Abstract
Background
Anterior cervical plate and cage fixation system (ACPC) used in anterior cervical corpectomy and fusion (ACCF) is reported to incur excess complications. This study aimed to introduce integrated fixation cage (IFC) into ACCF to eliminate the anterior cervical plate (ACP)-related complications.
Material/Methods
One validated intact and 3 ACCF-simulated C3–C7 cervical spine models were developed. In ACCF models, C5 was corpectomied and fixed by IFC or ACPC. For each model, 1.0 Nm moments of flexion, extension, lateral bending, and torsion were imposed on the C3 vertebra. The range of motion (ROM) of each segment and the stress distribution on screw-vertebra interface, bone graft, and cage-endplate were recorded and analyzed.
Results
ROMs of C3–C7 were not different in any motion condition between IFC and ACPC models. The maximal von Mises stress on screw-vertebra interface of the IFC model was lower than that of the ACPC models in flexion, extension, and lateral bending, but higher in rotation. The maximal von Mises stress on bone graft of the IFC model was higher compared with the ACPC models, except in flexion. The IFC model showed a higher maximal von Mises stress on cage-endplate interface in all motion planes.
Conclusions
Based on finite element analysis, IFC provided identical C3–C7 construct stability as ACPC. Compared with ACPC, IFC showed better biomechanical performance on screw-vertebra interface and bone graft, but worse biomechanical performance on cage-endplate interface.
MeSH Keywords: Biomechanical Phenomena, Cervical Vertebrae, Finite Element Analysis, Spinal Fusion
Background
Anterior cervical corpectomy and fusion (ACCF) is a common surgical intervention in treating numerous cervical spine diseases, including degenerative, traumatic, neoplastic, and infectious lesions [1,3] One limitation of ACCF is that it often leads to complications, such as postoperative dislodgement and migration of cage, pseudarthrosis, and segmental kyphosis [4]. Addition of an anterior cervical plate (ACP) is an effective solution to these complications, and has been widely used. ACP is reported to provide rigid segmental stability, thereby increasing the fusion rate, preventing segmental kyphosis, and reducing complications related to cage subsidence [4,5].
However, ACP also has some disadvantages. Firstly, affixing ACP extends the operation time, increases blood loss and cost, and makes revision surgery more difficult if the ACP has to be removed [6–8]. Secondly, ACP-induced compression to adjacent tissues may lead to esophageal and pharyngeal injury, dysphagia, and adjacent-level degeneration [9–12]. Furthermore, ACP or screw failure severely injures peripheral tissue, leading to serious consequences, even death [13,14]. These problems of ACP need to be resolved.
Several attempts have been made to tackle the ACP-related complications. Recently, the integrated interbody device has drawn critical attention. The integrated interbody consists of an interbody spacer, in which screw holes are set to allow fixation directly through the endplate. This design avoids using additional anterior internal fixation devices, thus theoretically circumventing the incidence of aforementioned ACP-related complications [15]. At the same time, it still provides sufficient segmental stability needed for cervical spinal fusion. This device has been proved and applied in anterior cervical discectomy and fusion; however, few published studies have investigated whether it is available in ACCF. In order to explore the possibility of using integrated fixation cage (IFC) instead of anterior cervical plate and cage fixation system (ACPC) in ACCF to avoid the clinical complications caused by ACP, in the present study, the IFC was tested in one-level ACCF by comparing it with ACPC. The finite element (FE) method was adopted here due to the difficulty in obtaining internal stress information from traditional biomechanical study of cadavers.
Material and Methods
FE molding of intact spine
The computed tomography (CT) images of cervical spine were obtained at 1-mm interval from a healthy male volunteer (age 31 years, weight 76 kg, and height 177 cm) who had not suffered any cervical disease. Then, the CT images were imported into the image-processing software (Mimics 19 and 3 Matic 11, Materialise, Inc., Belgium) and commercially available finite-element analysis software (Hypermesh 14.0, Altair Technologies, Inc., CA, USA) to construct the geometric structure of C3–C7 consisting of 5 vertebral bodies and 4 intervertebral disks.
The vertebral bodies consisted of cancellous bone, cortical bone, posterior bone structure, and endplate. The articular surface of the facet joint was covered with a layer of cartilage, between which the joint space was set to 0.5 mm. The intervertebral disks were partitioned into 3 parts: nucleus pulposus, annulus ground substance, and annulus fiber. The annulus fiber was a net structure constructed by a truss element comprising 20% of the ground substance volume and an inclination between 15° and 45° with respect to the horizontal planes [16]. Five major intervertebral ligaments, including anterior longitudinal ligament, posterior longitudinal ligaments, flaval ligaments, capsular ligaments, and interspinous ligaments, were constructed at corresponding anatomical positions.
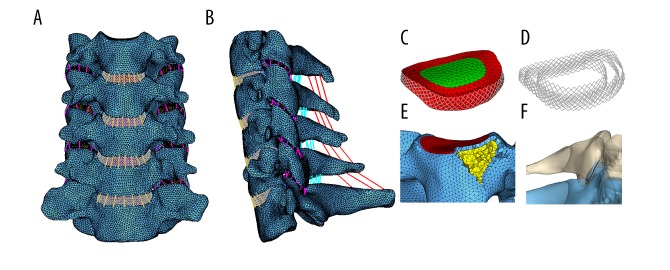
The cortical bone, cancellous bone, posterior bone structure, and cartilage were all meshed into tetrahedron elements (C3D4). The endplate was constructed with a 0.4-mm-thick shell element (S3) [17]. Nucleus pulposus and annulus ground substance were mesh into triangular prism elements (C3D6). Annulus fiber and all intervertebral ligaments were modeled as tension-only truss elements. The interfaces between the vertebral body, endplate, and intervertebral disk were assigned with a tie constraint. The facet joints were assigned with a frictionless sliding contact. Thus, an intact C3–C7 cervical spine FE model was constructed (Figure 1). The material properties of this FE model were in accordance with previous FE studies and are shown in Table 1.
Figure 1.
Finite element model of intact C3–C7 cervical spine. (A) Frontal view; (B) Lateral view; (C) Intervertebral disk; (D) Annulus fiber; (E) Vertebral body; (F) Facet joint.
Table 1.
Material properties for components used in current FE models.
| Material | Elastic modulus (MPa) | Poisson ratio | Cross-sectional area (mm2) |
|---|---|---|---|
| Bones | |||
| Cortical bone | 12000 | 0.3 | – |
| Cancellous bone | 100 | 0.2 | – |
| Endplate | 500 | 0.4 | – |
| Posterior structure | 3500 | 0.25 | – |
| Bone graft | 3500 | 0.3 | – |
| Facet cartilage | 10 | 0.4 | – |
| Implants | – | ||
| IFC/APC/screw | 110000 | 0.3 | |
| Ligaments | |||
| ALL | 30 | 0.4 | 6.1 |
| PLL | 20 | 0.4 | 5.4 |
| LF | 10 | 0.4 | 50.1 |
| ISL | 10 | 0.4 | 13.1 |
| CL | 10 | 0.4 | 46.6 |
| Intervertebral disc | |||
| Annulus fiber | 450 | 0.45 | |
| Annulus ground | Hyperelastic | ||
| Substance | Mooney-Rivlin | ||
| C10=0.56 | |||
| C01=0.14 | |||
| Nucleus pulposus | Hyperelastic | ||
| Mooney-Rivlin | |||
| C10=0.12 | |||
| C01=0.09 | |||
ALL – anterior longitudinal ligaments; PLL – posterior longitudinal ligaments; LF – ligamentum flavum; ISL – interspinous ligaments; CL – capsular ligaments.
Simulation of ACCF
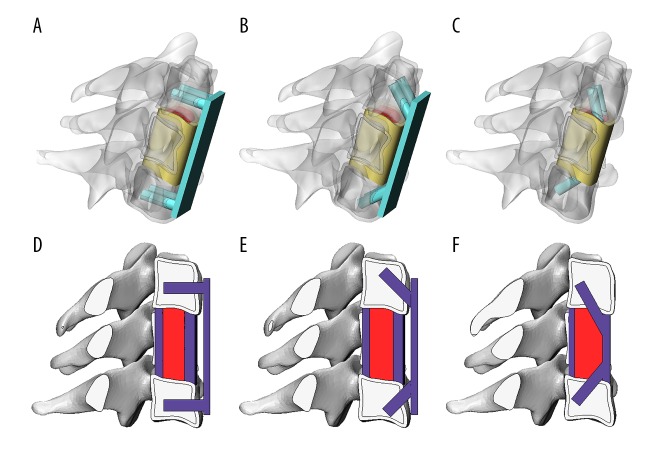
To simulate the ACCF clinical scenario, a one-level C5 vertebral body corpectomy was performed, and the adjoining intervertebral disk and ALL were totally excised. The IFC and cage of ACPC inserted with bone graft were installed between C4 inferior and C7 superior endplates according to clinical experiences. After installing the cage and bone graft, 4 titanium screws were fixed inside the C4 and C7 vertebral body along the direction of the screw holes in the IFC model. In the ACPC model, an ACP was rigidly fixed to the C4 and C6 vertebral body by 4 titanium screws with 2 different combinations of angulation within the vertebral bodies (C4, C7): (0°, 0°) and (45°, 45°).
The internal fixation devices and bone graft were designed and simplified based on the dimension of the intact model and titanium mesh cage used in clinical practice. The IFC, ACPC, screw, and bone graft were meshed into tetrahedron elements (C3D4). The screw-vertebra interface and screw-implant interface was assigned with a tie constraint. The endplate-cage, endplate-bone graft, and bone graft-screw interfaces were assigned with a nonlinear node–face contact with a friction coefficient of 0.07 [18]. Then, 3 kinds of ACCF FE models were constructed. The 3D and sagittal plane diagram of the ACCF models are shown in Figure 2. The material properties of implants and bone graft are shown in Table 1.
Figure 2.
3D and 2D view of implants used in ACCF models. A/D, ACPC (0°, 0°). B/E, ACPC (45°, 45°). C/F, IFC.
Keeping the inferior endplate of C7 fixed at 6 degrees of freedom, a moment of 1.0 Nm was applied at a reference point coupled with the superior surface of C3 in sagittal, coronal, and horizontal planes to simulate the spinal motions of flexion, lateral bending, and axial rotation. In addition, an axial compressive preload of 73.6 N was applied through the motion center of the cervical spine model to simulate the follow-load technique [19]. All the intact and ACCF FE models were solved in Abaqus 6.13 (Simulia, Inc., RI, USA) using the nonlinear method. The intact model was validated against the published FE [20,21] and in vitro studies [22]. The stability of the ACCF model was measured in terms of the segmental range of motion (ROM). The distribution of von Mises stress on screw-vertebra interface, bone graft, and cage-endplate interface was recorded and analyzed to assess the mechanical superiority of each fixation device.
Results
Model validation results for intact model
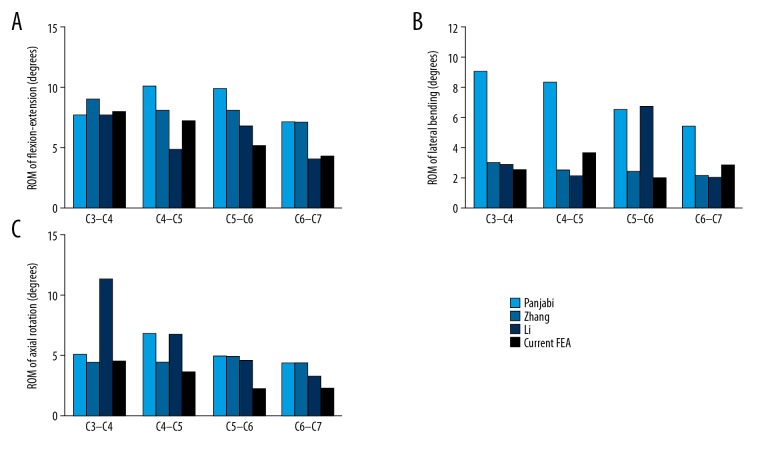
The ROM of each segment obtained from the intact model was compared with the results of Panjabi et al., Zhang et al., and Li et al. (Figure 3). The ROMs of the present intact model were all smaller than the average segmental ROMs of the in vitro experiment; the minimum difference in segmental ROM (C3–C4, C4–C5, C5–C6, and C6–C7) was flexion-extension (0°, 0°, 0°, 0°), lateral bending (4.5°, 2.9°, 2.9°, 1.1°), and rotation (0°, 1.8°, 1.6°, 1.3°). The difference was greater in lateral bending but was not obvious in the other 2 planes. In flexion–extension, all the segmental ROMs of intact model fell within the standard deviation of the results reported by Panjabi et al. [22]. Furthermore, the ROMs of the present study were found to be much closer to the data of Zhang et al. and Li et al., [20,21], who also used the FE cervical spine model. Combining the comparisons between in vitro and FE experiments, the FE model can be regarded as validated and could be used in the present study.
Figure 3.
Validation of the C3–C7 intact model. (A) Under flexion/extension moment of ±1.0 Nm; (B) Under lateral bending moment of ±1.0 Nm; (C) Under axial rotation moment of ±1.0 Nm.
Range of motion
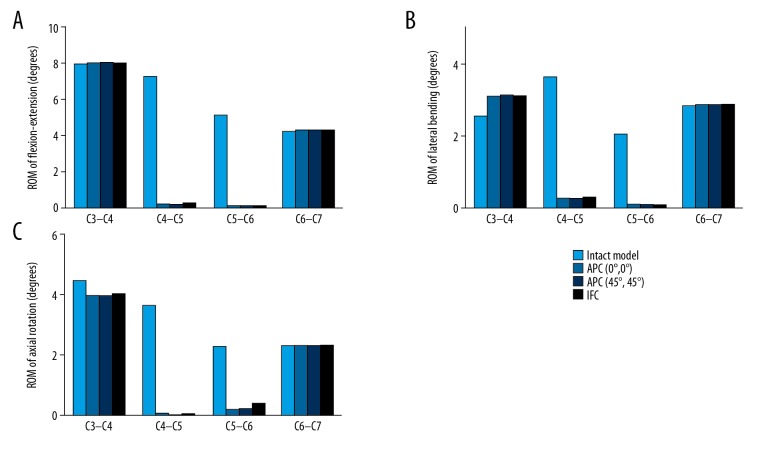
The ROM and construct stability were independent of the type of internal fixation devices. All the ACCF surgical operations completely fixed C4–C5 and C5–C6 segments but had no influence on C3–C4 and C6–C7 segments. The reduction in C4–C6 ROM in flexion-extension, lateral bending, and rotation was ACPC (0° screw; 96.6%, 93.4%, 96.0%), ACPC (45° screw; 96.8%, 93.6%, 96.3), and IFC (96.1%, 93.2%, 93.0%) (Figure 4).
Figure 4.
ROM in flexion-extension, lateral bending and axial rotation of ACCF models. (A) Under flexion/extension moment of ±1.0 Nm; (B) Under lateral bending moment of ±1.0 Nm; (C) Under axial rotation moment of ±1.0 Nm
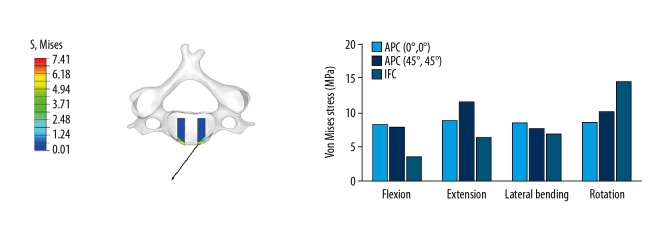
Stress distribution on screw-vertebra interface
The screw-vertebra stress was associated with the type of fixation device but showed the opposite trend in different motion direction. Compared with the ACPC (0° screw) and ACPC (45° screw) models, the max screw-vertebra stress of the IFC model was lower in flexion (56%, 54%), extension (28%, 45%), and lateral bending (20%, 11%), but higher in rotation (68%, 43%). The angular position of the screw altered the stress distribution on screw-vertebra, but not significantly: ACPC (45° screw) models showed higher max stress in extension (30%) and rotation (17%) and lower max stress in flexion (4%) and lateral bending (10%). The stress was mainly concentrated on the interface of screw and cortical bone in all models (Figure 5).
Figure 5.
Stress distribution on screw-vertebra interface in flexion, extension, lateral bending, and axial rotation.
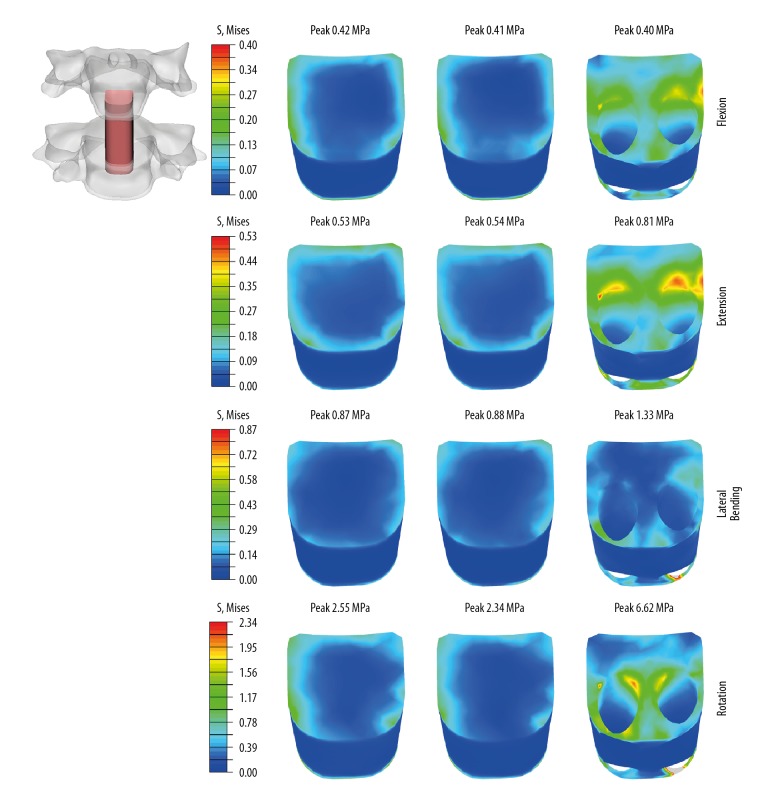
Stress distribution on bone graft
The graft stresses were dependent on the type of fixation device. Compared with the ACPC (0° screw) and ACPC (45°screw) models, the max bone graft stresses of the IFC model were higher in extension (53%, 50%), lateral bending (53%, 51%), and rotation (160%, 183%), and at the same level in flexion. No difference in bone graft stresses was observed between ACPC (0° screw) and ACPC (45° screw) models (Figure 6).
Figure 6.
Stress distribution on bone graft in flexion, extension, lateral bending, and axial rotation. Left, ACPC (0°, 0°); Middle, ACPC (45°, 45°); Right, IFC.
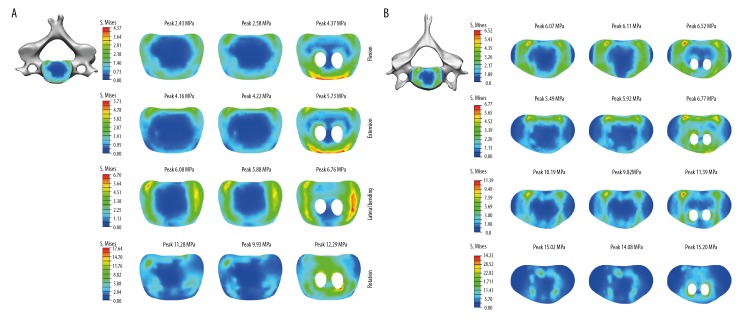
Stress distribution on the cage-endplate interface
The cage-endplate stresses were dependent on the type of fixation device. Compared with the ACPC (0° screw) and ACPC (45° screw) models, the C4 inferior cage-endplate stresses in the IFC model were higher in all motion planes: flexion (80%, 69%), extension (38%, 35%), lateral bending (11%, 15%), and rotation (9%, 24%). Similarly, the C6 superior cage-endplate stresses in the IFC model were also higher in all motion planes compared with the ACPC (0° screw) and ACPC (45° screw) models: flexion (7%, 7%), extension (23%, 14%), lateral bending (12%, 16%), and rotation (1%, 8%). The max endplate stresses of ACPC models were located on the endplate-cage interface in all motion directions. Similar to the ACPC models, the max endplate stresses of the IFC model also existed on the endplate-cage interface in flexion, extension, and lateral bending. However, it was located on the edge of the drilled hole of endplate in rotation (Figure 7).
Figure 7.
Stress distribution on cage-endplate interface in flexion, extension, lateral bending, and rotation. (A) Stress on C4 cage-endplate interface; (B) Stress on C6 cage-endplate interface; Left, ACPC (0°, 0°); Middle, ACPC (45°, 45°); Right, IFC.
Discussion
This FE study investigated the feasibility of using a novel internal anterior cervical fixation device (IFC) instead of ACPC in ACCF from the perspective of biomechanics. In this novel fixation device, ACP was removed to eliminate the adjacent tissue injury and stress-shielding effects, the operation-level stability was achieved by 4 screws directly through the vertebral endplate. Compared with the traditional anterior cervical plate and cage fixation system (ACPC), the IFC model in the present study showed slightly higher endplate–cage interface stress, which might increase the risk of cage subsidence. However, it demonstrated equivalent immediate postoperative stability, smaller screw-vertebra stress needed for long-term stability, and better graft stress condition necessitated by fusion.
IFC provided identical immediate postoperative stability as ACPC
Previous in vitro biomechanical and FE studies found that ACPC sharply decreased the ROM of operated spine in flexion-extension, lateral bending, and rotation after ACCF [23,24]. In current study, the ACPC and IFC decreased the ROM of operation level by up to 93% at least; this result is consistent with that of the previous studies, and confirmed the correctness of the FE ACCF-simulated models. No significant differences in reduction of ROM were detected between IFC and ACPC, which clearly indicates that the IFC can provide identical immediate spinal stability as ACPC. This finding determined that the IFC met the basic requirement of anterior cervical internal fixation according to the conclusion of Manohar M. Panjabi, et al.: the most important goal of internal fixation instrument was to provide stability to the unstable spine. The values of the decreased ROM (93%) in all groups were little higher than that of previously reported levels [23,24], which might be due to the model difference between cadavers and FE models and the different loading protocols in each study.
IFC might effectively reduce the risk of screw loosening
Except for the immediate stability, the clinical performance of an anterior cervical fixation device is also dependent on the long-term stability offered by the maintenance of the strength of the screw-vertebra fixation. Cases of delayed screw loosening or breakage have been reported in previously published studies [25,26]; in spite of successful spine arthrodesis, the incidence has been estimated as high as 5.2% [27]. To assess the morbidity of screw loosening, the stresses on the screw-vertebra interface were recorded in the present study, as too much stress on this region might be the leading cause in clinical series [23]. The results showed that the screw-vertebra interface stresses in the IFC model were obviously lower than those of the ACPC models in flexion-extension and lateral bending, and higher during rotation. It can thus be concluded that IFC can effectively reduce the risk of screw loosening in most motion conditions [28], and as such, IFC may provide an option to reduce screw-related complications.
IFC has the potential to improve the fusion rate
The final goal of ACCF is to realize solid bone fusion. To achieve this purpose, the optimal mechanical microenvironment between the endplate and the bone graft is of fundamental importance. According to the Wolff law [29], a certain stress stimulates bone formation, while the lack of stress promotes bone absorption. In the present study, the IFC model showed much higher bone graft stresses than the ACPC models in extension, lateral bending, and rotation. Although the optimal stress needed for bone graft-endplate fusion is unknown, this capacity of IFC may provide a new way to improve the fusion rate of ACCF.
IFC may have a slightly increased risk of subsidence
One concern with the use of IFC is the safety problem with vertebral endplates caused by the insertion of screws and endplate preparation. Several reports have shown that IFC had a higher subsidence rate than the plate-cage construct in ACDF [30,31]. In the present study, IFC increased the cage-endplate stresses in all motion planes compared with ACPC, and it seems that the higher subsidence rate of IFC would also occur in ACCF. However, the actual increased stresses were limited, and the max increased value was only 2.36 MPa. In the meta-analysis by Liu [31], IFC was shown to possess a higher cage subsidence rate but had no statistically significant difference in cervical lordosis and long-term complications. Hence, it could conceivably be that such a small change in stress neither severely aggravates the cage subsidence [32] nor causes critical neurologic symptoms. Nevertheless, optimization measures, such as end structure of cage and screw position, should be considered in designing the IFC model to decrease the potential risk of cage subsidence [33].
Limitations
The present study clearly had some limitations. The cage and bone graft were simplified in this study to accurately simulate the physiological postoperative contact between the endplate, cage, and bone graft instead of directly binding them as in the previous studies. The bone graft was designed as a uniform solid cylinder and the cage as a hollow circular cylinder, neglecting the meshes on the side wall and the spinous processes on end faces. In addition, the screws were designed as solid cylinders bound to the cage or plate, and the threads on the screws were not modeled [34]. These simplifications in the FE model improved the convergence but inevitably affected the actual stress distribution. Another noticeable limitation was that the ACCF models developed in the present study were not validated because of the lack of corresponding normal in vitro studies. Therefore, the results from these models should be interpreted carefully. However, in fact, completely duplicating the result of in vivo studies in FE analysis was impossible and not within the scope of this study. Notwithstanding these limitations, this study effectively shows the biomechanical differences between IFC and ACPCs in ACCF by maintaining the consistency of the experimental conditions in each ACCF model.
Conclusions
The IFC model provided identical construct stability as ACPC. At the same time, it decreased the stresses on the screw-vertebra interface and increased the stresses on bone graft and endplate. Taken together, these results suggest that it would be feasible to use IFC in ACCF to avoid the complications caused by ACP. In vitro and in vivo studies are needed to provide more reliable evidence for further evaluation.
Footnotes
Source of support: This project was supported by the Key Research Project of Shanxi Province (2017ZDCXL-SF-01-05)
References
- 1.Ghogawala Z. Anterior cervical option to manage degenerative cervical myelopathy. Neurosurg Clin N Am. 2018;29(1):83–89. doi: 10.1016/j.nec.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaidis I, Fouyas IP, Sandercock PA, et al. Surgery for cervical radiculopathy or myelopathy. Cochrane Database Syst Rev. 2010;(1):CD001466. doi: 10.1002/14651858.CD001466.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann S, Tschugg A, Obernauer J, et al. Cervical corpectomies: Results of a survey and review of the literature on diagnosis, indications, and surgical technique. Acta Neurochir (Wien) 2016;158(10):1859–67. doi: 10.1007/s00701-016-2908-z. [DOI] [PubMed] [Google Scholar]
- 4.Xiao SW, Jiang H, Yang LJ, et al. Anterior cervical discectomy versus corpectomy for multilevel cervical spondylotic myelopathy: A meta-analysis. Eur Spine J. 2015;24(1):31–39. doi: 10.1007/s00586-014-3607-1. [DOI] [PubMed] [Google Scholar]
- 5.Oliver JD, Goncalves S, Kerezoudis P, et al. Comparison of outcomes for anterior cervical discectomy and fusion with and without anterior plate fixation: A systematic review and meta-analysis. Spine (Phila Pa 1976) 2018;43(7):E413–22. doi: 10.1097/BRS.0000000000002441. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Wang H, Li X, et al. Comparison of a Zero-Profile anchored spacer (ROI-C) and the polyetheretherketone (PEEK) cages with an anterior plate in anterior cervical discectomy and fusion for multilevel cervical spondylotic myelopathy. Eur Spine J. 2016;25(6):1881–90. doi: 10.1007/s00586-016-4500-x. [DOI] [PubMed] [Google Scholar]
- 7.Yun DJ, Lee SJ, Park SJ, et al. Use of a zero-profile device for contiguous 2-level anterior cervical diskectomy and fusion: Comparison with cage with plate construct. World Neurosurg. 2017;97:189–98. doi: 10.1016/j.wneu.2016.09.065. [DOI] [PubMed] [Google Scholar]
- 8.Sellin JN, Burks SS, Levi AD. Fracture of fusion mass following anterior cervical plate removal: Case report. J Clin Neurosci. 2018;47:128–31. doi: 10.1016/j.jocn.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Ma L, Yang D, et al. Incidence and risk factors of postoperative adjacent segment degeneration following anterior decompression and instrumented fusion for degenerative disorders of the cervical spine. World Neurosurg. 2017;105:78–85. doi: 10.1016/j.wneu.2017.05.094. [DOI] [PubMed] [Google Scholar]
- 10.Joseph JR, Smith BW, Mummaneni PV, et al. Postoperative dysphagia correlates with increased morbidity, mortality, and costs in anterior cervical fusion. J Clin Neurosci. 2016;31:172–75. doi: 10.1016/j.jocn.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Halani SH, Baum GR, Riley JP, et al. Esophageal perforation after anterior cervical spine surgery: A systematic review of the literature. J Neurosurg Spine. 2016;25(3):285–91. doi: 10.3171/2016.1.SPINE15898. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Lu X, He H, et al. Longer plate-to-disc distance prevents adjacent-level ossification development but does not influence adjacent-segment degeneration. Spine (Phila Pa 1976) 2015;40(7):E388–93. doi: 10.1097/BRS.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 13.Wong DT, Fehlings MG, Massicotte EM. Anterior cervical screw extrusion leading to acute upper airway obstruction – Case report. Spine. 2005;30(22):E683–86. doi: 10.1097/01.brs.0000186861.82651.00. [DOI] [PubMed] [Google Scholar]
- 14.Kapu R, Singh M, Pande A, et al. Delayed anterior cervical plate dislodgement with pharyngeal wall perforation and oral extrusion of cervical plate screw after 8 years: A very rare complication. J Craniovertebr Junction Spine. 2012;3(1):19–22. doi: 10.4103/0974-8237.110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin M, Ma J, Huang Q, et al. The new Zero-P implant can effectively reduce the risk of postoperative dysphagia and complications compared with the traditional anterior cage and plate: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2016;17(1):430. doi: 10.1186/s12891-016-1274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo Z, Li Q, Jia Z, et al. Biomechanical consideration of prosthesis selection in hybrid surgery for bi-level cervical disc degenerative diseases. Eur Spine J. 2017;26(4):1181–90. doi: 10.1007/s00586-016-4777-9. [DOI] [PubMed] [Google Scholar]
- 17.Mo ZJ, Zhao YB, Du CF, et al. Does location of rotation center in artificial disc affect cervical biomechanics? Spine. 2015;40(8):E469–75. doi: 10.1097/BRS.0000000000000818. [DOI] [PubMed] [Google Scholar]
- 18.Chen SH, Zhong ZC, Chen CS, et al. Biomechanical comparison between lumbar disc arthroplasty and fusion. Med Eng Phys. 2009;31(2):244–53. doi: 10.1016/j.medengphy.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Ganbat D, Kim YH, Kim K, et al. Effect of mechanical loading on heterotopic ossification in cervical total disc replacement: A three-dimensional finite element analysis. Biomech Model Mechanobiol. 2016;15(5):1191–99. doi: 10.1007/s10237-015-0752-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QH, Teo EC, Ng HW, et al. Finite element analysis of moment-rotation relationships for human cervical spine. J Biomech. 2006;39(1):189–93. doi: 10.1016/j.jbiomech.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Lewis G. Influence of surgical treatment for disc degeneration disease at C5–C6 on changes in some biomechanical parameters of the cervical spine. Med Eng Phys. 2010;32(6):595–603. doi: 10.1016/j.medengphy.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Panjabi MM, Crisco JJ, Vasavada A, et al. Mechanical properties of the human cervical spine as shown by three-dimensional load-displacement curves. Spine (Phila Pa 1976) 2001;26(24):2692–700. doi: 10.1097/00007632-200112150-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hussain M, Natarajan RN, Fayyazi AH, et al. Screw angulation affects bone-screw stresses and bone graft load sharing in anterior cervical corpectomy fusion with a rigid screw-plate construct: A finite element model study. Spine J. 2009;9(12):1016–23. doi: 10.1016/j.spinee.2009.08.461. [DOI] [PubMed] [Google Scholar]
- 24.Isomi T, Panjabi MM, Wang JL, et al. Stabilizing potential of anterior cervical plates in multilevel corpectomies. Spine (Phila Pa 1976) 1999;24(21):2219–23. doi: 10.1097/00007632-199911010-00008. [DOI] [PubMed] [Google Scholar]
- 25.Anandaswamy TC, Pujari VS, Shivanna S, et al. Delayed pharyngoesophageal perforation following anterior cervical spine surgery: An incidental finding. J Anaesthesiol Clin Pharmacol. 2012;28(1):139–40. doi: 10.4103/0970-9185.92477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CC, Guo JH, Cheng YK, et al. Delayed anterior cervical screws migrating simultaneously to the lung and stomach. Spine J. 2016;16(4):e263–64. doi: 10.1016/j.spinee.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 27.Xie N, Yuan W, Ye XJ, et al. Anterior cervical locking plate-related complications; prevention and treatment recommendations. Int Orthop. 2008;32(5):649–55. doi: 10.1007/s00264-007-0369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fakhouri SF, Shimano MM, de Araujo CA, et al. Analysis of stress induced by screws in the vertebral fixation system. Acta Ortop Bras. 2014;22(1):17–20. doi: 10.1590/S1413-78522014000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frost HM. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994;64(3):175–88. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Shi S, Liu ZD, Li XF, et al. Comparison of plate-cage construct and stand-alone anchored spacer in the surgical treatment of three-level cervical spondylotic myelopathy: A preliminary clinical study. Spine J. 2015;15(9):1973–80. doi: 10.1016/j.spinee.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Liu WJ, Hu L, Wang JW, et al. Comparison of zero-profile anchored spacer versus plate-cage construct in treatment of cervical spondylosis with regard to clinical outcomes and incidence of major complications: A meta-analysis. Ther Clin Risk Manag. 2015;11:1437–47. doi: 10.2147/TCRM.S92511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailey CS, Sjovold SG, Dvorak MF, et al. The strength profile of the thoracolumbar endplate reflects the sagittal contours of the spine. Spine (Phila Pa 1976) 2011;36(2):124–28. doi: 10.1097/BRS.0b013e3181cc8a32. [DOI] [PubMed] [Google Scholar]
- 33.Nemoto O, Kitada A, Naitou S, et al. Stand-alone anchored cage versus cage with plating for single-level anterior cervical discectomy and fusion: A prospective, randomized, controlled study with a 2-year follow-up. Eur J Orthop Surg Traumatol. 2015;25(Suppl 1):S127–34. doi: 10.1007/s00590-014-1547-4. [DOI] [PubMed] [Google Scholar]
- 34.Meng L, Zhang Y, Lu Y. Three-dimensional finite element analysis of mini-external fixation and Kirschner wire internal fixation in Bennett fracture treatment. Orthop Traumatol Surg Res. 2013;99(1):21–29. doi: 10.1016/j.otsr.2012.07.015. [DOI] [PubMed] [Google Scholar]