylmercury. [Google Scholar]
- 36.Al-Saleh I., Al-Sedairi A., Elkhatib R. Effect of mercury (Hg) dental amalgam fillings on renal and oxidative stress biomarkers in children. Sci Total Environ. 2012;431:188–196. doi: 10.1016/j.scitotenv.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Shimoi K., Kasai H., Yokota N., Toyokuni S., Kinae N. Comparison between high-performance liquid chromatography and enzyme-linked Immunosorbent assay for the determination of 8-hydroxy-2′-deoxyguanosine in human urine. Cancer Epidemiol Biomarkers Prev. 2002;11:767–770. [PubMed] [Google Scholar]
- 38.Kaga M., Noda M., Ferracane J.L., Nakamura W., Oguchi H., Sano H. The in vitro cytotoxicity of eluates from dentin bonding resins and their effect on tyrosine phosphorylation of L929 cells. Dent Mater. 2001;17:333–339. doi: 10.1016/s0109-5641(00)00091-9. [DOI] [PubMed] [Google Scholar]
- 39.Engelmann J., Leyhausen G., Leibfritz D., Geurtsen W. Effect of TEGDMA on the intracellular glutathione concentration of human gingival fibroblasts. J Biomed Mater Res. 2002;63:746–751. doi: 10.1002/jbm.10465. [DOI] [PubMed] [Google Scholar]
- 40.Stanislawski L., Lefeuvre M., Bourd K., Soheili-Majd E., Goldberg M., Perianin A. TEGDMA-induced toxicity in human fibroblasts is associated with early and drastic glutathione depletion with subsequent production of oxygen reactive species. J Biomed Mater Res Part A. 2003;66A:476–482. doi: 10.1002/jbm.a.10600. [DOI] [PubMed] [Google Scholar]
- 41.Volk J., Engelmann J., Leyhausen G., Geurtsen W. Effects of three resin monomers on the cellular glutathione concentration of cultured human gingival fibroblasts. Dent Mater. 2006;22:499–505. doi: 10.1016/j.dental.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Yildiz M., Taysi S., Yildiz A., Bakan N., Akcay F., Kuskay S. Effect of composite resin filling material implantation into connective tissue of rabbits on oxidative stress in erythrocytes. Pain Clin. 2004;16:323–328. [Google Scholar]
- 43.Daokar S., Siddiqui S., AlJeaidi Z.A., Mustafa M., Mapari P.S., Nadeem F. Assessment of oxidative stress induced by various restorative materials: an in vivo biochemical study. J Int Oral Health. 2016;8:670–674. [Google Scholar]
- 44.Kleinsasser N.H., Wallner B.C., Harreus U.A. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent. 2004;32:229–234. doi: 10.1016/j.jdent.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Kleinsasser N.H., Schmid K., Sassen A.W. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials. 2006;27:1762–1770. doi: 10.1016/j.biomaterials.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Ogura H., Takeuchi T., Morimoto K. A comparison of the 8-hydroxydeoxyguanosine, chromosome aberrations and micronucleus techniques for the assessment of the genotoxicity of mercury compounds in human blood lymphocytes. Mutat Res Rev Genet. 1996;340:175–182. doi: 10.1016/s0165-1110(96)90047-0. [DOI] [PubMed] [Google Scholar]
- 47.Heil J., Reifferscheid G., Waldmann P., Leyhausen G., Geurtsen W. Genotoxicity of dental materials. Mutat Res Genet Tox. 1996;368:181–194. doi: 10.1016/s0165-1218(96)90060-9. [DOI] [PubMed] [Google Scholar]
- 48.Schweikl H., Schmalz G., Rackebrandt K. The mutagenic activity of unpolymerized resin monomers in Salmonella typhimurium and V79 cells. Mutat Res Genet Tox En. 1998;415:119–130. doi: 10.1016/s1383-5718(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 49.Issa Y., Watts D.C., Brunton P.A., Waters C.M., Duxbury A.J. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater. 2004;20:12–20. doi: 10.1016/s0109-5641(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 50.Geurtsen W., Lehman F., Spahl W., Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3- and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–480. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 51.Engelmann J., Janke V., Volk J. Effects of Bis-GMA on glutathione metabolism and apoptosis in human gingival fibroblasts invitro. Biomaterials. 2004;25:4573–4580. doi: 10.1016/j.biomaterials.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 52.Kasacka I., Łapińska J. Salivary cells in patients with dental amalgam and composite resin material restorations –A morphological investigation. Pol J Environ Stud. 2010;19:1223–1227. [Google Scholar]