Abstract
Introduction
Although emergency medicine (EM) training programmes have begun to be introduced in low- and middle-income countries (LMICs), minimal data exist on their effects on patient-centered outcomes in such settings. This study evaluated the impact of EM training and associated systems implementation on mortality among patients treated at the University Teaching Hospital-Kigali (UTH-K).
Methods
At UTH-K an EM post-graduate diploma programme was initiated in October 2013, followed by a residency-training programme in August 2015. Prior to October 2013, care was provided exclusively by general practice physicians (GPs); subsequently, care has been provided through mutually exclusive shifts allocated between GPs and EM trainees. Patients seeking Emergency Centre (EC) care during November 2012–October 2013 (pre-training) and August 2015–July 2016 (post-training) were eligible for inclusion. Data were abstracted from a random sample of records using a structured protocol. The primary outcomes were EC and overall hospital mortality. Mortality prevalence and risk differences (RD) were compared pre- and post-training. Magnitudes of effects were quantified using regression models to yield adjusted odds ratios (aOR) with 95% confidence intervals (CI).
Results
From 43,213 encounters, 3609 cases were assessed. The median age was 32 years with a male predominance (60.7%). Pre-training EC mortality was 6.3% (95% CI 5.3–7.5%), while post-training EC mortality was 1.2% (95% CI 0.7–1.8%), constituting a significant decrease in adjusted analysis (aOR = 0.07, 95% CI 0.03–0.17; p < 0.001). Pre-training overall hospital mortality was 12.2% (95% CI 10.9–13.8%). Post-training overall hospital mortality was 8.2% (95% CI 6.9–9.6%), resulting in a 43% reduction in mortality likelihood (aOR = 0.57, 95% CI 0.36–0.94; p = 0.016).
Discussion
In the studied population, EM training and systems implementation was associated with significant mortality reductions demonstrating the potential patient-centered benefits of EM development in resource-limited settings.
Keywords: Emergency medicine, Training, Mortality, Rwanda, Africa
African relevance
-
•
Emergency Centre mortality in Africa is higher than the global average
-
•
Minimal data exists on emergency medicine training effects on patient-centered outcomes in Africa
-
•
Initiating emergency medicine training in Kigali, Rwanda resulted in a 43% reduction in overall hospital mortality.
-
•
Emergency medicine training programmes may provide significant patient-centered benefits in African and similar contexts.
Introduction
Emergency care provided by residency-trained emergency medicine (EM) physicians has been associated with improved outcomes in high-income countries (HICs) [1]. In HIC trauma centers with EM residency training programme, fewer complications and overall shorter hospital stays have been documented as compared to centres without training programmes [2]. While EM training is robust in many HICs, formalised EM training and associated care in the majority of low- and middle-income countries (LMICs) is incipient in implementation [3].
Emergency Centre (EC) mortality in LMICs is high, with a meta-analysis demonstrating a median mortality prevalence of 1.8% [3]. Reasons for this are multifactorial and include higher burdens of under-nutrition [4], infectious diseases and injuries [5], [6], systems barriers, and insufficient numbers of trained emergency care personnel in LMICs [3], [7]. As health burdens are projected to increase disproportionately in LMICs [8], [9], there is consensus from advisory bodies that strengthened emergency care systems are crucially needed [10]. Thus, some LMICs have prioritised improving emergency care services [11], [12].
Although there is limited data, focused improvements have been achieved in the provision of emergency care in LMICs. Development and implementation of triage systems have been associated with decreased inpatient mortality in LMIC settings [13], and focused courses for injuries have been shown to facilitate improved outcomes in select countries [14]. Based on these data, some LMICs have initiated formalised EM training programmes. However, reports on these programmes tend to be descriptive and rarely include outcomes data [15], [16].
Rwanda is a LMIC in sub-Saharan Africa that has radically transformed its healthcare system through the creation of national insurance [17], community health expansion [18], and HIV treatment [19]. One of the nation’s recent health initiatives was the establishment of the first EM training programme at the University Teaching Hospital-Kigali (UTH-K). This study aimed to assess the impact of the introduction of formalised EM training and associated emergency care system implementation on patient-centered outcomes among cases treated at the UTH-K EC.
Methods
This retrospective interrupted time-series study was carried out at the UTH-K in Kigali, Rwanda. All patients presenting to the EC during the periods of November 2012 through October 2013 (pre-EM training implementation) and August 2015 through July 2016 (post-EM training implementation) were eligible for inclusion. Cases without identifiable medical records or lacking EC record documentation for the encounter of interest were excluded. The interrupted time-series approach was chosen as it is a validated method to evaluate healthcare improvement strategies while controlling for secular trends [20], [21]. The research activities were approved by the UTH-K ethics committee and the institutional review board of Rhode Island Hospital.
The UTH-K is the national public referral health center for Rwanda. The facility is a tertiary-care institution with approximately 40 EC and 500 inpatient beds with access to specialty and diagnostic services. As part of the Human Resources for Health (HRH) programme, which partnered academic medical centers in the United States with the Ministry of Health (MoH) to enhance the workforce across multiple specialties in Rwanda [22], an EM post-graduate diploma (PGD) programme was begun at the UTH-K in October 2013 with clinical rollout in November of that year. The PGD programme was transitioned into a comprehensive four-year EM residency-training programme in August 2015. Prior to initiation of the EM training programmes, EC care was provided exclusively by general practice physicians (GPs). Since the initiation of EM training, EC staffing has been oriented with specific shifts allocated between GPs and EM residents, the latter of which functioned with oversight by board certified international EM physicians [22], [23]. The international faculty precept all EM resident cases but do not serve as primary treating providers. During the November 2012–October 2013 study period (pre-EM training), twelve GPs were staffed at the UTH-K EC 24-hours a day. For the August 2015 through July 2016 study period (post-EM training), EC care was delivered by seven independently practicing GPs and fourteen EM residents with faculty oversight. Shifts for each provider type were staffed only by the specific type of provider (i.e. GP or EM resident providers).
Aside from the implementation of the EM training programmes, resources available for emergency care in the pre- and post-EM training periods were similar. No significant changes were made in the physical structure of the EC or the availability of equipment/supplies. The one exception was the introduction of point-of-care ultrasound (POCUS), which was phased in as EM residents were trained in its usage. In addition, several EM systems changes were passed in over time in conjunction with EM training implementation, including formalised triage, team-based resuscitation and the designation of higher acuity areas in the EC [23], [24].
Cases were identified and data were queried from institutional records via protocolised methods, as previously described [25], [26], [27], [28]. Briefly, using a multipoint composite index generated from an electronic hospital database, all EC cases during each month of the accruement periods were identified. Subsequently, all cases were coded with a unique identification number and were sampled at random until a sufficient number of records meeting inclusion criteria were identified (range: 135–165 records per month). Protocol-trained personnel abstracted data using a standardised instrument. Data procedures conformed to quality practices for chart review research [29].
Data included demographics, prior medical history, provider type, clinical presentation, case type, EC care, diagnoses, length of stay (LOS), and EC and inpatient outcomes. Clinical presentation included data on triage vital signs and mental status (using the Alert/Verbal/Pain/Unresponsive [AVPU] scale) [30]. Case types were coded as medical or injury. Provider type was defined based on the practitioner that treated each patient at presentation and was coded as either GP or EM resident. Time and date of first EC contact was used to categorise cases as treated during day (07 h00-19 h29 hours) or night (19 h30-06 h59 hours) and on weekdays (Monday through Friday) or weekend days (Saturday or Sunday). Data were entered into an electronic password-protected database [31]. Ten percent of included records were randomly selected and re-entered by personnel blinded to the initial abstraction.
Data analysis was performed using STATA version 15.0 (StataCorp; College Station, USA). Descriptive analyses were undertaken for the overall cohort and stratified by time period as pre and post-EM training implementation. Variables were described using frequencies with percentages or medians with associated interquartile ranges (IQR). Characteristics based on time period were compared using Pearson X2 tests for categorical variables and by Mann-Whitney or t-tests for non-normally and normally distributed continuous variables, respectively. To account for multiple testing, a significance level of p < 0.004 was utilised in analyses [32].
The primary outcome of interest was all-cause mortality. Discrete mortality outcomes were evaluated during EC care (all cases) and inpatient care (admitted cases) periods. As well, overall mortality, (aggregating EC and inpatient outcomes), was used. The primary predictor variable was the period of care defined as the reference of pre-EM training (November 2012–October 2013) or comparator of post-EM training and systems implementation (August 2015–July 2016).
Mortality outcomes based on three-month periods before and after training initiation were explored using proportions with 95% confidence intervals (CI). Logistic regression models yielding odds ratios (OR) were used to quantify magnitudes of effect. Multivariable regression analyses, adjusted a priori for covariates known to be associated with mortality, were used to calculate adjusted odds ratios (aOR). Covariates included: age [3], type of illness [33], [34], EC LOS [35], presence of critical hypoxia (SpO2 ≤ 88%) [36] and shock index [37], [38]. Age was modeled as a continuous parameter. Shock index was utilised as binary with a cut-point at 0.9 [39]. All regression models included a temporal variable based on year and month to control for secular trends in outcomes in the study setting. Stemming from identified differences in EC LOS during the study periods, a post-hoc sensitivity analysis comparing inpatient mortality during inpatient care days 2–4 was undertaken to evaluate if the changes in observed EC mortality could be accounted for through differential categorisation of patient status as EC patients versus inpatients.
Additional secular trends in mortality outcomes were explored using proportions and risk differences (RDs) with statistical significance assessed using two-sample tests of proportions. Specific comparisons were undertaken for the pre- and post-EM training periods and included variables on treatment initiation during day versus night hours and weekdays versus weekend days. Mortality outcomes were also assessed by matched time periods pre- and post-EM training to evaluate for temporal trends and impacts of seasonality. Sensitivity analyses using the initial regression models with the addition of the presence of altered mental status (AMS) were carried out. For data quality assessment using the double-entered records, inter-rater reliability (IRR) was calculated via Cohen’s κappa (κ), and interpreted using standard criteria [40].
Results
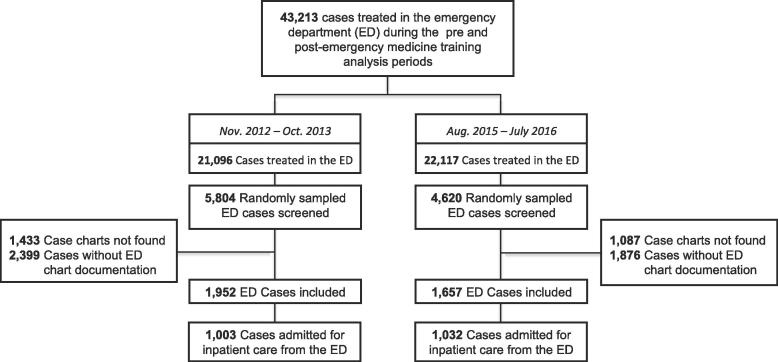
Among 43,213 EC cases during the study periods, 10,424 were randomly selected and screened. Of these, a total of 3,609 cases met inclusion and were analysed. There were 1952 pre-EM training cases and 1657 post-EM training cases sampled (Fig. 1).
Fig. 1.
Study Flow Diagram.
In the overall population, the median age was 32 years (IQR: 22, 50) and the majority of cases were male (60.7%). There were similar proportions of medical and injury cases in the overall cohort. Definitive outcome data were present for 85.3% of cases. In the pre- versus post-EM training cohorts, there were no significant differences in cases based on age, gender, case type, or prior medical history. As compared to the pre-EM training cases, those in the post-EM training cohort had a significantly lower prevalence of AMS (Table 1).
Table 1.
Case Characteristics.
| Characteristics | Overall (N = 3,609) n (%) | Pre-EM Training (N = 1,952) n (%) | Post-EM Training (N = 1,657) n (%) | p |
|---|---|---|---|---|
| Gender | ||||
| Male | 2190 (60.7) | 1176 (60.3) | 1014 (61.2) | |
| Female | 1415 (39.2) | 774 (39.6) | 641 (38.7) | 0.830 |
| Unknown | 4 (0.1) | 2 (0.1) | 2 (0.1) | |
| Age (years (IQR)) | 32 (22, 50) | 32 (23, 50) | 32 (22, 49) | 0.522 |
| Prior medical history | ||||
| No | 1257 (34.8) | 665 (34.1) | 592 (35.7) | |
| Yes | 1182 (32.8) | 660 (33.8) | 522 (31.5) | 0.319 |
| Unknown | 1170 (32.4) | 627 (32.1) | 543 (32.8) | |
| Case type | ||||
| Injury | 1672 (46.3) | 886 (45.4) | 786 (47.4) | |
| Medical | 1882 (52.2) | 1029 (52.7) | 853 (51.5) | 0.085 |
| Unknown | 55 (1.5) | 37 (1.9) | 18 (1.1) | |
| EC presentation Illness Severity | ||||
| Shock index ≥ 0.9 (n = 2491) | 756 (30.4) | 407 (30.2) | 349 (30.5) | 0.875 |
| Hypoxia (Sp02 < 89%) (n = 2366) | 163 (6.9) | 94 (4.8) | 69 (4.2) | 0.636 |
| Altered mental status* (n = 2064) | 367 (17.9) | 207 (21.7) | 160 (14.4) | <0.001 |
| EC treating physician type | ||||
| General Practice | 2473 (68.5) | 1952 (100) | 900 (54.3) | |
| Emergency Medicine Resident | 475 (13.2) | 0 (0.0) | 475 (28.7) | - |
| Unknown | 661 (18.3) | 0 (0.0) | 282 (17.0) | |
| Treatment Outcomes | ||||
| EC LOS (days (IQR)) | 1 (0, 3) | 1 (0, 4) | 1 (0, 2) | <0.001 |
| EC Outcome | ||||
| Discharged | 854 (23.7) | 522 (26.7) | 323 (20.0) | |
| Admitted to hospital | 2035 (56.4) | 1003 (51.4) | 1032 (62.2) | |
| Transferred | 54 (1.5) | 40 (2.1) | 14 (0.9) | <0.001 |
| Died | 142 (3.9) | 123 (6.3) | 19 (1.2) | |
| Eloped | 7 (0.2) | 2 (0.1) | 5 (0.3) | |
| Unknown | 517 (14.3) | 262 (13.4) | 255 (15.4) | |
| Inpatient LOS (days (IQR)) | 8 (4, 18) | 10 (5, 21) | 7 (3, 16) | <0.001 |
| Inpatient Outcome | ||||
| Discharged | 1653 (81.2) | 811 (80.8) | 842 (81.6) | |
| Transferred | 134 (6.6) | 65 (6.5) | 69 (6.7) | |
| Died | 232 (11.4) | 116 (11.6) | 116 (11.2) | 0.637 |
| Eloped | 3 (0.2) | 2 (0.2) | 1 (0.1) | |
| Unknown | 13 (0.6) | 9 (0.9) | 4 (0.4) | |
| Overall care LOS (days (IQR)) | 6 (2, 15) | 7 (2, 16) | 6 (2, 14) | 0.248 |
| Overall Outcome | ||||
| Discharged | 2507 (69.5) | 1333 (68.3) | 1174 (70.8) | |
| Transferred | 188 (5.2) | 105 (5.4) | 83 (5.0) | |
| Died | 374 (10.4) | 239 (12.2) | 135 (8.2) | 0.001 |
| Eloped | 10 (0.3) | 4 (0.2) | 6 (0.4) | |
| Unknown | 530 (14.7) | 271 (13.9) | 259 (15.6) | |
Note. EC, emergency centre; LOS, length of stay; IQR, interquartile range; *AMS indicates a mental status other than Alert on AVPU scale.
In the post-EM training sample, there was a significantly shorter EC LOS, a greater proportion of admitted cases, and a lower prevalence of transfers as compared to the pre-EM training sample. Inpatient LOS was significantly shorter for the post-EM training cohort as compared to the pre-EM training cohort; however there were no significant differences for other inpatient outcomes (Table 1).
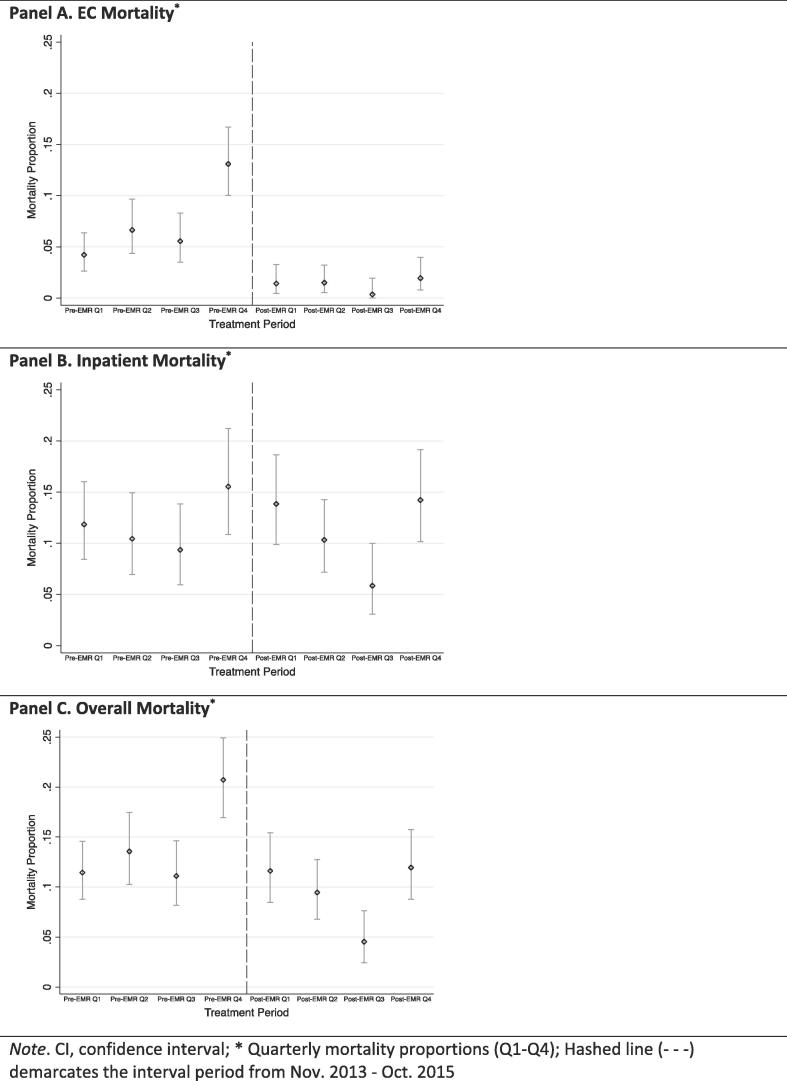
EC mortality decreased from 6.3% (95% CI 5.3–7.5%) during the pre-EM training period to 1.2% (95% CI 0.7–1.8%) during the post-EM training period (Table 1). The improved EC survival remained when the sample was stratified based on chronologic and temporally matched time periods, as shown in Fig. 2 (Panel A) and Supplement A, respectively. In multivariable regression analyses, there was a 93% reduced likelihood of EC mortality for cases treated in the post-EM training cohort as compared to those in the pre-EM training sample (aOR = 0.07, 95% CI 0.03–0.17; p < 0.001) (Table 2).
Fig. 2.
Mortality Outcomes by Chronological Time. Note. CI, confidence interval; * Quarterly mortality proportions (Q1–Q4); Hashed line (- - -) demarcates the interval period from Nov. 2013 - Oct. 2015.
Table 2.
Mortality Likelihood Post-Emergency Medicine Training Implementation.
| Outcome | OR§ (95% CI) | p | aOR§,# (95% CI) | p |
|---|---|---|---|---|
| EC Mortality | 0.19 (0.12, 0.30) | <0.001 | 0.07 (0.03, 0.17) | <0.001 |
| Inpatient Mortality | 0.97 (0.76, 1.23) | 0.785 | 0.80 (0.53, 1.81) | 0.951 |
| Overall Mortality | 0.68 (0.55, 0.83) | <0.001 | 0.57 (0.36, 0.94) | 0.016 |
EC, emergency centre; OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval.
Pre-Emergency Medicine training case comprised the reference groups for mortality outcomes.
Multivariable models adjusted for treatment year and month, patient age, type of illness, EC length of stay, shock index and hypoxia.
Inpatient mortality was not significantly different during the pre- versus post-EM training periods (11.6% versus 11.2%) (Table 1). This was maintained when the cohorts were compared by three-month time periods chronologically (Fig. 2, Panel B) and temporally matched (Supplement A). Similarly, there was no significant difference pre- versus post-EM training in the likelihood of inpatient mortality identified after adjusting for potential confounders via regression modeling (Table 2).
Overall hospital mortality was 12.2% (95% CI 10.9–13.8%) and 8.2% (95% CI 6.9–9.6%) for the pre- and post-EM training cohorts respectively. Overall hospital mortality in the post-EM training cohort was reduced during all stratified time periods evaluated as compared to the pre-EM training cohort (Fig. 2, Panel C & Supplement A). Overall hospital mortality likelihood was reduced by 43% among the post-EM training cases as compared to the pre-EM training cases (aOR = 0.57, 95% CI 0.36–0.94; p < 0.016) (Table 3).
Table 3.
Secular Trends in Mortality Pre- Versus Post-Emergency Medicine Training Implementation.
| Emergency Centre Mortality |
||||
|---|---|---|---|---|
| Pre-EM Training % (95% CI) |
Post-EM Training % (95% CI) |
Risk Difference % (95% CI) |
p | |
| Treatment Initiation Time | ||||
| Day hours | 8.1 (5.8, 10.3) | 1.1 (0.2, 2.0) | −7.0 (−9.4, −4.5) | <0.001 |
| Night hours | 8.7 (6.3, 11.0%) | 1.9 (0.8, 3.1) | −6.7 (−9.3, −4.1) | <0.001 |
| Initial Treatment Day | ||||
| Weekday | 7.3 (5.9, 8.8) | 1.4 (0.1, 2.1) | −5.9 (−7.5, −4.4) | <0.001 |
| Weekend day | 7.1 (4.5, 9.6) | 1.3 (0.0, 2.5) | −5.8 (−8.6, −3.0) | <0.001 |
| Inpatient Mortality | ||||
| Pre-EM Training | Post-EM Training | Risk Difference | p | |
| % (95% CI) | % (95% CI) | % (95% CI) | ||
| Treatment Initiation Time | ||||
| Day hours | 13.2 (9.8, 16.7) | 10.7 (7.7, 13.7) | −2.5 (−7.1, 2.1) | 0.281 |
| Night hours | 11.5 (8.0, 15.0) | 12.1 (8.9, 15.2) | 0.6 (−4.1, 5.2) | 0.819 |
| Initial Treatment Day | ||||
| Weekday | 10.7 (8.5, 12.9) | 10.9 (8.7, 13.0) | 0.2 (−2.9, 3.3) | 0.901 |
| Weekend day | 15.1 (10.4, 19.9) | 12.7 (8.3, 17.1) | −2.4 (−8.9, 4.1) | 0.466 |
| Overall Mortality | ||||
| Pre-EM Training | Post-EM Training | Risk Difference | p | |
| % (95% CI) | % (95% CI) | % (95% CI) | ||
| Treatment Initiation Time | ||||
| Day hours | 16.5 (13.5, 19.6) | 9.2 (6.8, 11.7) | −7.2 (−19.6, −3.4) | <0.001 |
| Night hours | 15.5 (12.4, 18.5) | 10.7 (8.2, 13.3) | −4.7 (−8.7, −0.8) | 0.019 |
| Initial Treatment Day | ||||
| Weekday | 13.8 (11.9, 15.6) | 9.5 (7.8, 11.3) | −4.2 (−6.8, −1.7) | 0.001 |
| Weekend day | 15.4 (11.8, 18.9) | 10.0 (6.7, 13.3) | −5.4 (−10.2, −0.5) | 0.034 |
The same patterns in outcomes were found in secular analyses when cases were stratified and compared by treatment time or treatment day (Table 3). Additionally, intra-cohort analyses of secular trends for mortality outcomes based on treatment time and day identified no significant differences in outcomes between samples (Supplement B). In post-hoc sensitivity analysis assessing early inpatient deaths (days 2–4 as inpatients) there was no significant difference in mortality between the pre- and post-EM cohorts (1.7% versus 1.9%; p = 0.79). Regression sensitivity models reproduced the same trends in likelihoods of mortality across the outcomes of interest as the primary analyses (Supplement C). Among double entered records, IRR was excellent, κ = 0.95 (standard error 0.04).
Discussion
This study provides key data on the impact of formalised EM training and systems development on mortality in an LMIC setting. The results demonstrate an association between the implementation of EM training with formalised EM care systems and reduced overall hospital mortality, which was driven predominately by improved EC survival. Although limited by design aspects, these findings highlight improvements in a patient-centered outcome with EM training and systems development in an LMIC, and support further research and investment in EM in resource-limited settings.
ECs in LMICs treat high-risk patients. A meta-analysis found that the median EC mortality was 4.8% among reports from Africa, a figure one-hundred fold greater than EC mortality from the United States [3], [41]. Adding to this, with the limited access to trained emergency care personnel in LMICs [42], the potential for EM training to improve outcomes is substantial. The current results represent the first available data demonstrating an association between implementation of formal EM training with formalised EM care systems and mortality reductions in an LMIC. Although no studies have shown this previously, multiple reports have illustrated benefits with forms of EM development. A study from Tanzania, which assessed patient outcomes before and after the installation of a full-capacity EC, found that hospital mortality was reduced post-intervention. In the Tanzania report, impacts on mortality were not assessed based on implementation of EM training specifically [43]. Additionally, use of the South African Triage Scale in Botswana improved triage accuracy [44], implementation of trauma protocols in Colombia increased use of vital EC treatments [45] and emergency paediatric care in Malawi and Sierra Leone have been associated with reduced child mortality [46], [47]. The concordance of the present data with the earlier reports from alternative settings suggest validity in the findings and adds support for investment in EM systems in LMICs.
In the analysis, a 4% absolute reduction for overall hospital mortality was found between study periods. Although during the studied time periods mortality among hospitals in Rwanda was down trending, the magnitude of absolute reduction in the current cohort was twice as great as the national trend, and the association of improved survival with EM implementation was statistically significant when adjusting for temporal variables, suggesting that alternative beneficial factors existed [48], [49]. Paramount among these was the MoH HRH programme, which supported not only EM training initiatives but also ones in internal medicine, surgery, anaesthesia, obstetrics and gynaecology, and paediatrics [22]. With this programme, it is likely that survival gains were derived from multiple factors, such as improved care delivery and resuscitation during the emergent phases of illnesses, more rapid access to consultant services, and enhanced efficiency in care delivery. For example there was a significant reduction in EC LOS between study periods, a factor that has been shown to be associated with improved outcomes [35]. Taking into account however, the EC-specific mortality reductions which drove the overall survival benefits, the data suggests that implementation of EM training and systems were likely a key factor in the observed outcomes.
Although the MoH HRH programme was engaged in training practitioners across a number specialties, the EM training programme was the only de novo specialty in the Rwanda setting [22]. Given this, it is reasonable to expect larger relative impacts in emergency care outcomes. As the EM programme employed multiple educational and systems changes, including formal didactics, clinical oversight and improved EC organisation, the significant improvements in EC specific outcomes are likely valid [23], [24], [25], [27], [28].
There were some significant differences between the pre- and post-EM training cohorts. However, in sensitivity analyses adjusting for differences, the trends between EM training and improved survival were maintained. Furthermore, in evaluation of inpatient mortality, there was no significant difference in early deaths between the time periods studied, making re-categorisation of case status unlikely to account for the EC gains. The secular trends in pre- and post-EM training outcomes demonstrated that overall mortality risks were consistently reduced regardless of treatment time or day assessed. These results suggest that EM training and systems implementation rather than external factors were associated with the beneficial observed outcomes.
Although rigorous methods were used [29], the retrospective design resulted in an inability to identify a proportion of eligible cases and some missing data among included cases, which may have introduced bias. However, given that records were missing at random, there were minimal significant differences between periods, and outcome data were attained for more than 85% of cases, it is likely that the sample is representative of the population of interest. Although the multivariable analyses controlled for illness severity and temporal factors, unmeasured confounders such as disease specific characteristics and availability of treatments or procedural services could have influenced the findings. The interrupted time-series design did not allow for the assessment of causality, limiting the ability to definitively evaluate whether EM training and system development reduced mortality [50]. Moreover, the available data did not allow for evaluation of specific training or systems interventions to which survival gains could be attributable and it is possible that the driving factors were multifactorial, potentially stemming from improved staffing resources and abilities or variations in treatments. Even though specific interventions and all possible confounders were not identifiable, the present data provides a pragmatic analysis on the impact of EM training and associated EM systems implementation. Additionally, although temporal factors were evaluated and controlled for in the analyses it is possible broader changes in survival over time in Rwanda could have impacted the results. Finally, the research was performed at a single tertiary-care hospital with external support, which may limit the generalisability to health venues with fewer resources.
The presented data, demonstrating that implementation of EM training with formalised EM systems were associated with a significant reduction in mortality, predominately driven by enhanced EC survival, and supports investment in EM education and systems development. However, with the inherent design limitations in the time-series data, the findings would be bolstered through prospective evaluations in alternative LMIC settings. Given the health burdens in emergency populations, controlled randomised cross-over trials evaluating EM training implementation will be the most ethically appropriate methods for future research.
Conflict of interest
The authors declared no conflicts of interest.
Dissemination of results
Preliminary results from this work were presented at the American College of Emergency Medicine, Scientific Assembly, October, 2017 in Washington D.C., USA. The final results from this research were shared with staff members at the University Teaching Hospital in Kigali, Rwanda through presentations within the emergency centre by local staff affiliated with the study activities. It will be further discussed at the forthcoming African Conference on Emergency Medicine, also in Kigali, Rwanda.
Authors’ contributions
All authors contributed equally to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Funding
Funding was provided through grants from the University Emergency Medicine Foundation, Providence, Rhode Island. The funders had no role in the study design, data collection or reporting processes.
Footnotes
Peer review under responsibility of African Federation for Emergency Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.afjem.2018.10.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Holliman C.J., Mulligan T.M., Suter R.E. The efficacy and value of emergency medicine: a supportive literature review. Int J Emerg Med. 2011;4:44. doi: 10.1186/1865-1380-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor S.F., Gerhardt R.T., Simpson M.P. An association between Emergency Medicine residencies and improved trauma patient outcome. J Emerg Med. 2005;29:123–127. doi: 10.1016/j.jemermed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Obermeyer Z., Abujaber S., Makar M. Emergency care in 59 low- and middle-income countries: a systematic review. Bull World Health Organ. 2015;93:577–586G. doi: 10.2471/BLT.14.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed T., Hossain M., Sanin K.I. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. 2012;61(Suppl 1):8–17. doi: 10.1159/000345165. [DOI] [PubMed] [Google Scholar]
- 5.Haagsma J.A., Graetz N., Bolliger I. The global burden of injury: incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj Prev. 2016;22:3–18. doi: 10.1136/injuryprev-2015-041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global AIDS Update 2016. [cited 2016 January 27]; Available from: <http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf/>; 2016.
- 7.Baelani I., Jochberger S., Laimer T. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care. 2011;15:R10. doi: 10.1186/cc9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith J., Haile-Mariam T. Priorities in global emergency medicine development. Emerg Med Clin North Am. 2005;23:11–29. doi: 10.1016/j.emc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson P.D., Suter R.E., Mulligan T. World Health Assembly Resolution 60.22 and its importance as a health care policy tool for improving emergency care access and availability globally. Ann Emerg Med. 2012;60(35–44) doi: 10.1016/j.annemergmed.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Pek J.H., Lim S.H., Ho H.F. Emergency medicine as a specialty in Asia. Acute Med Surg. 2016;3:65–73. doi: 10.1002/ams2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen L.S., Geduld H.I., Nagurney J.T., Wallis L.A. Africa's first emergency medicine training program at the University of Cape Town/Stellenbosch University: history, progress, and lessons learned. Acad Emerg Med. 2011;18:868–871. doi: 10.1111/j.1553-2712.2011.01131.x. [DOI] [PubMed] [Google Scholar]
- 13.Molyneux E., Ahmad S., Robertson A. Improved triage and emergency care for children reduces inpatient mortality in a resource-constrained setting. Bull World Health Organ. 2006;84:314–319. doi: 10.2471/blt.04.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali J., Adam R., Butler A.K. Trauma outcome improves following the advanced trauma life support program in a developing country. J Trauma. 1993;34:890–898. doi: 10.1097/00005373-199306000-00022. discussion 898–899. [DOI] [PubMed] [Google Scholar]
- 15.Wen L.S., Geduld H.I., Tobias Nagurney J., Wallis L.A. Perceptions of graduates from Africa's first emergency medicine training program at the University of Cape Town/Stellenbosch University. CJEM. 2012;14:97–105. doi: 10.2310/8000.2012.110639. [DOI] [PubMed] [Google Scholar]
- 16.Nowacki A.K., Landes M., Azazh A., Puchalski Ritchie L.M. A review of published literature on emergency medicine training programs in low- and middle-income countries. Int J Emerg Med. 2013;6:26. doi: 10.1186/1865-1380-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karara G., Verbeke F., Nyssen M. The role of hospital information systems in universal health coverage monitoring in Rwanda. Stud Health Technol Inform. 2015;216:193–197. [PubMed] [Google Scholar]
- 18.Condo J., Mugeni C., Naughton B. Rwanda's evolving community health worker system: a qualitative assessment of client and provider perspectives. Hum Resour Health. 2014;12:71. doi: 10.1186/1478-4491-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nsanzimana S., Prabhu K., McDermott H. Improving health outcomes through concurrent HIV program scale-up and health system development in Rwanda: 20 years of experience. BMC Med. 2015;13:216. doi: 10.1186/s12916-015-0443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penfold R.B., Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13:S38–44. doi: 10.1016/j.acap.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 21.MacBride-Stewart S., Marwick C., Houston N. Evaluation of a complex intervention to improve primary care prescribing: a phase IV segmented regression interrupted time series analysis. Br J Gen Pract. 2017;67:e352–e360. doi: 10.3399/bjgp17X690437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binagwaho A., Kyamanywa P., Farmer P.E. The human resources for health program in Rwanda–new partnership. N Engl J Med. 2013;369:2054–2059. doi: 10.1056/NEJMsr1302176. [DOI] [PubMed] [Google Scholar]
- 23.Mbanjumucyo G., DeVos E., Pulfrey S., Epino H.M. State of emergency medicine in Rwanda 2015: an innovative trainee and trainer model. Int J Emerg Med. 2015;8:20. doi: 10.1186/s12245-015-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbanjumucyo G., Henwood P.C. Focused assessment with sonography for HIV-associated tuberculosis (FASH) case series from a Rwandan district hospital. Afr J Emerg Med. 2016;6(4):198–201. doi: 10.1016/j.afjem.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aluisio A.R., Umuhire O.F., Mbanjumucyo G. Epidemiologic characteristics of pediatric trauma patients receiving prehospital care in Kigali, Rwanda. Pediatr Emerg Care. 2017 doi: 10.1097/PEC.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 26.Kearney A.S., Kabeja L.M., George N. Development of a trauma and emergency database in Kigali, Rwanda. Afr J Emerg Med. 2016;6:185–190. doi: 10.1016/j.afjem.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbanjumucyo G., George N., Kearney A. Epidemiology of injuries and outcomes among trauma patients receiving prehospital care at a tertiary teaching hospital in Kigali, Rwanda. Afr J Emerg Med. 2016;6:191–197. doi: 10.1016/j.afjem.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aluisio A.R., Garbern S., Wiskel T. Mortality outcomes based on ED qSOFA score and HIV status in a developing low income country. Am J Emerg Med. 2018 doi: 10.1016/j.ajem.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaji A.H., Schriger D., Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014;64:292–298. doi: 10.1016/j.annemergmed.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Kelly C.A., Upex A., Bateman D.N. Comparison of consciousness level assessment in the poisoned patient using the alert/verbal/painful/unresponsive scale and the Glasgow Coma Scale. Ann Emerg Med. 2004;44:108–113. doi: 10.1016/j.annemergmed.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streiner D.L. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr. 2015;102:721–728. doi: 10.3945/ajcn.115.113548. [DOI] [PubMed] [Google Scholar]
- 33.Naidoo D.K., Rangiah S., Naidoo S.S. South African family practice - an evaluation of the triage early warning score in an urban accident and emergency department in KwaZulu-Natal. S Afr Fam Pract. 2014;56:69–73. [Google Scholar]
- 34.Massaut J., Valles P., Ghismonde A. The modified South African triage scale system for mortality prediction in resource-constrained emergency surgical centers: a retrospective cohort study. BMC Health Serv Res. 2017;17:594. doi: 10.1186/s12913-017-2541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Qahtani S., Alsultan A., Haddad S. The association of duration of boarding in the emergency room and the outcome of patients admitted to the intensive care unit. BMC Emerg Med. 2017;17:34. doi: 10.1186/s12873-017-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raman S., Prince N.J., Hoskote A., Ray S., Peters M.J. Admission PaO2 and mortality in critically ill children: a cohort study and systematic review. Pediatr Crit Care Med. 2016;17:e444–e450. doi: 10.1097/PCC.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 37.Berger T., Green J., Horeczko T. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14:168–174. doi: 10.5811/westjem.2012.8.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balhara K.S., Hsieh Y.H., Hamade B. Clinical metrics in emergency medicine: the shock index and the probability of hospital admission and inpatient mortality. Emerg Med J. 2017;34:89–94. doi: 10.1136/emermed-2015-205532. [DOI] [PubMed] [Google Scholar]
- 39.Mutschler M., Nienaber U., Munzberg M. The Shock Index revisited - a fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Crit Care. 2013;17:R172. doi: 10.1186/cc12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 41.Tang N., Stein J., Hsia R.Y., Maselli J.H., Gonzales R. Trends and characteristics of US emergency department visits, 1997–2007. JAMA. 2010;304:664–670. doi: 10.1001/jama.2010.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsia R.Y., Mbembati N.A., Macfarlane S., Kruk M.E. Access to emergency and surgical care in sub-Saharan Africa: the infrastructure gap. Health Policy Plan. 2012;27:234–244. doi: 10.1093/heapol/czr023. [DOI] [PubMed] [Google Scholar]
- 43.Sawe H.R., Mfinanga J.A., Mwafongo V., Reynolds T.A., Runyon M.S. Trends in mortality associated with opening of a full-capacity public emergency department at the main tertiary-level hospital in Tanzania. Int J Emerg Med. 2015;8:24. doi: 10.1186/s12245-015-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mullan P.C., Torrey S.B., Chandra A., Caruso N., Kestler A. Reduced overtriage and undertriage with a new triage system in an urban accident and emergency department in Botswana: a cohort study. Emerg Med J. 2014;31:356–360. doi: 10.1136/emermed-2012-201900. [DOI] [PubMed] [Google Scholar]
- 45.Kesinger M.R., Puyana J.C., Rubiano A.M. Improving trauma care in low- and middle-income countries by implementing a standardized trauma protocol. World J Surg. 2014;38:1869–1874. doi: 10.1007/s00268-014-2534-y. [DOI] [PubMed] [Google Scholar]
- 46.Clark M., Spry E., Daoh K., Baion D., Skordis-Worrall J. Reductions in inpatient mortality following interventions to improve emergency hospital care in Freetown, Sierra Leone. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robison J.A., Ahmad Z.P., Nosek C.A. Decreased pediatric hospital mortality after an intervention to improve emergency care in Lilongwe, Malawi. Pediatrics. 2012;130:e676–682. doi: 10.1542/peds.2012-0026. [DOI] [PubMed] [Google Scholar]
- 48.Rwanda Annual Health Statistics Booklet. Accessed at <http://www.moh.gov.rw/fileadmin/templates/HMIS_Docs/Rwanda_Annual_Health_Statistics_Booklet_2013_signed.pdf/>; 2013.
- 49.Republic of Rwanda, Annual Health Statistics Booklet. 2015. Accessed at: <http://www.moh.gov.rw/fileadmin/templates/hmis_reports/2015_20Annual_20Statistical_20booklets_20V13_20Signed.pdf/>; 2015.
- 50.Grimshaw J., Campbell M., Eccles M., Steen N. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract. 2000;17(Suppl 1):S11–16. doi: 10.1093/fampra/17.suppl_1.s11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.