Abstract
Background
Circular RNAs (circRNAs) are novel non-coding RNAs that have important roles in tumor progression. This study aimed to measure the levels of hsa_circ_0000885 in serum samples and tumor tissue from patients with osteosarcoma compared with controls and to evaluate the findings with disease-free survival and overall survival.
Material/Methods
Fifty pairs of osteosarcoma tissues and matched adjacent normal tissue were obtained from patients who underwent the same chemotherapy regimen before surgery. Blood samples were obtained from 30 patients with osteosarcoma before and after chemotherapy, 25 patients with osteosarcoma before and after surgery, 27 patients with benign bone tumors, and 25 age-matched and sex-matched healthy controls. Circular RNA sequencing and quantitative real-time polymerase chain reaction (qRT-PCR) were used to analyze hsa_circ_0000885 expression.
Results
Hsa_circ_0000885 expression was significantly increased in tissue and serum samples from patients with osteosarcoma, compared with controls, with significantly increased expression levels in patients with Enneking stage IIB and III osteosarcoma, compared with early-stage osteosarcoma. Patients with high serum and tumor levels of hsa_circ_0000885 had lower rates of disease-free survival and overall survival. The serum expression levels of hsa_circ_0000885 were significantly higher in patients with osteosarcoma compared with patients with benign bone tumors or healthy controls.
Conclusions
Hsa_circ_0000885 was upregulated in osteosarcoma, and it could serve as a good prognostic biomarker indicating poor clinical outcomes of osteosarcoma. Hsa_circ_0000885 was upregulated in serum of osteosarcoma patients and could serve as a good diagnostic biomarker for osteosarcoma.
MeSH Keywords: Biological Markers; Osteosarcoma; RNA, Untranslated
Background
Osteosarcoma (OS) is the most common primary bone tumor in children and adolescents and is characterized by early metastasis and rapid progression, which results in a high mortality rate [1,2]. Despite improvements in radiotherapy, adjuvant chemotherapy and surgery, due to the aggressive behavior of osteosarcoma, the overall survival rate remains poor [3–5]. The behavior and progression of osteosarcoma is a complex process that involves molecular and epigenetic changes [6]. Therefore, the identification of new biomarkers and the molecular mechanisms involved in osteosarcoma tumorigenesis and progression are needed.
With the development of high-throughput sequencing technology, several non-coding RNAs had recently been identified [7]. Circular RNA (circRNA) is a novel RNA that is formed by a covalently closed loop and lacks the ability for protein encoding, which is more stable than corresponding linear mRNAs, and plays important roles in oncogenesis and tumor progression [8–10]. The circRNA, hsa_circ_001569, has been reported to be expressed at high levels in colorectal cancer, and act as a sponge for the microRNA, miR 145, that targets the E2F5, BAG4, and FMNL2 genes to promote the progression of colorectal cancer [11]. Also, hsa_circ_0001649, hsa_circ_0000190, and hsa_circ_0013958 have been identified as potential prognostic biomarkers in hepatocellular carcinoma [12], gastric cancer [13], and adenocarcinoma of the lung [14], respectively. These findings indicate that circRNAs might be promising candidates as new diagnostic or prognostic biomarkers in malignancy.
Therefore, this study aimed to measure the levels of hsa_circ_0000885 in serum samples and tumor tissue from patients with osteosarcoma compared with controls and to evaluate the findings with disease-free survival and overall survival.
Material and Methods
Patients and specimens
This study was approved by the Ethics Committee of The Affiliated Hospital of Qingdao University and conformed to the Ethical Guidelines of the Declaration of Helsinki. Written informed consent was obtained from all the participants. A total of 50 pairs of osteosarcoma tissues and their matched adjacent normal tissue were obtained from patients who underwent the same chemotherapy regimen before surgery and underwent complete resection surgery at the Department of Orthopedics of the Affiliated Hospital of Qingdao University. Blood samples from 30 patients with osteosarcoma before and after chemotherapy, 25 patients with osteosarcoma before and after surgery, 27 patients with benign bone tumors (13 patients with giant cell tumor of bone, 14 patients with fibrous dysplasia), and 25 age-matched and sex-matched healthy controls were collected. The clinical and pathological features of the patients with osteosarcoma are shown in Table 1.
Table 1.
Correlations between the clinical parameters of patients with osteosarcoma and hsa_circ_0000885 expression in osteosarcoma tissues.
| Variables | Total number | hsa_circ_0000885 expression low | hsa_circ_0000885 expression high | P-value |
|---|---|---|---|---|
| Gender | 0.285 | |||
| Men | 27 | 12 | 15 | |
| Women | 23 | 13 | 10 | |
| Age (years) | 0.500 | |||
| <25 | 31 | 16 | 15 | |
| ≥25 | 19 | 9 | 10 | |
| Enneking stage | 0.006 | |||
| I, IIA | 15 | 12 | 3 | |
| IIB, III | 35 | 13 | 22 | |
| Lung metastasis | 0.009 | |||
| No | 38 | 23 | 15 | |
| Yes | 12 | 2 | 10 | |
| Tumor size (cm) | 0.500 | |||
| <8 | 29 | 15 | 14 | |
| ≥8 | 21 | 10 | 11 | |
| ALP | 0.284 | |||
| Normal | 21 | 9 | 16 | |
| Abnormal | 29 | 12 | 13 | |
OS – osteosarcoma; ALP – alkaline phosphatase; hsa_circ_0000885 expression high/low – the expression of hsa_circ_0000885 in osteosarcoma tissue was higher/lower than the median.
Circular RNA sequencing
Total RNA was extracted from four osteosarcoma tissues and four adjacent normal tissues by TRIzol (Invitrogen, Carlsbad, CA, USA). RNAs were digested with RNase R (Epicentre Technologies, Madison, WI, USA) to remove the linear RNAs and enrich the circular configuration. The sequencing analysis was performed on an Illumina HiSeq 2000 Platform (Illumina, San Diego, CA, USA). The sequencing results were analyzed with Cluster 3.0 software (University of Tokyo, Human Genome Center).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the hsa_circ_0000885 expression using a SYBR green kit (Takara Japan, Minato-ku, Tokyo, Japan). The expression of hsa_circ_0000885 was normalized to GAPDH expression. The primers used included:
hsa_circ_0000885, Forward: 3′-ACTGCCAGAAAGTGTGTCCC-5′;
hsa_circ_0000885, Reverse: 3′-CGGGCCTCGTTTTGAACATC-5′;
GAPDH, Forward: 5′-AATGGGCAGCCGTTAGGAAA-3′;
GAPDH, Reverse: 5′-TGAAGGGGTCATTGATGGCA-3′.
The results were analyzed using the 2−ΔΔCT method and compared using paired Student’s t-tests.
Statistical analysis
Data were analyzed using SPSS version 18.0 software (SPSS, Chicago, IL, USA). Data were presented as the mean ± standard deviation (SD). Statistical significance was analyzed by the Student’s t-test or the chi-squared (χ2) test. Progression-free survival and overall survival were analyzed by Kaplan-Meier survival analysis. Receiver operating characteristic (ROC) curves were used to investigate the efficiency of as hsa_circ_001569 as a biomarker. P<0.05 was considered as statistically significant.
Results
Circular RNA (circRNA), hsa_circ_0000885, expression was upregulated in osteosarcoma tissues
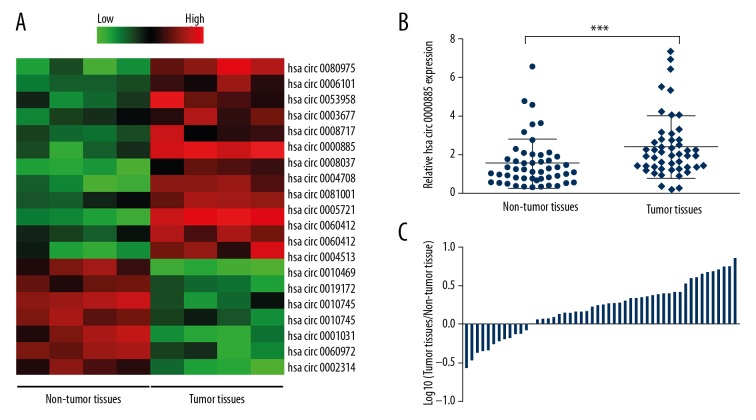
Circular RNA sequencing was performed to detect the circRNA profile in ostesarcoma tissues and adjacent non-tumor tissues. Results showed that there were 12 upregulated circRNAs and seven downregulated circRNAs with fold-changes >2.0 and P-values <0.05 between the osteosarcoma tissues and adjacent non-tumor tissues (Figure 1A). Then, quantitative real-time polymerase chain reaction (qRT-PCR) analysis performed in osteosarcoma tissues and adjacent non-tumor tissues confirmed that hsa_circ_0000885 expression was upregulated in osteosarcoma tissues, which supported the findings from the circRNA profile (Figure 1B, 1C).
Figure 1.
hsa_circ_0000885 expression was upregulated in osteosarcoma tissue. (A) The heatmap shows the circRNA expression profiles of osteosarcoma tissues and adjacent non-tumor tissues. Red and green indicate high and low expression, respectively. (B) Expression levels of hsa_circ_0000885 in 50 pairs of osteosarcoma tissues and adjacent non-tumor tissues, analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) and normalized to GAPDH. The results were analyzed using the 2−ΔΔCT method and compared using paired Student’s t-tests. (C) Relative hsa_circ_0000885 expression ratios in osteosarcoma tissues versus adjacent non-tumor tissues shown on the logarithmic scale. Data are shown as the means ± standard deviation (SD). *** P<0.001.
High hsa_circ_0000885 expression was correlated with poor clinical prognosis
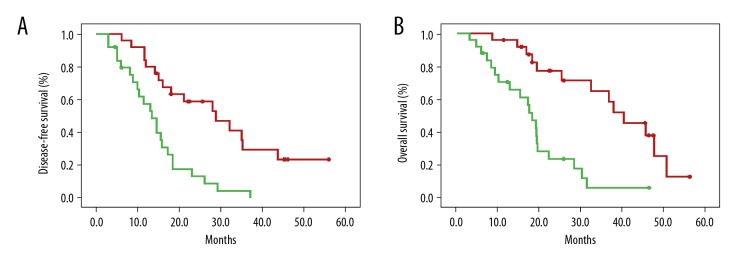
The hsa_circ_0000885 expression levels were divided into low hsa_circ_0000885 expression or high hsa_circ_0000885 expression, based on the median expression level. Analysis of the correlation between clinicopathological variables and hsa_circ_0000885 showed that hsa_circ_0000885 expression was increased in patients with Enneking stage IIB or III osteosarcoma in patients with lung metastasis (Table 1). Kaplan-Meier survival analysis also showed that patients with high levels of hsa_circ_0000885 had reduced disease-free survival and overall survival rates (Figure 2A, 2B). The Cox proportional hazards regression model showed that hsa_circ_0000885 was an independent prognostic factor for overall survival but not for disease-free survival (Table 2).
Figure 2.
Increased hsa_circ_0000885 expression was correlated with poor clinical prognosis. (A) Disease-free survival (DFS) in patients with high hsa_circ_0000885 expression and low hsa_circ_0000885 expression (p<0.001). (B) Overall survival of patients with high hsa_circ_0000885 expression and a low hsa_circ_0000885 expression (p<0.001). Red line, hsa_circ_0000885 low expression group; green line, hsa_circ_0000885 high expression group; +, censored points.
Table 2.
Factors influencing the disease-free survival (DFS) and overall survival (OS) in patients with osteosarcoma.
| Variables | Disease-free survival | Overall survival | ||
|---|---|---|---|---|
| HR | P-value | HR | P-value | |
| Univariate analysis | ||||
| Gender (Male/Female) | 0.873 | 0.679 | 0.954 | 0.895 |
| Age (<25, ≥25 years) | 0.897 | 0.739 | 0.752 | 0.437 |
| Enneking stage (I, IIA or IIB, III) | 3.504 | 0.002 | 4.001 | 0.002 |
| Lung metastasis (yes/no) | 6.090 | <0.001 | 5.757 | <0.001 |
| Tumor size (≤5, >5 cm) | 1.328 | 0.396 | 1.030 | 0.936 |
| hsa_circ_0000885 expression (low, high) | 3.356 | <0.001 | 4.247 | <0.001 |
| ALP (normal, abnormal) | 1.111 | 0.747 | 1.249 | 0.533 |
| Multivariate analysis | ||||
| Enneking stage (I, IIA or IIB, III) | 2.164 | 0.091 | 2.226 | 0.121 |
| Lung metastasis (yes, no) | 3.382 | 0.005 | 2.996 | 0.021 |
| hsa_circ_0000885 expression (high, low) | 1.924 | 0.099 | 2.458 | 0.047 |
OS – osteosarcoma; ALP – alkaline phosphatase; hsa_circ_0000885 expression high/low – the expression of hsa_circ_0000885 in osteosarcoma tissue was higher/lower than the median.
hsa_circ_0000885 was upregulated in the serum of patients with osteosarcoma
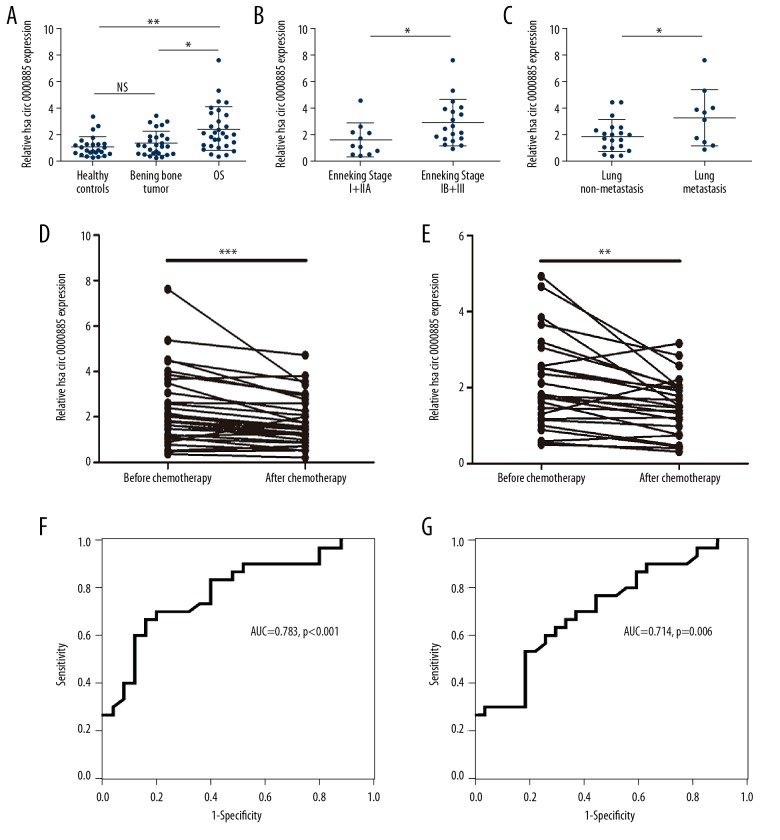
Serum samples from 30 patients with osteosarcoma, 27 patients with benign bone tumors and 25 age-matched and sex-matched healthy controls were studied. The result showed that levels of hsa_circ_0000885 expression were increased in the serum of patients with osteosarcoma when compared with patients with benign bone tumors or healthy controls (Figure 3A). Serum levels of hsa_circ_0000885 were also increased in patients with osteosarcoma who had Enneking stage IIB and III and patients with lung metastases (Figure 3B, 3C). Also, the expression level of hsa_circ_0000885 significantly decreased following chemotherapy or following surgery (Figure 3D, 3E). The receiver operating characteristic (ROC) curve was used to estimate the diagnostic value of serum hsa_circ_0000885 levels in distinguishing between patients with osteosarcoma and healthy individuals (Figure 3F). ROC curve analysis showed that serum levels of hsa_circ_0000885 also distinguished specifically between patients with osteosarcoma and benign bone tumors (Figure 3G). These results suggested that hsa_circ_0000885 could act as a good diagnostic biomarker in OS.
Figure 3.
Serum hsa_circ_0000885 levels were increased in serum from patients with osteosarcoma. (A) Expression levels of hsa_circ_0000885 in 30 patients with osteosarcoma, 27 patients with benign bone tumors, and 25 age-matched and sex-matched healthy controls were analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and normalized to the GAPDH level. (B) Relative expression of hsa_circ_0000885 in 11 patients with osteosarcoma, Enneking stage I or IIA and 19 patients with osteosarcoma, Enneking stage IB or III. (C) Relative expression of hsa_circ_0000885 in 20 patients with osteosarcoma without metastasis and 10 patients with osteosarcoma with lung metastasis. (D) Relative expression of hsa_circ_0000885 in 30 patients with osteosarcoma before and after chemotherapy. (E) Relative expression of hsa_circ_0000885 in 30 patients with osteosarcoma before and after surgery. (F) Receiver operating characteristic (ROC) curve shows the diagnostic value of hsa_circ_0000885 in distinguishing between patients with osteosarcoma from healthy individuals. (G) ROC curve shows the diagnostic value of hsa_circ_0000885 in distinguishing between patients with osteosarcoma and benign bone tumors.
Discussion
Recently, several functional circular RNAs (circRNAs) have been discovered and studied in human disease, including malignancy [15]. Some circRNAs have been reported to be dysregulated and serve as tumor suppressors or tumor promoters in several tumors, including osteosarcoma [16–18]. The findings of the present study showed that expression of the novel circRNA, hsa_circ_0000885, was upregulated in osteosarcoma by circular RNA sequencing and verified using quantitative real-time polymerase chain reaction (qRT-PCR). Also, increased expression of hsa_circ_0000885 was correlated with Enneking stage and with the presence of lung metastasis. Survival analysis showed that hsa_circ_0000885 expression was also correlated with disease-free survival and overall survival rates of patients with osteosarcoma and that hsa_circ_0000885 was an independent prognostic factor for overall survival.
Aberrant levels of some circRNAs in the serum from patients with malignancy, including osteosarcoma, has been previously reported [19–21]. For example, hsa_circ_0081001 was upregulated in osteosarcoma cell lines, tissue, and serum, and was shown to be correlated with poor prognosis, possible as a more sensitive biomarker than alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) in patients with osteosarcoma [19]. In patients with hepatocellular carcinoma (HCC), the area under the receiver operating characteristic curve (AUC-ROC) of 0.973 for circRNA_104075 was shown to be a new diagnostic biomarker with a sensitivity of 96.0% and a specificity of 98.3% [20]. CircPRMT5 was shown to promote epithelial-mesenchymal transition (EMT) in urothelial carcinoma cells and might have potential, as a prognostic biomarker and therapeutic target for patients with urothelial carcinoma of the bladder [21].
The findings of the present study showed that hsa_circ_0000885 expression levels were significantly increased in the serum from patients with osteosarcoma when compared with patients with benign bone tumors and with healthy controls. Also, hsa_circ_0000885 expression was significantly increased in osteosarcoma tissue when compared with normal adjacent tissue. Serum levels of hsa_circ_0000885 significantly decreased following chemotherapy and surgery. Serum hsa_circ_0000885 expression levels were also relatively increased in patients with osteosarcoma with Enneking stage IIB or III tumors and in patients with lung metastasis. ROC curve suggested that hsa_circ_0000885 may act as a good diagnostic biomarker for OS.
Conclusions
Hsa_circ_0000885 was upregulated in osteosarcoma, and it could serve as a good prognostic biomarker indicating poor clinical outcomes of osteosarcoma. Hsa_circ_0000885 was upregulated in serum of osteosarcoma patients and could serve as a good diagnostic biomarker for osteosarcoma.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 2.Shi ZW, Wang JL, Zhao N, et al. Single nucleotide polymorphism of hsa-miR-124a affects risk and prognosis of osteosarcoma. Cancer Biomark. 2016;17(2):249–57. doi: 10.3233/CBM-160637. [DOI] [PubMed] [Google Scholar]
- 3.Chiappetta C, Mancini M, Lessi F, et al. Whole-exome analysis in osteosarcoma to identify a personalized therapy. Oncotarget. 2017;8(46):80416–28. doi: 10.18632/oncotarget.19010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He JP, Hao Y, Wang XL, et al. Review of the molecular pathogenesis of osteosarcoma. Asian Pac J Cancer Prev. 2014;15(15):5967–76. doi: 10.7314/apjcp.2014.15.15.5967. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Huang J, Ni J, et al. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle. 2017;16(6):578–87. doi: 10.1080/15384101.2017.1288324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne TS, Khanna C. A review of the association between osteosarcoma metastasis and protein translation. J Comp Pathol. 2012;146(2–3):132–42. doi: 10.1016/j.jcpa.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Almeida RA, Fraczek MG, Parker S, et al. Non-coding RNAs and disease: The classical ncRNAs make a comeback. Biochem Soc Trans. 2016;44(4):1073–78. doi: 10.1042/BST20160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333–38. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 9.Taborda MI, Ramirez S, Bernal G. Circular RNAs in colorectal cancer: Possible roles in regulation of cancer cells. World J Gastrointest Oncol. 2017;9(2):62–69. doi: 10.4251/wjgo.v9.i2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett SP, Salzman J. Circular RNAs: Analysis, expression and potential functions. Development. 2016;143(11):1838–47. doi: 10.1242/dev.128074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie H, Ren X, Xin S, et al. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7(18):26680–91. doi: 10.18632/oncotarget.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei K, Bai H, Wei Z, et al. The mechanism and function of circular RNAs in human diseases. Exp Cell Res. 2018;368(2):147–58. doi: 10.1016/j.yexcr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zhao Y. Circular RNAs: Characteristics, function, and role in human cancer. Histol Histopathol. 2018;33(9):887–93. doi: 10.14670/HH-11-969. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Xin Y. Circular RNAs: A new frontier for cancer diagnosis and therapy. J Hematol Oncol. 2018;11(1):21. doi: 10.1186/s13045-018-0569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caiment F, Gaj S, Claessen S, et al. High-throughput data integration of RNA-miRNA-circRNA reveals novel insights into mechanisms of benzo[a]pyrene-induced carcinogenicity. Nucleic Acids Res. 2015;43(5):2525–34. doi: 10.1093/nar/gkv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song YZ, Li JF. Circular RNA hsa_circ_0001564 regulates osteosarcoma proliferation and apoptosis by acting miRNA sponge. Biochem Biophys Res Commun. 2018;495(3):2369–75. doi: 10.1016/j.bbrc.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Huang K, Jie Z, et al. CircFAT1 sponges miR-375 to promote the expression of Yes-associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17(1):170. doi: 10.1186/s12943-018-0917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Shi W, Jiang C. Overexpressing circular RNA hsa_circ_0002052 impairs osteosarcoma progression via inhibiting Wnt/beta-catenin pathway by regulating miR-1205/APC2 axis. Biochem Biophys Res Commun. 2018;502(4):465–71. doi: 10.1016/j.bbrc.2018.05.184. [DOI] [PubMed] [Google Scholar]
- 19.Kun-Peng Z, Chun-Lin Z, Jian-Ping H, et al. A novel circulating hsa_circ_0081001 act as a potential biomarker for diagnosis and prognosis of osteosarcoma. Int J Biol Sci. 2018;14(11):1513–20. doi: 10.7150/ijbs.27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Xu Y, Qian Z, et al. circRNA_104075 stimulates YAP-dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis. 2018;9(11):1091. doi: 10.1038/s41419-018-1132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Chen RX, Wei WS, et al. PRMT5 Circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin Cancer Res. 2018;24(24):6319–30. doi: 10.1158/1078-0432.CCR-18-1270. [DOI] [PubMed] [Google Scholar]