Abstract
Background and Objectives:
Recently, a 22G Franseen needle for EUS-guided fine-needle biopsy (EUS-FNB) with three novel symmetric heels has been developed to adequately obtain a core tissue.
Methods:
All 38 consecutive patients with pancreatic masses who underwent EUS-FNB using a Franseen needle were investigated retrospectively to assess the efficacy and safety of EUS-FNB using the Franseen needle. Then, the EUS-FNB outcomes and histological assessments of the tissue obtained by EUS-FNB using the Franseen needle and EUS-FNA using the conventional end-cut type needle for each of the 30 pancreatic ductal adenocarcinoma cases were compared.
Results:
An accurate histological diagnosis of the Franseen needle was achieved with a mean of 2 passes in 97.4% of patients. Although the accurate histological diagnosis rate of pancreatic ductal adenocarcinoma was not significantly different (96.7% vs. 93.3%, P = 0.55), the mean number of passes in the Franseen needle was significantly less than that in the conventional needle (2.1 ± 0.4 vs. 3.2 ± 0.8, P < 0.001). The presence of desmoplastic fibrosis with neoplastic cellular elements and venous invasion were significantly higher (96.7% vs. 40.0%, P < 0.001 and 23.3% vs. 0%, P < 0.01, respectively) and the amount of obtained tissue was significantly larger with the Franseen needle (2.13 mm2 vs. 0.45 mm2, P < 0.001).
Conclusions:
EUS-FNB using the Franseen needle enables the acquisition of a larger amount of tissue sample and achieves an accurate histological diagnosis with a smaller number of passes than the conventional end-cut type needle.
Keywords: Endoscopic ultrasound, fine needle biopsy, histology, pancreatic cancer
INTRODUCTION
EUS-guided fine-needle biopsy (EUS-FNB) is an accurate and safe procedure for establishing a pathological diagnosis of pancreatic masses.[1,2,3,4,5,6] However, although the high histological diagnostic accuracy has been reported, this still depends on endoscopic skills, pathological diagnostic skills, number of needle passes, and the presence of rapid onsite cytological evaluation (ROSE).[7,8,9,10] The easy achievement of accuracy rate in pathological diagnosis approximated 100% with 1 or 2 passes is ideal for EUS-FNB. Recently, the acquisition of a large amount of tissue sample has been regarded as important for achieving a high diagnostic accuracy and for reducing the number of needle passes, particularly if ROSE is not available.[11,12,13] The use of a large-caliber 19G needle and several useful puncture techniques such as the door knocking method for obtaining sufficient tissue sample has also been reported.[14,15] However, there are some technical issues with the use of a large-caliber 19G needle owing to its stiffness. Moreover, the efficacy of the puncture techniques is limited. Thus, the development of needles with good maneuverability for EUS-FNB, which can obtain a large amount of tissue sample with fewer needle passes, has been required.
Recently, a 22G needle with 3 novel symmetric heels for EUS-FNB, which is called a Franseen needle, has been developed. Our animal experimental study has shown better tissue acquisition abilities of the Franseen needle than the conventional 22G needles, which consist of the end-cut type needles with a beveled tip.[16] Herein, we assessed the efficacy and safety of EUS-FNB using the Franseen needle for pancreatic masses in daily clinical practice. We also retrospectively compared the outcomes and assessments of the tissue obtained by EUS-FNB using the Franseen needle with those obtained by EUS-FNA using the conventional end-cut type needle.
METHODS
Novel Franseen needle design
A 22G Franseen needle (Acquire, Boston Scientific Corp., Natick, MA, USA) has 3 novel symmetric heels designed to maximize tissue capture and minimize fragmentation [Figure 1]. This needle was developed to adequately obtain a core tissue and improve the diagnostic yield. To appropriately acquire a core tissue, it is important to not only cut the tissue but also collect the tissue in the needle tract. In this respect, the Franseen needle has three symmetrical needle points, providing greater control at the puncture site and stability to the tip than the conventional needle. The electropolished strain-resistant cutting edges are fully formed to maximize sharpness of the needle and to cut the tissue from three different angles, creating a circular cut. Cobalt chromium, which has the reputation of being a highly durable alloy, is used as the material for the needle, allowing repeat punctures without needle dysfunction.
Figure 1.
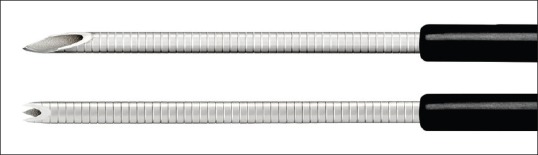
Upper needle: A conventional 22G end-cut type needle with beveled tips. Lower needle: A novel 22-guage Franseen needle with 3 symmetric heels (Courtesy of Boston Scientific Corp.)
Patients
This was a retrospective study conducted at a tertiary referral center where more than 100 EUS-FNBs are performed yearly (Tokyo Medical University Hospital). All 38 consecutive patients with pancreatic masses (25 men and 13 women; median age 62 years; range 42–89) who underwent diagnostic EUS-FNB using a 22G Franseen needle between September 2016 and January 2017 were investigated retrospectively [Table 1]. The final clinical diagnoses of pancreatic masses are shown in Table 1. The final clinical diagnoses of malignant tumors were based on the histological diagnoses of surgically resected specimens or EUS-FNB diagnoses positive for malignancy with compatible radiological findings and clinical data. The clinical diagnoses of benign disease were based on EUS-FNB diagnoses that were negative for malignancy and clinical data that indicated no deterioration on follow-up. Written informed consent for EUS-FNB was obtained from all the patients. This retrospective study was approved by the Institutional Review Board (No. 2016-199).
Table 1.
Patient characteristics and final diagnoses
| Pancreatic masses (n=38) | |
|---|---|
| Age (years) | |
| Mean±SD | 66.2±12.3 |
| Range | 42-89 |
| Gender | |
| Male | 25 |
| Female | 13 |
| Site of pancreatic mass | |
| Head | 18 |
| Body | 12 |
| Tail | 8 |
| Size of masses on EUS (mm) | |
| Mean±SD | 28.5±10.8 |
| Range | 6-63 |
| <20 | 12 |
| ≥20 | 26 |
| Final diagnosis | |
| Pancreatic adenocarcinoma | 30 |
| Neuroendocrine tumor | 3 |
| Solid pseudopapillary neoplasm | 2 |
| Autoimmune pancreatitis | 3 |
SD: Standard deviation
Procedure
EUS-FNB was performed using a curved linear array echoendoscope (GF-UCT240 or GF-UCT260; Olympus Medical Systems, Tokyo, Japan) under moderate sedation. All FNB punctures were performed by experts on the EUS-FNB procedure (>5 years of EUS-FNB experience) or by trainees (<5 years of EUS-FNB experience) under the direction of experts. The pancreatic mass was visualized under EUS. After careful evaluation, including assessment of the regional vasculature with the color Doppler function, the pancreatic mass was punctured through the transgastric or transduodenal route. Then, the central stylet was removed and 20 mL negative syringe suction was applied at the first puncture. If blood contamination was extensive macroscopically, a slow pull technique or no suction was applied at the second puncture. The needle was moved to-and-fro within the pancreatic mass more than 10 times, using the fanning technique basically. The obtained tissue specimens were immediately fixed in 10% neutral-buffered formalin solution for histological examination by releasing the syringe and reinserting the stylet. The number of FNB passes was decided on the basis of the macroscopic visible core, which is defined as white or yellow pieces of obtained tissue with an apparent bulk, without ROSE. Basically, 2 FNB passes were performed, but an additional puncture was performed if the tissue specimens obtained in the 2 FNB passes were considered insufficient for pathological diagnosis.
Tissue specimen handling
At our institution, only histological analyses were performed without cytological analyses. The fixed tissue specimen was routinely processed and embedded in paraffin in the histological tissue specimen handling room. The paraffin-embedded tissues were cut into 3 μm slices. Only sections that contained mostly tissue specimen were processed into slides. Thus, one slide was prepared for one pass. The tissue sections were stained with hematoxylin and eosin for evaluation by a pathologist. Immunohistochemical procedures were performed if necessary.
Comparison of histological assessments
Until August 2016, we used a conventional 22G end-cut type needle with beveled tips (Expect SL, Boston Scientific Corp.) [Figure 1] for EUS-FNA. The procedure and specimen handling method was the same as those for EUS-FNB using the Franseen needle. We retrospectively investigated 30 consecutive patients with a final clinical diagnosis of pancreatic ductal adenocarcinoma in whom EUS-FNA using the conventional 22G needle was performed between February 2016 and August 2016. Thereafter, the EUS-FNAB outcomes for each of the 30 pancreatic ductal adenocarcinoma cases were compared. The outcomes included (1) accuracy of histological diagnosis, (2) presence of desmoplastic fibrosis with neoplastic cellular elements, (3) amount of obtained tissue, and (4) presence of venous, lymphatic, or nerve invasion. All of these histological factors were evaluated with hematoxylin and eosin staining sections, supported by several special and immunohistochemical stains; alpha-SMA (Dako; clone 1A4; 1:100 dilution) stain was used for assessment for presence of desmoplastic fibrosis; elastic van Gieson and CD31 (Dako; clone JC70A; 1:20 dilution) stains for venous invasion; Podoplanin (Roche; clone D2-40; Ready to use) stain for lymphatic invasion; S-100 protein (Roche; clone Polyclonal; Ready to use) for nerve invasion, respectively. The total amount of obtained tissue was evaluated as follows. Initially, one slide was selected for each case, which includes the largest amount of tissue. Then, three large tissue clots were selected in each slide. Each area of a tissue clot was calculated by measuring both the major axis and the minor axis [Figure 2]. The amount of tissue obtained by each needle was determined as the total area of the 3 tissue clots. The total amount of tissue obtained was compared between the two treatment groups.
Figure 2.
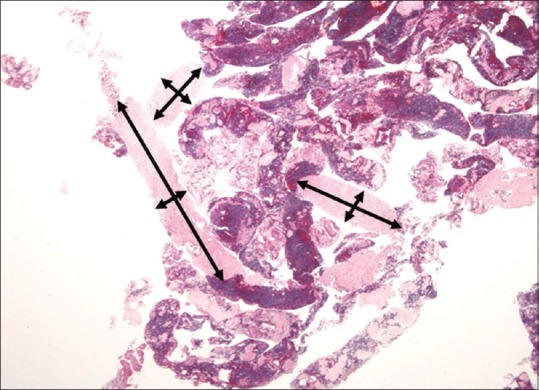
Three large tissue clots were selected in a slide, and the area of the tissue clot was calculated by measuring the major axis and the minor axis (H and E, ×20)
Statistical analysis
Continuous variables pertaining to the baseline characteristics of the two treatment groups were presented as means ± standard deviation and were compared using the Student's t-test or the Wilcoxon rank-sum test as appropriate. Categorical variables were compared using the Chi-squared or Fisher's exact test. Statistical analyses were performed using StatMate III (ATMS, Tokyo, Japan). A value of P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Details of the procedure and outcomes of EUS-FNB using the Franseen needle for 38 pancreatic masses are shown in Table 2. Although almost all cases (34/38, 89.5%) were performed by a trainee, EUS-FNB was technically successful in all the 38 patients without needle dysfunction or needle changes regardless of the puncture route (i.e., transgastric or transduodenal). The needle was well visualized on EUS during the procedure in all cases. An accurate histological diagnosis was achieved with a mean of two passes (range: 1–3) in 37 of the 38 patients (97.4%) without ROSE. A failure in obtaining a diagnosis occurred in one patient with pancreatic ductal adenocarcinoma because the amount of tissue obtained was too tiny. There were no procedural or late adverse events in all the patients.
Table 2.
Outcomes of EUS-guided fine-needle biopsy using a Franseen needle
| Pancreatic masses (n=38), n (%) | |
|---|---|
| Procedure | |
| Trainee | 34 (89.5) |
| Expert | 4 (10.5) |
| Technical success | 38 (100) |
| Puncture route | |
| Transgastric | 21 (55.3) |
| Transduodenal | 17 (44.7) |
| Number of punctures | |
| Mean±SD | 2.0±0.5 |
| Range | 1-3 |
| Needle dysfunction | 0 |
| Needle change | 0 |
| Diagnostic accuracy | 37 (97.4) |
| Adverse events | 0 |
SD: Standard deviation
For the comparison of the patients and masses between the Franseen needle and the conventional needle, the characteristics of the patients and pancreatic masses were not significantly different [Table 3].
Table 3.
Patient characteristics
| Franseen needle (n=30) | Conventional end-cut type needle (n=30) | P | |
|---|---|---|---|
| Age (years) | |||
| Mean±SD | 64.7±12.5 | 69.0±9.5 | 0.13 |
| Range | 42-89 | 49-86 | |
| Gender | |||
| Male | 19 | 20 | 0.79 |
| Female | 11 | 10 | |
| Site of pancreatic mass | |||
| Head | 15 | 15 | 0.93 |
| Body | 9 | 10 | |
| Tail | 6 | 5 | |
| Size of masses on EUS (mm) | |||
| Mean±SD | 30.6±10.7 | 27.7±7.2 | 0.21 |
| Range | 15-47 | 14-40 | |
| <20 | 7 | 6 | 0.75 |
| ≥20 | 23 | 24 | |
SD: Standard deviation
The comparison of the EUS-FNAB outcomes is shown in Table 4. The technical success rate was 100% (30/30) in both needles. Although the rate of accurate histological diagnosis was not significantly different between the Franseen needle and the conventional needle (96.7% vs. 93.3%, P = 0.55), the mean number of passes in the Franseen needle was significantly less than that in the conventional needle (2.1 ± 0.4 vs. 3.2 ± 0.8, P < 0.001). Regarding the histological assessment of the obtained tissue samples, the numbers of cases showing the presence of desmoplastic fibrosis with neoplastic cellular elements and venous invasion were significantly higher with the Franseen needle than with the conventional needle (96.7% vs. 40%, P < 0.001 and 23.3% vs. 0%, P < 0.01, respectively).
Table 4.
Comparison of EUS-guided fine-needle aspiration biopsy outcomes
| Franseen needle (n=30), n (%) | Conventional end-cut type needle (n=30), n (%) | P-value | |
|---|---|---|---|
| Procedure | |||
| Trainee | 27 (90) | 24 (80) | 0.47 |
| Expert | 3 (10) | 6 (20) | |
| Technical success | 30 (100) | 30 (100) | 1 |
| Puncture route | |||
| Transgastric | 16 (53.3) | 16 (53.3) | 1 |
| Transduodenal | 14 (46.7) | 14 (46.7) | |
| Number of passes | |||
| Mean±SD | 2.1±0.4 | 3.2±0.8 | <0.001 |
| Range | 1-3 | 2-5 | |
| Diagnostic accuracy | 29 (96.7) | 28 (93.3) | 0.55 |
| Presence of desmoplastic fibrosis | 29 (96.7) | 12 (40) | <0.001 |
| Presence of venous invasion | 7 (23.3) | 0 | <0.01 |
| Presence of lymphatic invasion | 3 (10) | 0 | 0.05 |
| Presence of nerve invasion | 1 (3.3) | 0 | 0.33 |
| Amount of obtained tissue (mm2) | |||
| Mean | 2.24±1.37 | 0.43±0.33 | <0.001 |
| Median | 2.13 | 0.45 | <0.001 |
| Range | 0.70-6.71 | 0.04-1.23 | |
| Adverse events | 0 | 0 | 1 |
SD: Standard deviation
The scatter plot showing the amount of obtained tissue is shown in Figure 3. The amount of obtained tissue with the Franseen needle was significantly larger than that with the conventional needle (median, 2.13 mm2 vs. 0.45 mm2, P < 0.001).
Figure 3.
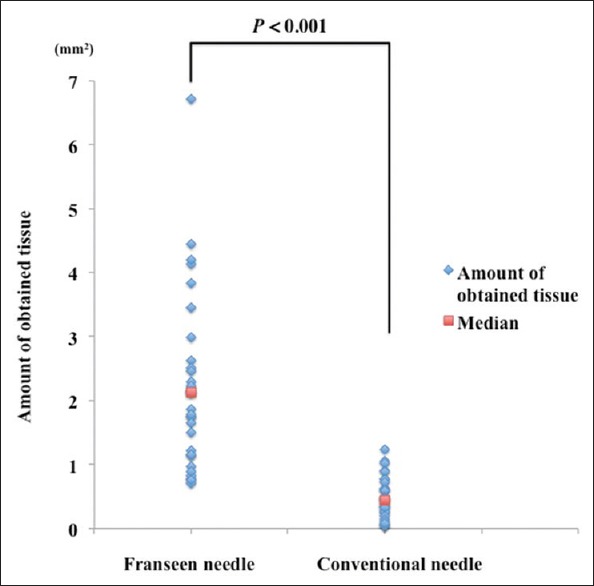
Scatter plot of the amount of obtained tissues evaluated by the total area of the 3 tissue clots for EUS-FNAB using each needle
DISCUSSION
It was worth noting that EUS-FNB using the novel 22G Franseen needle achieved an accurate histological diagnosis in 37 of the 38 patients with pancreatic masses at an average of 2 passes without ROSE. Although there were concerns that the shape of the needle tip might make needle puncture difficult in several patients, particularly for the transduodenal puncture, needle punctures were possible in all the patients without needle dysfunction or the need for exchange with a thinner needle. EUS-FNB using the Franseen needle was performed safely without adverse events. The present results revealed that EUS-FNB using the Franseen needle is nearly ideal for the pathological diagnosis of pancreatic masses.
In this study, the diagnostic yield using the conventional needle was high at 92.3%. And so there was no significant difference in the diagnostic yield between the Franseen needle and the conventional needle. However, the median area of the tissue sample in the slide obtained using the Franseen needle for histopathological evaluation was about 5 times larger than that using the conventional needle. This suggests the extremely superior capability of the Franseen needle to obtain larger tissue samples. Bang et al. reported that the median total tissue area obtained using the Franseen needle was 2.94 mm2 as evaluated using specialized digital software.[17] In the present study, the amount of tissue obtained as evaluated by the median total area of the three tissue clots (i.e., 2.13 mm2) showed a nearly similar value. Notably, the least amount of obtained tissue using the Franseen needle in this study (i.e., 0.70 mm2) was still larger than the median amount of obtained tissue using the conventional needle (i.e., 0.45 mm2). This indicates the high probability of tissue sample acquisition using the Franseen needle. Recently, Bang et al. showed the similar date in the prospective randomized study comparing 22G FNB needle with FNA needle.[18]
Obtaining a large amount of tissue sample in EUS-FNB for pancreatic masses has the following advantages. First, it enables macroscopic onsite evaluation (MOSE). Although ROSE has been reported to improve the diagnostic accuracy and decrease the number of passes,[7,9,19] a cytopathologist is not regularly available because of labor shortages even at high-volume centers. Iwashita et al. in their study reported the efficacy of MOSE as an alternative to ROSE wherein the number of passes is decided on the basis of the macroscopic visible core.[20] In their report, a conventional 19G needle was used to obtain a visible core. However, there are some technical issues regarding the use of a 19G needle. The Franseen needle enables MOSE using a 22G needle owing to its ability to obtain a visible core. In this study, MOSE could be performed in all the patients, resulting in the low number of passes (average: 2 passes) with a good diagnostic accuracy rate.
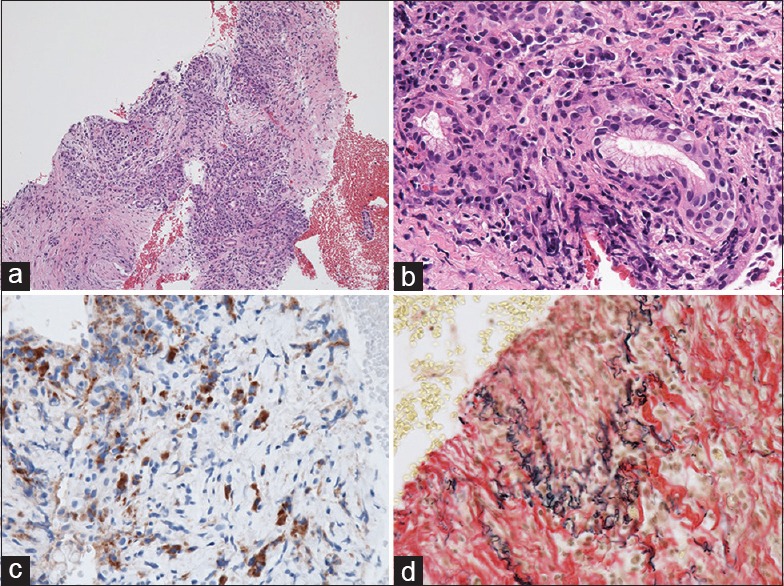
Second, a large amount of tissue sample greatly helps a pathologist to make a more accurate pathological diagnosis. Although a high diagnostic yield of EUS-FNA using a conventional needle has been reported, most of these reports were from high-volume centers wherein experienced endosonographers perform the EUS-FNA and pathologists specializing in pancreatic and biliary diseases make the pathological diagnosis. When only a small amount of a fragmented atypical epithelial cluster is obtained, it may be difficult for a general pathologist to differentiate the cancer cells from the gastric foveolar epithelial cells or normal pancreatic acinar cells that have atrophied owing to the inflammation[21,22] [Figure 4a]. Although pancreatic ductal adenocarcinoma has a high clinical malignant potential, cellular atypia is occasionally unremarkable. If a tissue sample for histological examination is not available, cytological evaluation alone makes it even more difficult to arrive at a pathological diagnosis, incurring a risk of making a false-positive or a false-negative diagnosis. Pancreatic ductal adenocarcinoma proliferates invasively with accompanying desmoplastic reactions. When the tissue obtained shows atypical cells that are proliferating invasively in the pancreas with an irregularly distributed fibrosis, making a pathological diagnosis of invasive pancreatic ductal adenocarcinoma is easy even for an inexperienced pathologist. In the present study, a pathological diagnosis could be readily made from the tissue sample containing desmoplastic reactions obtained using the Franseen needle in most patients (96.2%) [Figure 4b]. Furthermore, venous, lymphatic, or nerve invasion, which helps a pathologist make a definitive diagnosis of adenocarcinoma, was present in 8 patients (26.7%) [Figure 5]. Obtaining a large amount of tissue sample would also be beneficial to pathologists specializing in pancreatic and biliary diseases because it reduces the time required for diagnosis, and immunohistochemical assay for p53 or Ki-67 is no longer necessary, thereby reducing the medical cost.
Figure 4.
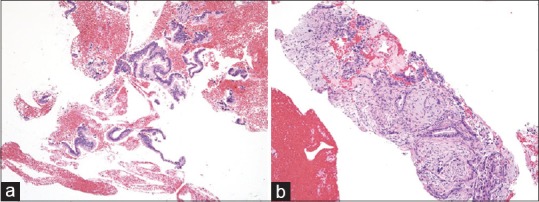
(a) A small amount of fragmented adenocarcinoma cell clusters obtained using a conventional end-cut type needle (H and E, ×100), which is difficult to differentiate from contaminated gastric foveolar epithelium. The evaluation of invasive growth is impossible based on this section. (b) A core tissue including the desmoplastic fibrosis with neoplastic cellular elements obtained using a novel Franseen needle (H and E, ×100). Destructive invasion growth is apparent, leading to an accurate diagnosis for malignancy
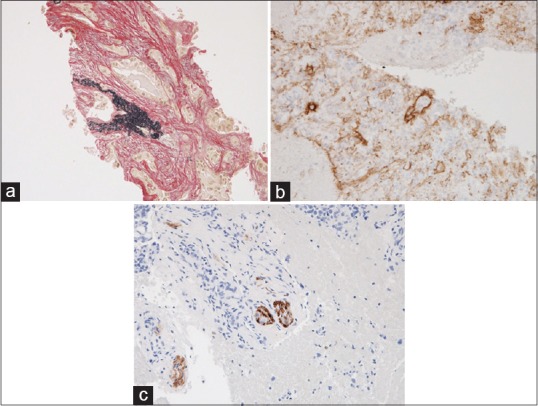
Figure 5.
A large amount of core tissue including the venous, lymphatic, or nerve invasion, which helps a pathologist to make a definitive pathological diagnosis of adenocarcinoma. (a) Venous invasion of adenocarcinoma evaluated by elastic Van Gieson staining (×200). (b) Lymphatic invasion of adenocarcinoma evaluated by immunohistochemical assessment of D2-40 (×200). (c) Nerve invasion evaluated by immunohistochemical assessment of S-100 (×200)
Third, recently molecular pathological studies have been actively performed for various types of cancer. Unfortunately, the development of molecular-targeted agents for pancreatic cancer is lagging behind other types of cancer because of the difficulty in obtaining a large amount of biopsy samples.[23] A clinical trial of personalized anticancer treatment according to molecular profiling of pancreatic cancer has indicated that EUS-FNA frequently provides an insufficient amount of tissue sample for molecular testing because pancreatic carcinoma may be relatively hypocellular.[24] However, if a large amount of tissue sample can be easily obtained, the remaining tissue sample after making a pathological diagnosis can be used for molecular pathological studies. In the future, tailor-made treatments may also be possible for pancreatic cancer.
In terms of adverse events, accidental bleeding from obtaining a large amount of tissue sample is a concern with the use of the Franseen needle. Bang et al. reported one bleeding adverse event (3.3%) consisting of arterial mucosal bleeding that required endoscopic hemostasis using two clips.[17] Although no bleeding requiring hemostasis occurred in the present study, oozing from the puncture site appears to have increased with the Franseen needle. Thus, needle puncture should be performed after ensuring that intervening blood vessels are avoided from the puncture route using a color Doppler function. An exception is in patients with coagulopathy or receiving antithrombotic agents, in whom the Franseen needle may need to be avoided.
Franseen needle also raises a possibility for pathological diagnosis of another pancreatic disease. Histological assessment is critical for the diagnosis of autoimmune pancreatitis (AIP). However, it has been reported that the accurate histological diagnosis rate of EUS-FNA is not very high. In their multicenter study, Kanno et al. reported that the histological diagnosis of lymphoplasmacytic sclerosing pancreatitis (type 1 AIP) could be achieved in 57.7% of patients.[25] Improvement in the diagnostic yield for AIP requires the acquisition of high-quality histological core tissue sample and confirmation of the markedly increased number of IgG4-positive plasma cells, infiltration of lymphocyte plasma cells, storiform fibrosis, and obliterative phlebitis [Figure 6]. Taken together, the Franseen needle is a very promising needle, which can greatly contribute to improving the histological diagnosis of AIP.
Figure 6.
Obtained core tissue by EUS-guided fine-needle biopsy using a Franseen needle for lymphoplasmacytic sclerosing pancreatitis (type 1 autoimmune pancreatitis). (a) Storiform fibrosis (H and E, ×100). (b) Extensive infiltration of lymphocyte plasma cells (H and E, ×400). (c) Markedly increased numbers of IgG4-positive plasma cells (×400). (d) Obliterative phlebitis evaluated by elastic van Gieson staining (×200)
Recently, a novel fork-tip 22G needle has also been developed to improve the ability to achieve an accurate histological diagnosis. Kandel et al. reported that this needle is superior to a conventional needle in obtaining tissue samples according to the standard scoring criteria for histology.[26] In their report, the fork-tip needle allowed architecturally intact tissue samples to be obtained in 69% of patients. Furthermore, a novel 20G needle has also been developed with the aim of increasing the puncture performance closer to a 22G needle while maintaining the ability to obtain tissue samples closer to a 19G needle.[16] A well-designed randomized controlled trial is required to compare these novel needles with the Franseen needle.
There are several limitations in this study. One, some technical biases regarding the suction or stroke method could not be completely avoided because of the retrospective nature of the study involving single-center enrollment.[27] However, the effects of these technical biases may be limited. Two, regarding the histological assessment of the obtained tissue samples, some studies used the objective way of quantifying the tissue components using specialized digital software.[17,18] However, the obtained tissue was subjectively assessed and compared by the experienced pathologist only with hematoxylin and eosin staining, supported by several special and immunohistochemical stains because we do not have the objective way such as the specialized digital software. Three, in this study we compared the FNB needle with the FNA needle only in the patients with pancreatic adenocarcinoma. It is unknown whether these results adapt to other pancreatic solid masses such as AIP, neuroendocrine tumor, lymphoma, or extra-pancreatic masses such as gastrointestinal stromal tumor. Four, the Franseen needle is promising to the molecular profiling of pancreatic cancer for risk stratification and personalized anti-cancer therapy. However, in this study the molecular assessment was not carried out. It is unknown whether the Franseen needle has advantage in the molecular profiling.
CONCLUSIONS
EUS-FNB using the novel Franseen needle for pancreatic masses enables the acquisition of a larger amount of tissue sample than EUS-FNA using the conventional end-cut type needle. Moreover, the Franseen needle achieves a more accurate histological diagnosis with a small number of passes using MOSE. Evaluation of the efficacy and safety of the Franseen needle for other organs and a comparative study in terms of effectiveness using a prospective randomized controlled trial between the Franseen needle and other novel needles are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Edward Barroga, Associate Professor and Senior Medical Editor from the Department of International Medical Communications of Tokyo Medical University for editing the manuscript.
REFERENCES
- 1.Binmoeller KF, Thul R, Rathod V, et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–7. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 2.Itoi T, Tsuchiya T, Itokawa F, et al. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: A single-center experience. Dig Endosc. 2011;23(Suppl 1):34–8. doi: 10.1111/j.1443-1661.2011.01142.x. [DOI] [PubMed] [Google Scholar]
- 3.Wani S, Muthusamy VR, Komanduri S. EUS-guided tissue acquisition: An evidence-based approach (with videos) Gastrointest Endosc. 2014;80:939–59. e7. doi: 10.1016/j.gie.2014.07.066. [DOI] [PubMed] [Google Scholar]
- 4.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing proCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 5.Huang JY, Chang KJ. Improvements and innovations in endoscopic ultrasound guided fine needle aspiration. J Hepatobiliary Pancreat Sci. 2015;22:E37–46. doi: 10.1002/jhbp.232. [DOI] [PubMed] [Google Scholar]
- 6.Hijioka S, Hara K, Mizuno N, et al. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2016;51:923–30. doi: 10.1007/s00535-016-1164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 8.Itoi T, Sofuni A, Itokawa F, et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23(Suppl 1):17–21. doi: 10.1111/j.1443-1661.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 9.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N. Factors influencing the accuracy of EUS FNA: Do educational elements matter? J Gastroenterol. 2017;52:263. doi: 10.1007/s00535-016-1276-z. [DOI] [PubMed] [Google Scholar]
- 11.Levy MJ, Wiersema MJ. EUS-guided trucut biopsy. Gastrointest Endosc. 2005;62:417–26. doi: 10.1016/j.gie.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 13.Wittmann J, Kocjan G, Sgouros SN. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and trucut needle biopsy: A prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 14.Larghi A, Verna EC, Ricci R, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: A prospective study. Gastrointest Endosc. 2011;74:504–10. doi: 10.1016/j.gie.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Mukai S, Itoi T, Ashida R, et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–7. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Mukai S, Itoi T, Katanuma A, et al. An animal experimental study to assess the core tissue acquisition ability of endoscopic ultrasound-guided histology needles. Endosc Ultrasound. 2017 doi: 10.4103/eus.eus_16_17. doi: 10.4103/eus.eus_16_17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 18.Bang JY, Hebert-Magee S, Navaneethan U, et al. EUS-guided fine needle biopsy of pancreatic masses can yield true histology: Results of a randomised trial. Gut. 2017;pii:gutjnl. doi: 10.1136/gutjnl-2017-315154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Weynand B, Deprez P. Endoscopic ultrasound guided fine needle aspiration in biliary and pancreatic diseases: Pitfalls and performances. Acta Gastroenterol Belg. 2004;67:294–300. [PubMed] [Google Scholar]
- 22.Deshpande V, Mino-Kenudson M, Brugge WR, et al. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: Diagnostic criteria and pitfalls. Am J Surg Pathol. 2005;29:1464–71. doi: 10.1097/01.pas.0000173656.49557.48. [DOI] [PubMed] [Google Scholar]
- 23.Zagouri F, Sergentanis TN, Chrysikos D, et al. Molecularly targeted therapies in metastatic pancreatic cancer: A systematic review. Pancreas. 2013;42:760–73. doi: 10.1097/MPA.0b013e31827aedef. [DOI] [PubMed] [Google Scholar]
- 24.Chantrill LA, Nagrial AM, Watson C, et al. Precision medicine for advanced pancreas cancer: The individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clin Cancer Res. 2015;21:2029–37. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
- 25.Kanno A, Masamune A, Fujishima F, et al. Diagnosis of autoimmune pancreatitis by EUS-guided FNA using a 22-gauge needle: A prospective multicenter study. Gastrointest Endosc. 2016;84:797–8040. doi: 10.1016/j.gie.2016.03.1511. [DOI] [PubMed] [Google Scholar]
- 26.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest Endosc. 2016;84:1034–9. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 27.Katanuma A, Itoi T, Baron TH, et al. Bench-top testing of suction forces generated through endoscopic ultrasound-guided aspiration needles. J Hepatobiliary Pancreat Sci. 2015;22:379–85. doi: 10.1002/jhbp.201. [DOI] [PubMed] [Google Scholar]


