Abstract
Viperin (virus inhibitory protein, endoplasmic reticulum-associated, interferon-inducible) is a widely distributed protein that is expressed in response to infection and causes antiviral effects against a broad spectrum of viruses. Viperin is a member of the radical S-adenosyl-l-methionine (SAM) superfamily of enzymes, which typically employ a 4Fe-4S cluster to reductively cleave SAM to initiate chemistry. Though the specific reaction catalyzed by viperin remains unknown, it has been shown that expression of viperin causes an increase in the fluidity of lipid membranes so as to impede the budding of nascent viral particles from the membrane which inhibits propagation of the infection. Herein we show that expression of the human viperin homolog induces a dramatically elongated morphology of the host E. coli cells. Mutation of an essential cysteine that coordinates the radical SAM cluster abrogates this effect. Thus the native radical SAM activity of viperin is likely occurring in the host bacteria, indicating the elusive substrate is shared between both bacteria and humans, significantly narrowing the range of potential candidate substrates and providing a convenient bacterial platform from which future studies can occur.
Graphical Abstract
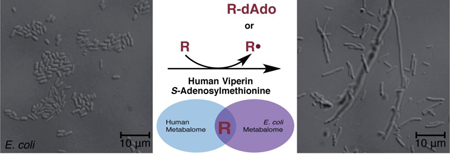
Viperin is one of many proteins upregulated in response to viral infection and has been shown to effectively inhibit viral replication of a variety of RNA and DNA viruses1. It is even expressed in response to some bacterial and protozoan infections highlighting its significant role in immune-mediated responses2. This effect has garnered significant attention though the specific mechanism by which it acts has yet to be elucidated.
The catalytic nature of viperin is suggested by its inclusion in the diverse and ancient radical SAM family of proteins that is characterized by a canonical CxxxCxxC motif that coordinates an iron-sulfur cluster containing four iron atoms and four sulfur atoms in a cubane structure (4Fe-4S), which is capable of reducing SAM to methionine and a highly reactive 5′-deoxyadenosyl radical3–5. This radical species is then used to initiate a rich variety of radical-mediated reactions, leaving open the possibility for many potential radical SAM-type reactions that could be catalyzed by viperin. A defining feature of this class of enzymes that can be diagnostic, however, is the uncoupled reductive cleavage of SAM into methionine and 5′-deoxyadenosine (dAdo) even in the absence of the corresponding substrate, and viperin has been shown to possess this activity in its purified form demonstrating its ability to catalyze radical mediated reactions3, 4. Tellingly, the antiviral activity of viperin is dependent upon the presence of this radical SAM architecture including the unusual viperin activity reportedly co-opted by human cytomegalovirus to increase its infectivity6–8.
Through pioneering efforts by the Cresswell lab, viperin was discovered to cause changes in lipid membranes, increasing their fluidity and thus disrupting the so-called lipid rafts that viruses depend upon for proper entry, assembly, and budding9. In these studies the viral particles themselves were imaged crowding the cell surface from which they were unable to escape to cause further infection. It was also shown that viperin interacts with farnesyl pyrophosphate synthase (FPPS) and that overexpression of FPPS can recover membrane rigidity allowing for viral release9. More recently it has been established that viperin does not directly inhibit FPPS and is thus likely acting more generally to affect lipid metabolism10. This connection of viperin to lipid metabolism or modification was further strengthened by the finding that viperin localizes to lipid droplets in an essential step for viral inhibition11.
To study this enzyme we first attempted to obtain the human version in a form more amenable to purification and in vitro handling than has previously been reported by creating a chimera of maltose binding protein (MBP) and a truncated version of viperin (residues 44–361) lacking the N-terminal membrane binding helices11. As with other proteins fused with MBP, this viperin chimera allowed for significant overexpression and stability to harsh conditions like that used in 4Fe-4S cluster reconstitution12. This MBP-viperin chimera displayed the characteristic uncoupled cleavage of SAM in vitro indicating the catalytic ability of viperin is maintained (Fig. 1)3, 13.
Figure 1.
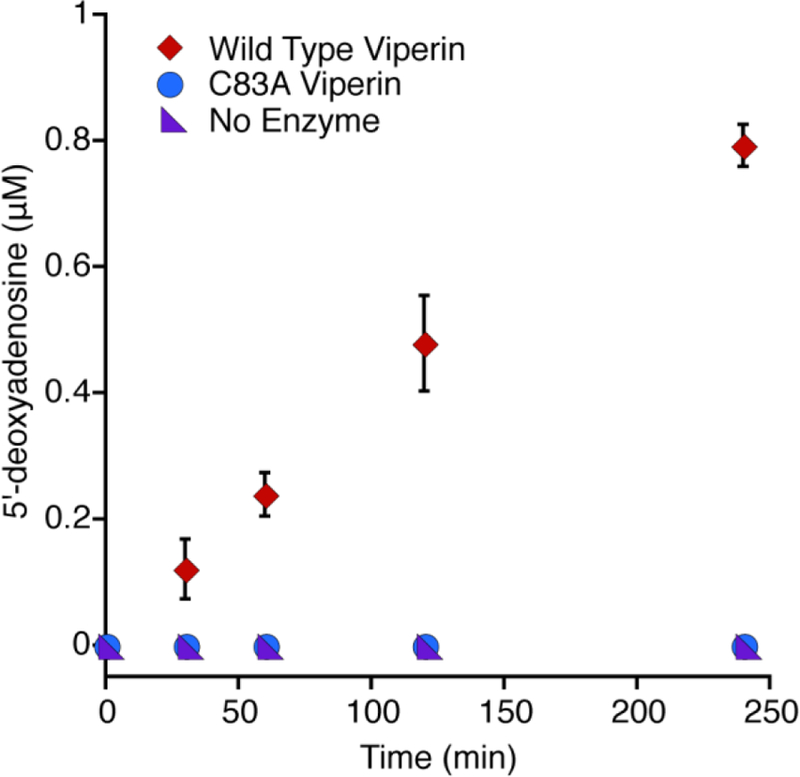
The production of 5′-deoxyadenosine by wild type viperin is depicted. The C83A mutant and no-enzyme control show no detectable formation of 5′-deoxyadenosine.
In a serendipitous finding, cultures expressing this chimera were seen to settle quickly and prompted our attention to potential changes in the E. coli cell structure that may have been caused by the activity of viperin. We thus observed these cells using a confocal microscope and found a dramatic elongation in those cells bearing the wild type viperin construct, whereas those with an empty plasmid showed only normal morphology (compare panels A and B in Fig. 2, Supporting Fig. 1).
Figure 2.
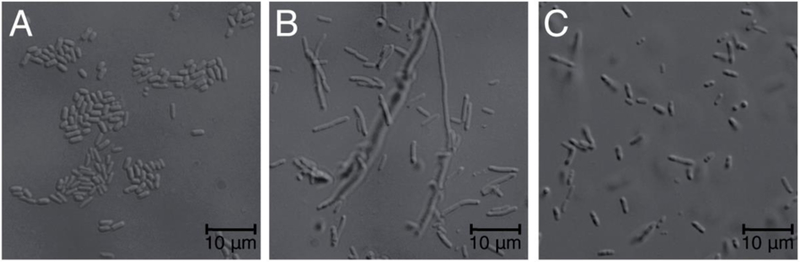
Images of E. coli containing empty plasmid (A), wild type viperin (B), and C83A-viperin (C).
To confirm the elongated phenotype of the E. coli heterologously expressing wild type viperin is the result of the radical SAM activity of viperin, a mutant of one of the conserved 4Fe-4S cluster coordinating cysteine residues, C83A, was constructed to directly implicate the 4Fe-4S cluster in the elongation activity (using numbering for full length wild type human viperin). It has previously been reported that this cysteine residue along and the other two cluster-ligating cysteine residues are essential for viperin to bind the 4Fe-4S cluster14. The purified C83A MBP-viperin chimera was here shown to be incapable of uncoupled cleavage of SAM confirming the radical SAM activity was abolished (Fig. 1). Indeed in E. coli expressing this site-directed variant the cell morphology more closely resembles that of wild type E. coli (panel C, Fig. 2, Supporting Fig. 1).
This radical SAM dependent change in the structure of E. coli strongly suggests the native activity of viperin is occurring even in bacterial cells that lack many of the proteins and cellular organelles to which viperin has been associated, significantly narrowing the range of potential substrates which may be acted upon by viperin1. The change in morphology itself is highly suggestive that viperin is interfering with the dividing mechanism of E. coli which is known to be dependent upon proper membrane composition for correct assembly of the divisome, and so this observation is supportive of previous studies in human cells showing one of the key aspects of viperin’s activity is likely a reaction capable of effecting changes in lipid metabolism or composition15.
The recently described crystal structure of viperin has shown a number of conserved positively charged residues which have been suggested to be used to bind to a nucleotide substrate16. This finding of viperin activity in bacteria is in keeping with viperin acting upon a small molecule substrate that must be shared between humans and bacteria. While larger molecules such as proteins are less likely to bear sufficient similarity between human and bacterial homologs to act as substrates by this human enzyme, at this point the possibility can not be excluded.. The specific transformation that occurs can not be predicted a priori, though the formation of dAdo as a reductive cleavage product is highly suggestive of a role for viperin either in H-atom abstraction or radical addition involving 5′-dAdo radical (Scheme 1). Further studies will be required to identify this substrate, and E. coli may be useful as a convenient model in which to test for viperin activity and uncover more quickly the potential antiviral therapies that can be inspired by this enigmatic enzyme.
Scheme 1.
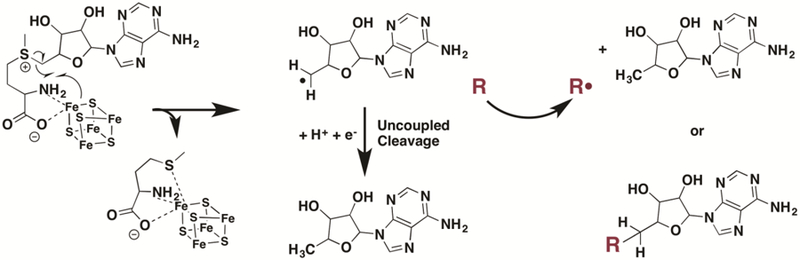
Proposed radical SAM reactivity of viperin.
Supplementary Material
ACKNOWLEDGMENT
The work reported in this publication was supported by National Institutes of General Medical Sciences of the National Institutes of Health grant R01 GM72623 and GM120638 awarded to VB. The content is solely the responsibility of the authors and does not necessarily represent the official views for the National Institutes of Health. No competing financial interests have been declared.
ABBREVIATIONS
- SAM
S-adenosyl-L-methionine
- 4Fe-4S
four iron four sulfur cluster
- dAdo
5′-deoxyadenosine
- FPPS
farnesyl pyrophosphate synthase
- MBP
maltose binding protein
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. This includes methods used and additional images of E. coli as in Figure 2.
No competing financial interests have been declared.
REFERENCES
- (1).Seo JY, Yaneva R, and Cresswell P (2011) Cell Host Microbe 10, 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Fitzgerald KA (2011) J. Interferon. Cytokine Res 31, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Broderick JB, Duffus BR, Duschene KS, and Shepard EM (2014) Chem. Rev 114, 4229–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Duschene KS, and Broderick JB (2010) FEBS Lett 584, 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Shaveta G, Shi J, Chow VT, and Song J (2010) Biochem. Biophys. Res. Commun 391, 1390–1395. [DOI] [PubMed] [Google Scholar]
- (6).Jiang D, Guo H, Xu C, Chang J, Gu B, Wang L, Block TM, and Guo JT (2008) J. Virol 82, 1665–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Upadhyay AS, Vonderstein K, Pichlmair A, Stehling O, Bennett KL, Dobler G, Guo JT, Superti-Furga G, Lill R, Overby AK, and Weber F (2014) Cell Microbiol 16, 834–848. [DOI] [PubMed] [Google Scholar]
- (8).Seo JY, Yaneva R, Hinson ER, and Cresswell P (2011) Science 332, 1093–1097. [DOI] [PubMed] [Google Scholar]
- (9).Wang X, Hinson ER, and Cresswell P (2007) Cell Host Microbe 2, 96–105. [DOI] [PubMed] [Google Scholar]
- (10).Makins C, Ghosh S, Roman-Melendez GD, Malec PA, Kennedy RT, and Marsh EN (2016) J. Biol. Chem 291, 26806–26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hinson ER, and Cresswell P (2009) Proc. Natl. Acad. Sci. U S A 106, 20452–20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kapust RB, and Waugh DS (1999) Protein Sci 8, 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bruender NA, Young AP, and Bandarian V (2015) Biochemistry 54, 2903–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Haldar S, Paul S, Joshi N, Dasgupta A, and Chattopadhyay K (2012) PLoS One 7, e31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Natale P, Pazos M, and Vicente M (2013) Environ. Microbiol 15, 3169–3182. [DOI] [PubMed] [Google Scholar]
- (16).Fenwick MK, Li Y, Cresswell P, Modis Y, and Ealick SE (2017) Proc. Natl. Acad. Sci 114, 6806–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


