Abstract
Background
The aim of this study was to investigate the correlations of C-reactive protein (CRP), interleukin-6 (IL-6), and insulin resistance (IR) with cerebral infarction in hypertensive patients.
Material/Methods
A total of 80 patients with cerebral infarction admitted to our hospital from March 2016 to November 2017 were selected and divided into 2 groups according to the diameter of cerebral infarction, namely, lacunar cerebral infarction group (n=40) and cerebral infarction group (n=40). The levels of high-sensitivity CRP (hs-CRP) and IL-6, homeostasis model assessment of IR (HOMA-IR) index and blood pressure level were compared between the 2 groups. The correlations of hs-CRP level, IL-6 level, and IR with the diameter of cerebral infarction, as well as the relationships of hs-CRP level and IR with the neurological function score after cerebral infarction were analyzed.
Results
The levels of hs-CRP and IL-6 in the cerebral infarction group were significantly higher than those in the lacunar cerebral infarction group (P<0.05). The cerebral infarction group had a markedly higher HOMA-IR index than the lacunar cerebral infarction group (P<0.05), but it had remarkably decreased systolic blood pressure and diastolic blood pressure compared with those in the lacunar cerebral infarction group (P<0.05). There were positive correlations of hs-CRP level, IL-6 level, and IR with the diameter of cerebral infarction (P<0.05). The hs-CRP level and IR had positive correlations with the neurological function score after cerebral infarction (P<0.05).
Conclusions
In hypertensive patients complicated with cerebral infarction, the body’s inflammatory factors, and IR are positively correlated with the diameter of cerebral infarction, as well as the neurological prognosis of the patients.
MeSH Keywords: Interleukin-6, Metabolic Syndrome X, White Coat Hypertension
Background
Cerebrovascular diseases, as one of the major causes affecting human life and health at present, are one of the top 3 major fatal diseases that lead to the death in the world’s population [1]. With the aging of population and the changes of people’s life and diets in China, the morbidity, disability, and mortality rates of cerebrovascular diseases are significantly increased [2], seriously affecting the quality of life and life safety of people in China, especially the middle-aged and elderly [3]. Meanwhile, the incidence rate of hypertension in the middle-aged and elderly population has been at a high level. Hypertension is mainly treated with long-term drug conservative therapy of internal medicine. However, with the extension of the course of the disease, the long-term blood pressure elevation will lead to impairment of endothelial function [4], and hypertension acts as an independent risk factor for the onset of cerebral infarction. Even hypertensive patients with stable blood pressure control previously have an obviously higher incidence rate of cerebral infarction than normal population [5].
High-sensitivity C-reactive protein (hs-CRP), which can effectively reflect the inflammatory conditions of the body [6], has extremely high sensitivity to the mild aseptic inflammation, and has been widely applied to clinical detection of inflammatory conditions in various diseases. Insulin resistance (IR) is considered as a related risk factor for various medical diseases such as diabetes, hyperlipidemia and hypertension. In addition, recent studies have confirmed that the pathogenesis of IR in cerebral infarction has attracted more and more clinical attention [7]. To better guide clinical treatment and improve the prognosis of hypertensive patients complicated with cerebral infarction, this study mainly explores the correlations of the changes in the body’s inflammatory factors and IR with the occurrence of cerebral infarction in hypertensive patients, especially the correlation between their changing trends.
Material and Methods
General data
A total of 80 patients with cerebral infarction admitted to our hospital from March 2016 to November 2017 were selected. Before patients were included into groups, all of them and their family members signed the voluntary inclusion agreement, which was then submitted to the Hospital Ethics Committees for approval. All patients were diagnosed by magnetic resonance imaging (MRI) combined with clinical manifestations before inclusion. Patients were aged over 50 years old, with previous normal neurological and mental function, in line with emergency department admission, and they received MRI. Patients were excluded for the following: complicated with severe mental illness, serious cardiovascular diseases, previous brain-related surgical treatment, intracranial tumor, systemic infection, diabetes, chronic obstructive pulmonary disease, immune system diseases, expected survival time ≤24 hours, cerebral hernia after the onset or hemorrhagic brain disease. Patients were divided into 2 groups according to the diameter of cerebral infarction, with 40 cases in each group. In the lacunar cerebral infarction group, there were 26 males and 14 females aged 50–80 years old, with an average age of 68.9±1.3 years old, including 21 smokers, the course of hypertension was 10–35 years, with an average of 18.1±1.3 years, and the body mass index (BMI) was 23–31 kg/m2, with an average of 28.1±0.3 kg/m2. In the cerebral infarction group, there were 27 males and 13 females aged 50–80 years old, with an average age of 68.8±1.3 years old, including 20 smokers, the course of hypertension was 10–35 years, with an average of 18.0±1.3 years, and the BMI was 23–31.5 kg/m2, with an average of 28.2±0.3 kg/m2. There were no statistically significant differences in gender, age, smoking history, course of hypertension and BMI (P>0.05). This study has been pre-approved by the ethical committee of Linyi Central Hospital. All patients have signed the consent forms before recruitment in this study.
Material and Methods
All the included patients were first diagnosed with hypertension in line with the diagnostic criteria for hypertension of the World Health Organization in 1978, or had definite hypertension previously and received drug therapy regularly. Moreover, brain infarction was confirmed by brain MRI before the patients were re-included into groups. Improvement of the blood supply of cerebral ischemia site and promotion of the recovery of neurological function as early as possible were taken as the treatment standard such as early thrombolysis, vascular interventional recanalization, and anticoagulation. Furthermore, antihypertensive treatment for hypertension should be paid attention to, and the patients should be treated with symptomatic and supporting treatment after the onset of the disease, such as strengthening the nutrition and psychological intervention of the patients, maintaining the internal environmental balance, and improving the balance of oxygen supply and demand of the body. After the patients were included into groups, their elbow venous blood was immediately sent for testing. The LX-20 automatic biochemical analyzer (Beckman, USA) was adopted for determination of hs-CRP and interleukin-6 (IL-6) levels in the body. At the same time, insulin-related indicators were detected, and the homeostasis model assessment of IR (HOMA-IR) index was calculated.
Observation indicators
The levels of inflammatory factors (hs-CRP and IL-6), HOMA-IR index, and blood pressure level were compared between the 2 groups. The correlations of hs-CRP level, IL-6 level, and IR with the diameter of cerebral infarction, as well as the relationships of hs-CRP level and IR with the neurological function score after cerebral infarction, were analyzed.
Evaluation criteria
The area of cerebral infarction was assessed in accordance with the results of brain MRI, which was performed using the 1.5 T superconducting MRI (GE). Additionally, a comprehensive diagnosis was conducted through the T1 weighted imaging (WI), T2WI, diffusion WI (DWI), and MRA4 models. Among them, infarct lesions with a diameter of 1.5 cm or below were considered as lacunar infarction, and infarct lesions with a diameter of over 1.5 cm were considered as cerebral infarction. Inflammatory factor indicators were mainly detected as follows: IL-6 was determined via enzyme-linked immunosorbent assay (0.37–0.46 μg/L), hs-CRP was determined via latex-enhanced immunoturbidimetry (≤10 mg/L), IR was expressed as HOMA-IR index [with 1 as the clinical reference value, HOMA-IR=fasting blood glucose level (mmol/L) x fasting insulin level (mu/L)], and neurological assessment was conducted using the National Institutes of Health Stroke Scale (NIHSS) score. Assessment was made by the middle-level physicians and above with more than 5 years of clinical experience in neurology twice at an interval of more than 8 hours on the same day to take the average as standard for all assessment scores. The score ranges from 0 to 42 points: the higher the score, the more serious the neurological impairment.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 21.0 software (IBM) was adopted for statistical analysis. The measurement data were expressed as mean ± standard deviation (χ̄±s), and t test was used for comparisons of the mean between the 2 groups, such as inflammatory factor (hs-CRP and IL-6) levels, HOMA-IR index, and blood pressure. Analyses on the correlations of hs-CRP, IL-6 and IR with the size of cerebral infarction and the neurological function score after cerebral infarction were performed by the correlation coefficient method. P<0.05 suggested that the difference was statistically significant.
Results
Comparisons of the levels of inflammatory factors (hs-CRP and IL-6) between the 2 groups
The levels of hs-CRP and IL-6 in the cerebral infarction group were significantly higher than those in the lacunar cerebral infarction group (P<0.05) (Table 1).
Table 1.
Comparisons of the levels of inflammatory factors (hs-CRP and IL-6) between the 2 groups (χ±s).
| IL-6 (μg/L) | Hs-CRP (mmol/L) | |
|---|---|---|
| Lacunar cerebral infarction group | 0.53±0.04 | 11.15±0.16 |
| Cerebral infarction group | 1.11±0.11 | 26.83±0.86 |
| t | 31.340 | 113.368 |
| p | 0.000 | 0.000 |
Comparisons of HOMA-IR index and blood pressure between the 2 groups
The cerebral infarction group had a markedly higher HOMA-IR index than the lacunar cerebral infarction group (P<0.05), but it had remarkably decreased systolic blood pressure and diastolic blood pressure compared with those in the lacunar cerebral infarction group (P<0.05) (Table 2).
Table 2.
Comparisons of HOMA-IR index and blood pressure between the 2 groups (χ±s).
| HOMA-IR index | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | |
|---|---|---|---|
| Lacunar cerebral infarction group | 1.13±0.01 | 135.6±11.3 | 82.1±5.6 |
| Cerebral infarction group | 1.41±0.02 | 106.2±3.4 | 71.3±1.7 |
| t | 79.796 | 15.757 | 11.671 |
| p | 0.000 | 0.000 | 0.000 |
IR – insulin resistance.
Analysis on correlation between the hs-CRP level and the diameter of cerebral infarction
There was a positive correlation between the hs-CRP level and the diameter of cerebral infarction (r=0.9678, P=0.000, P<0.05) (Figure 1).
Figure 1.
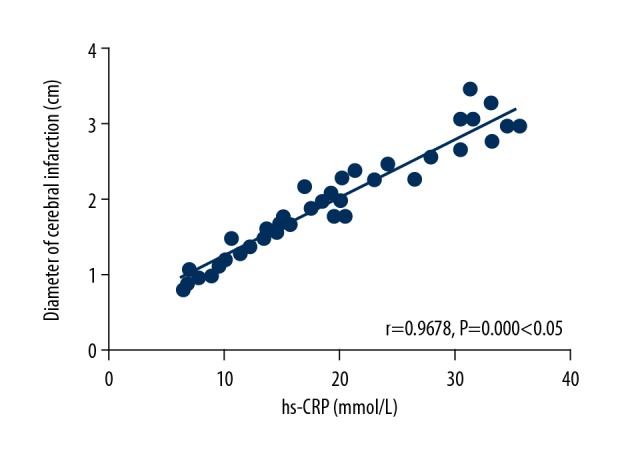
Analysis on correlation between the hs-CRP level and the diameter of cerebral infarction. There is a positive correlation between the hs-CRP level and the diameter of cerebral infarction (P<0.05). CRP – C-reactive protein.
Analysis on correlation between the IL-6 level and the diameter of cerebral infarction
There was a positive correlation between the IL-6 level and the diameter of cerebral infarction (r=0.9489, P=0.000, P<0.05) (Figure 2).
Figure 2.
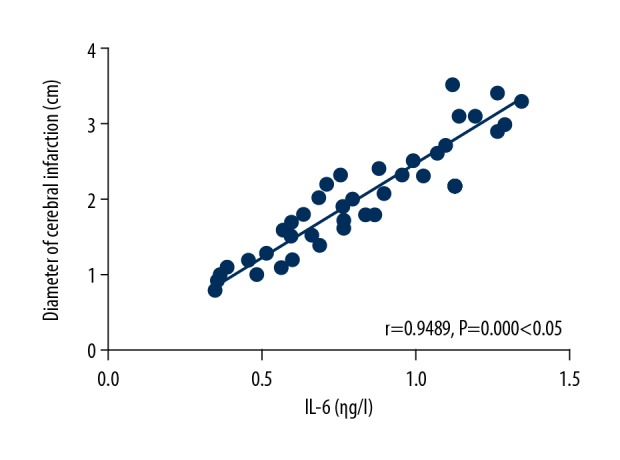
Analysis on correlation between the interleukin (IL)-6 level and the diameter of cerebral infarction. There is a positive correlation between the IL-6 level and the diameter of cerebral infarction (P<0.05).
Analysis on correlation between IR and the diameter of cerebral infarction
IR had a positive correlation with the diameter of cerebral infarction (r 0.9263, P=0.000, P<0.05) (Figure 3).
Figure 3.
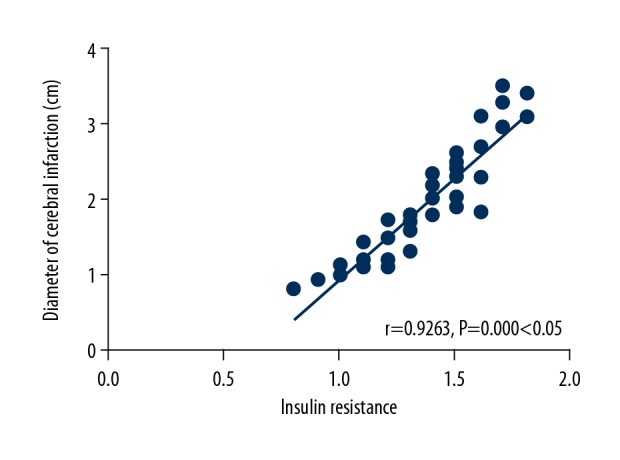
Analysis on correlation between insulin resistance (IR) and the diameter of cerebral infarction. IR has a positive correlation with the diameter of cerebral infarction (P<0.05).
Analysis on correlation between the hs-CRP level and the neurological function score after cerebral infarction
The hs-CRP level was positively correlated with the neurological function score after cerebral infarction (r=0.9776, P=0.000, P<0.05) (Figure 4).
Figure 4.
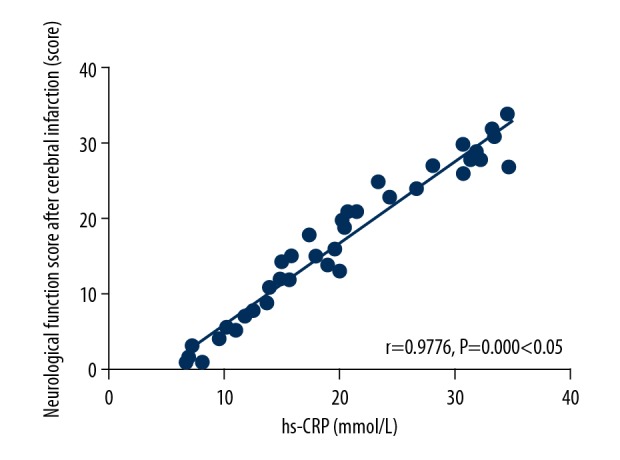
Analysis on correlation between the hs-CRP level and the neurological function score after cerebral infarction. The hs-CRP level is positively correlated with the neurological function score after cerebral infarction (P<0.05). CRP – C-reactive protein.
Analysis on correlation between IR and the neurological function score after cerebral infarction
IR was positively correlated with the neurological function score after cerebral infarction (r=0.9442, P=0.000, P<0.05) (Figure 5).
Figure 5.
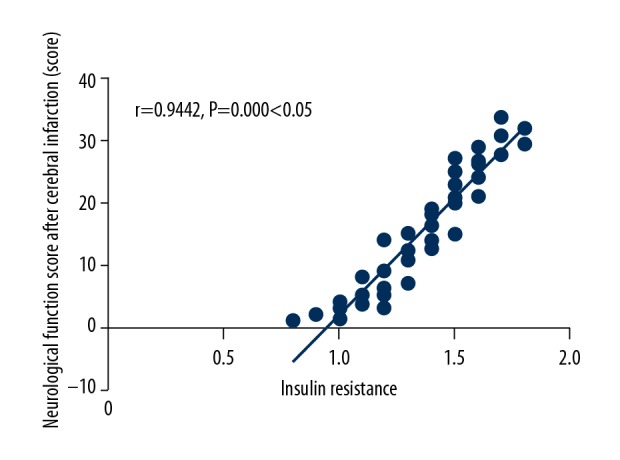
Analysis on correlation between insulin resistance (IR) and the neurological function score after cerebral infarction. IR is positively correlated with the neurological function score after cerebral infarction (P<0.05).
Discussion
Cerebral infarction, as the most common cerebrovascular disease diagnosed in the clinic at present, has high morbidity, disability, and mortality rates, and shows a younger trend [8]. According to studies, the incidence rate is 6–8% in people aged over 45 years old [9], and about 1% of people aged less than 45 years old suffer from this disease [10]. The occurrence of this disease has a great negative impact on the patients and their families. Hypertension is a related factor leading to hyaline degeneration, atherosclerosis and even intimal rupture of the arterial wall [11]. At the same time, the long-term blood pressure elevation can also lead to the adhesion and aggregation of platelets, thus resulting in thrombus formation. It has been recognized by the majority of scholars that hypertension acts as an independent risk factor for cerebral infarction. Hypertension has certain negative influence on the severity and prognosis of the disease after cerebral infarction [12].
The inflammatory factors in hypertensive patients complicated with cerebral infarction were investigated in this study, and it was found that the levels of hs-CRP and IL-6 in the cerebral infarction group were significantly higher than those in the lacunar cerebral infarction group. Comparisons of HOMA-IR index and blood pressure between the 2 groups at the same time revealed that the HOMA-IR index in the cerebral infarction group was markedly higher than that in the lacunar cerebral infarction group, while the systolic blood pressure and diastolic blood pressure were remarkably lower than those in the lacunar cerebral infarction group. The results indicated that in hypertensive patients complicated with cerebral infarction whose infarct size is in line with clinical diagnosis for cerebral infarction, namely, the diameter of the lesions is equal to or greater than 1.5 cm, the levels of inflammatory related factors in the body after the onset of the disease are higher, and the IR in the body is more serious. In addition, studies on the correlations of hs-CRP, IL-6 and IR with the diameter of cerebral infarction suggested that hs-CRP, IL-6 and IR are positively correlated with the diameter of cerebral infarction, that is, the larger the diameter of cerebral infarction, the more obvious the body’s inflammatory response, and the more serious the IR in the body. Finally, studies on the inflammatory factor hs-CRP, IR and the neurological function score after cerebral infarction have revealed that there was a positive correlation between these indexes and the neurological function of patients after onset of the disease, suggesting that the more serious the neurological impairment of hypertensive patients complicated with cerebral infarction, the more obvious the body’s inflammatory responses, and the more serious the IR in the body.
Hs-CRP is the most sensitive indicator of the body’s inflammatory responses, which has a certain impact on the occurrence and development of various medical diseases such as hypertension, diabetes, stem cells, and hyperlipidemia [13]. At 2 hours after cerebral infarction, there will be obvious pathophysiological changes in brain tissues, which lead to the increased IL level and aggravated IR in the body at the same time [14]. Among the increased inflammatory factors, the increase in IL-6 level is the most obvious. Besides, IR is aggravated, leading to increased CRP level [15]. With the increase of the diameter of the lesions, the infarct size enlarges, especially the cerebral ischemia and hypoxia sites, thus making the aforementioned pathophysiological process more obvious [16]. Furthermore, the large number of CRPs produced will further activate the body complement system, which aggravates vascular endothelial cell damage to activate the coagulation system [17], leads to further enlargement of the thrombus [18], and aggravates the progression of the disease and the disturbance of local blood circulation, thereby leading to the occurrence of more severe cerebral ischemia and hypoxia [19]. Therefore, the level of hs-CRP in hypertensive patients complicated with cerebral infarction is correlated with the diameter of the infarction [20–21], and it is of a certain value in predicting the prognosis of neurological function of the patients.
Conclusions
In summary, in hypertensive patients complicated with cerebral infarction, the body’s inflammatory factors and IR are positively correlated with the diameter of cerebral infarction, as well as the neurological prognosis of the patients.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Wang X, Shen B, Sun D, et al. Aspirin ameliorates cerebral infarction through regulation of TLR4/NF-κB-mediated endoplasmic reticulum stress in mouse model. Mol Med Rep. 2018;17:479–87. doi: 10.3892/mmr.2017.7879. [DOI] [PubMed] [Google Scholar]
- 2.DuPont JJ, Jaffe IZ. 30 years of the mineralocorticoid receptor: The role of the mineralocorticoid receptor in the vasculature. J Endocrinol. 2017;234:T67–82. doi: 10.1530/JOE-17-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao J, Chen G, He H, et al. Therapeutic effects of breviscapine in cardiovascular diseases: A Review. Front Pharmacol. 2017;23:289–93. doi: 10.3389/fphar.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd DP. Neurological complications of Behçet’s syndrome. J Neurol. 2017;264:2178–83. doi: 10.1007/s00415-017-8436-9. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin Sci (Lond) 2017;131:425–37. doi: 10.1042/CS20160604. [DOI] [PubMed] [Google Scholar]
- 6.Towfighi A, Cheng EM, Ayala-Rivera M, et al. Randomized controlled trial of a coordinated care intervention to improve risk factor control after stroke or transient ischemic attack in the safety net: Secondary stroke prevention by Uniting Community and Chronic care model teams Early to End Disparities (SUCCEED) BMC Neurol. 2017;17:24. doi: 10.1186/s12883-017-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Cui C, Yang X, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–85. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P, Kumar A, Srivastava MK, et al. Association of transforming growth factor Beta-1-509C/T gene polymorphism with ischemic stroke: A meta-analysis. Basic Clin Neurosci. 2016;7:91–96. doi: 10.15412/J.BCN.03070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang XH, Lin KX, Zhang YX, et al. Correlating interleukin-10 promoter gene polymorphisms with human cerebral infarction onset. Neural Regen Res. 2015;10:18091813. doi: 10.4103/1673-5374.170308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doll DN, Engler-Chiurazzi EB, Lewis SE, et al. Lipopolysaccharide exacerbates infarct size and results in worsened post-stroke behavioral outcomes. Behav Brain Funct. 2015;11:32. doi: 10.1186/s12993-015-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang SH, Lee H, Kim JS, et al. Association between Helicobacter pylori infection and cerebral small vessel disease. Korean J Fam Med. 2015;36:227–32. doi: 10.4082/kjfm.2015.36.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu H, Ni M, Chen JK, et al. Targeting α7 nicotinic acetylcholine receptor to combat inflammation in cardio-cerebral-vascular diseases. Curr Drug Targets. 2017;18:1779–84. doi: 10.2174/1389450116666150825123247. [DOI] [PubMed] [Google Scholar]
- 13.Tchalla AE, Wellenius GA, Travison TG, et al. Circulating vascular cell adhesion molecule-1 is associated with cerebral blood flow dysregulation, mobility impairment, and falls in older adults. Hypertension. 2015;66:340–46. doi: 10.1161/HYPERTENSIONAHA.115.05180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fluri F, Grünstein D, Cam E, et al. Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats. Exp Neurol. 2015;265:142–51. doi: 10.1016/j.expneurol.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Kono S, Kurata T, Sato K, et al. Neurovascular protection by telmisartan via reducing neuroinflammation in stroke-resistant spontaneously hypertensive rat brain after ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:537–47. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Yamashita T, Kurata T, et al. Telmisartan ameliorates inflammatory responses in SHR-SR after tMCAO. J Stroke Cerebrovasc Dis. 2014;23:2511–19. doi: 10.1016/j.jstrokecerebrovasdis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Min LJ, Mogi M, Tsukuda K, et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens. 2014;27:1036–44. doi: 10.1093/ajh/hpu015. [DOI] [PubMed] [Google Scholar]
- 18.Gurbuzer N, Gozke E, Ayhan Basturk Z. Gamma-glutamyl transferase levels in patients with acute ischemic stroke. Cardiovasc Psychiatry Neurol. 2014;20:170–76. doi: 10.1155/2014/170626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pires PW, Girgla SS, Moreno G, et al. Tumor necrosis factor-α inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats. Am J Physiol Heart Circ Physiol. 2014;307:H658–69. doi: 10.1152/ajpheart.00018.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rooy MJ, Pretorius E. Obesity, hypertension and hypercholesterolemia as risk factors for atherosclerosis leading to ischemic events. Curr Med Chem. 2014;21:2121–29. doi: 10.2174/0929867321666131227162950. [DOI] [PubMed] [Google Scholar]
- 21.Moller K, Boltze J, Pösel C, et al. Sterile inflammation after permanent distal MCA occlusion in hypertensive rats. J Cereb Blood Flow Metab. 2014;34:307–15. doi: 10.1038/jcbfm.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]


