Abstract
Background and Objectives:
Subepithelial lesions (SELs) of the upper part of the digestive tract are rare, and it can be difficult to characterize them. Recently, contrast-enhanced endosonography (EUS) and elastometry have been reported as useful adjuncts to EUS and EUS-guided fine needle aspiration (EUS-FNA) in cases of pancreatic mass and lymph node involvement. The aim of this retrospective analysis was to evaluate whether contrast-enhanced EUS can discriminate benign submucosal lesions from malignant ones. We describe our retrospective experience using the contrast agent SonoVue® (Bracco Imaging, Milan, Italy) in an attempt to increase the diagnostic yield.
Patients and Methods:
Between May 2011 and September 2014, 14 patients (5 men, 9 women; median age 64 years, range 31–80 years) with SELs of the stomach or esophagus underwent EUS with SonoVue® (low mechanical index). There were 3 esophageal lesions and 11 gastric lesions. Mean size of the lesions was 30 mm (range 11–50 mm). They were discovered after anemia (n = 5), dysphagia (n = 1), and pain (n = 4) and during follow-up for resected gastrointestinal stromal tumors (GISTs) (n = 1) and a standard upper gastrointestinal endoscopy (n = 3). On endoscopic sonograms, 10 of these lesions were hypoechoic and located in the fourth layer (muscularis), and 4 were in the second or third layer (mucosa and submucosa). Contrast enhancement was assessed in the early phase (after several seconds) and late phase (>30 seconds); a final diagnosis was made based on the findings of EUS-FNA using a 19-gauge ProCore (Cook Medical, Bloomington, IN) (n = 9) or 22-gauge FNA system (Cook Medical) (n = 1), the resected specimen (n = 3), or deep biopsy (n = 1). Different immunostaining was used in the pathologic studies (RNA was analyzed later using the C-kit, CD-117, CD-34, desmin, DOG-1, α-smooth actin, caldesmon, PS-100, and Ki-67 antibodies).
Results:
Final diagnoses were leiomyoma (n = 4), GIST (n = 5), schwannoma (n = 1), inflammatory tumor of Helvig (n = 1), pancreas rest (n = 2), and fibrosis (n = 1). No complications occurred. All 5 GISTs showed enhancement in the early and late phases, whereas the 8 remaining lesions did not show any enhancement. Only 1 leiomyoma showed heterogeneous enhancement.
Limitations:
The monocentric and retrospective study design and small number of patients.
Conclusions:
In cases of SELs of the stomach or esophagus, SonoVue® could be a complementary tool to endosonography to differentiate GISTs (early and clear enhancement) from other SELs (few or no enhancement), such as leiomyomas or pancreatic rest. These results are similar to those of the few, small studies published on this topic, but more studies with a larger number of patients are needed to confirm these findings.
Keywords: Contrast enhancement, diagnosis, endosonography, esophagus, subepithelial lesions of the stomach
INTRODUCTION
Subepithelial lesions (SELs) of the upper part of the digestive tract are rare, and it can be difficult to characterize them. Preliminary studies showed interest in using endosonography (EUS) with a contrast agent for other diseases, such as metastasis of the pancreas or lymph nodes.[1,2] SELs are quite rare during upper gastrointestinal (GI) endoscopy. Some SELs are benign, such as lipoma, leiomyoma, or schwannoma, whereas others are potentially malignant, such as GI stromal tumors (GISTs), which are the most common mesenchymal tumors in the GI tract (about 3% of all GI tumors and 5%–6% of all mesenchymal tumors).[3,4]
It is of highest importance to differentiate malignant lesions from benign ones in order to provide appropriate treatment (i.e., surgery, medication [imatinib], or follow-up). Even if EUS can be used to easily differentiate SELs from extrinsic compression, discrimination between malignant and benign lesions remains difficult.[5,6] EUS-guided fine needle aspiration (EUS-FNA) increases diagnostic accuracy, but sometimes tissue sampling is insufficient.[7] Deep forceps biopsy is likely to increase histopathologic accuracy, but complications, such as bleeding, perforation, etc., can occur.
The aim of this retrospective analysis was to evaluate whether contrast-enhanced EUS (CE-EUS) can discriminate benign submucosal lesions from malignant ones. We describe our experience using the contrast agent SonoVue® (Bracco Imaging, Milan, Italy) in an attempt to increase the diagnostic yield.
PATIENTS AND METHODS
Patients
Between May 2011 and September 2014, 14 patients (5 men, 9 women; median age 64 years, range 31–80 years) with an SEL of the stomach or esophagus were enrolled in this retrospective analysis. Patients with contraindications, such as severe heart failure, severe chronic obstructive pulmonary disease, known allergic disposition to SonoVue®, pregnancy, and age younger than 18 years, were excluded from the study.
Equipment
The echoendoscope used was the Hitachi/Pentax EG-3870UTK (Hitachi Medical Corp., Tokyo, Japan), and the ultrasound processor used was the Hitachi Preirus (Hitachi Medical Corp.). First, standard B-mode EUS was performed, and the size, location, and echogenicity of the lesions were recorded. Second, lesions in the submucosal upper GI tract were observed and documented 2 minutes after injecting SonoVue®. Standardized presets were established for EUS and CE-EUS to assess perfusion of these lesions using a lower acoustic power. We used the harmonic imaging technique because CE-EUS offers better imaging resolution than power Doppler imaging.
Contrast enhancement: Injection
All patients with subepithelial lesions received 5 mL of SonoVue® (8 μmL) through the peripheral vein catheter, followed by 10 mL of saline flush. The low mechanical index did not lead to destruction of the microbubbles of sulfur hexafluoride or cause it to remain in the blood vessels; in this way, dynamic investigation of the perfusion characteristics and analysis of microvascularization are possible.[8]
Contrast-enhanced endosonography: Image analysis
Ultrasound video sequences were recorded and analyzed using the software of the ultrasound processor (Hitachi Preirus, Hitachi Medical Corp.). Analysis of the recorded video data was simple. Contrast enhancement was evaluated in the early phase (after several seconds) and in late phase (>30 seconds), and subsequently, the enhancement patterns were classified as enhancement or no enhancement. All enhancement patterns were initially determined and noted onsite by experienced endosonographers.
Final diagnosis
After CE-EUS, the lesions were immediately punctured with a 19-gauge ProCore needle (Cook Medical, Bloomington, IN) (n = 9) or 22-gauge fine needle aspiration (FNA) system (Cook Medical) (n = 1) under guidance by EUS. In 3 patients, endoscopic resection was performed by mucosectomy or endoscopic submucosal dissection. In 1 patient, deep biopsy after mucosal opening was conducted. Regardless of the technique used, all specimens were subjected to pathological diagnosis. Different immunostaining was used in the pathologic studies (C-kit, CD-117, CD-34, desmin, DOG-1, α-smooth actin, caldesmon, PS-100, and Ki-67 antibodies). Genetic analysis (molecular studies with DNA) was performed in 3 patients.
RESULTS
All lesions were evaluated by upper GI endoscopy and CE-EUS. There were 3 esophageal lesions and 11 gastric lesions. They were discovered after anemia (n = 5), dysphagia (n = 1), and pain (n = 4), and during standard upper GI endoscopy (n = 3) and follow-up for resected GIST (n = 1). The median lesion size was 30 mm (range 11–50 mm).
On endoscopic sonograms, 10 of these lesions were hypoechoic and located in the fourth layer (muscularis) [Table 1], and 4 were in the second or third layer (mucosa and submucosa). After injection of the contrast agent, the lesions of 6 patients were classified as hyperenhanced, and those of 8 patients were hypoenhanced.
Table 1.
Feature of lesions
| Patients characteristics | GIST (n=5) | Non-GIST (n=5) Schwanoma (n=1) Leiomyoma (n=4) |
|---|---|---|
| Age (year) | 72 | 61 |
| Gender (male/female) | 3 male/2 female | 5 female |
| Lesion size (average) | 35 mm | 41 mm |
| Enhancement, n (%) | 5 (100) | 1 (20) a leiomyoma |
GIST: Gastrointestinal stromal tumors
The final diagnoses (after EUS-FNA or resection) were leiomyoma (n = 4), GIST (n = 5), schwannoma (n = 1), inflammatory tumor of Helvig (n = 1), pancreas rest (n = 2), and fibrosis (n = 1). The 4 lesions located in the first three layers (C1, C2, and C3 types, meaning mucosal or submucosal lesions) did not show any enhancement. Those lesions were an inflammatory tumor of Helvig (n = 1), pancreas rest (n = 2), and fibrosis (n = 1). The 5 GISTs showed enhancement in the early and late phases [Figures 1-4]. Only 1 leiomyoma showed heterogeneous enhancement. The main results are summarized in Table 2.
Figure 1.
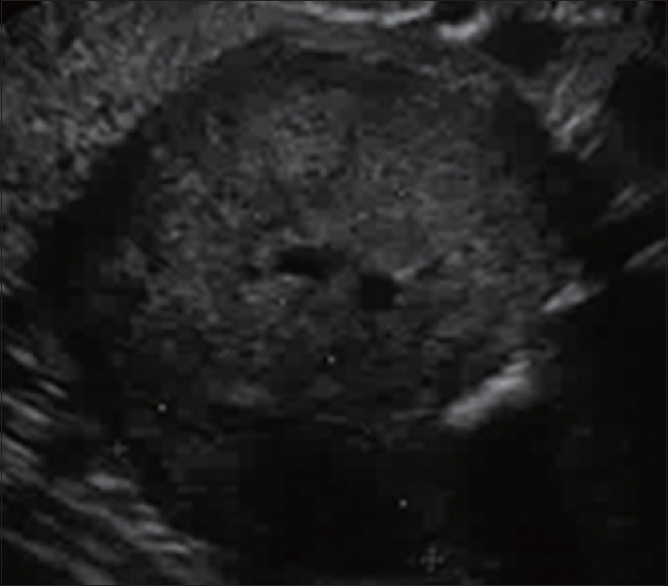
Gastrointestinal stromal tumors (submucosal tumors in the fourth layer)
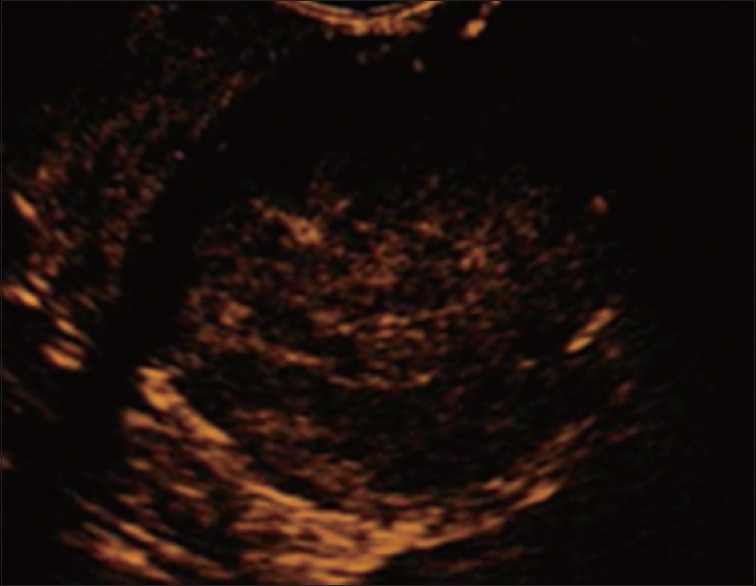
Figure 4.
Final diagnosis is made by endosonography-guided fine need aspiration (19-gauge needle)
Table 2.
Feature of lesions
| Enhancement (n=6) (%) | No enhancement (n=8) (%) | Location in digestive layers | |
|---|---|---|---|
| GIST (n=5) | 100 | 0 | C4 |
| Leiomyoma (n=4) | 25 | 75 | C4 (n=3) |
| C2 (n=1) | |||
| Pancreas rest (n=2) | 0 | 100 | C1C2 |
| Schwanoma (n=1) | 0 | 100 | C4 |
| Pseudo inflammatory tumor helvig (n=1) | 0 | 100 | C1C2C3 |
| Fibrosis (non-conclusive) | 0 | 100 | C3 |
GIST: Gastrointestinal stromal tumors
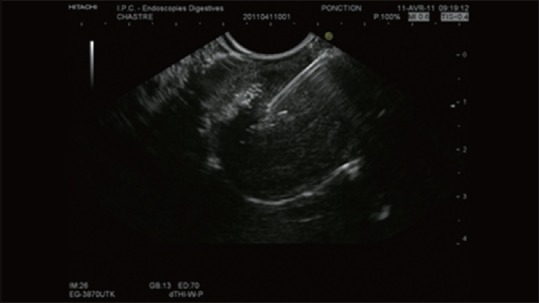
Figure 2.
The same lesion in Figure 1 before the injection of SonoVue®
Figure 3.
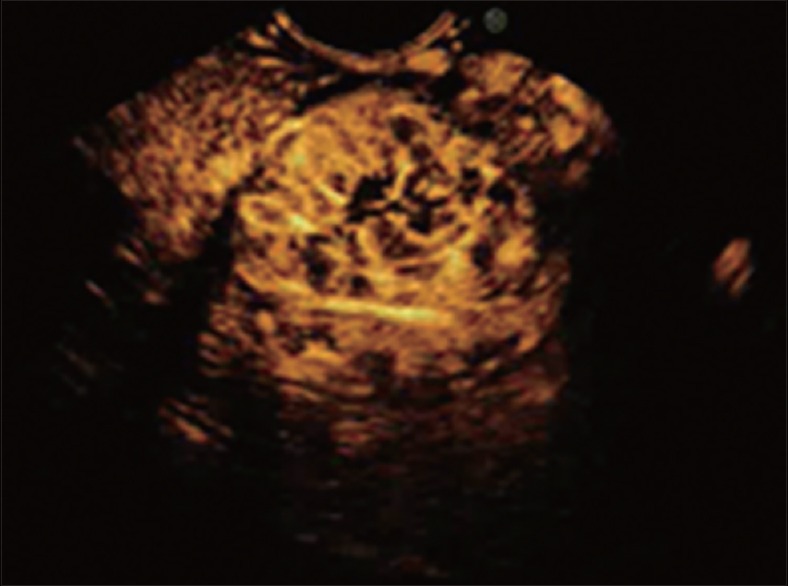
After injection of the contrast agent, the gastrointestinal stromal tumors appear as enhanced lesions
Diagnosis of GIST was suspected in 6 patients (based on the endoscopic sonographic appearance, location in C4, and enhancement), and the final diagnoses were GIST in 4 patients, leiomyoma in 1 patient, and inconclusive in 1 patient. We were unable to differentiate between a GIST and leiomyoma because of moderate enhancement, and the final diagnosis was a GIST. Diagnosis of leiomyoma was made based on findings from EUS and CE-EUS, and it was confirmed in all cases by histology [Figure 5].
Figure 5.
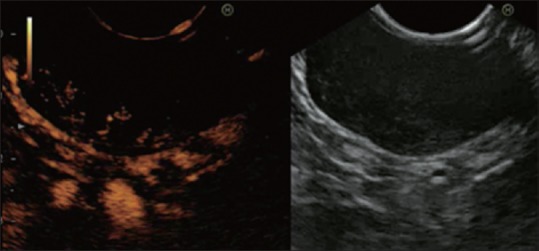
A typical leiomyoma and submucosal tumors without enhancement
Limitations
This was a monocentric, retrospective study with few patients, similar to the other rare studies on this topic. In addition, no interobserver agreement testing was performed to assess the reproducibility between the different endosonographers.
DISCUSSION
EUS is widely used to diagnose SELs because it is superior to other modalities (computed tomography [CT] and endoscopy). Standard EUS can be used to locate these lesions in different layers (mucosal C1 and C2, submucosal C3, and muscularis C4 types) with quite poor accuracy (between 30% and 66%).[9,10] Most often lesions of C1, C2, and C3 types are benign and correspond to pancreas rest, fibrosis, and inflammatory lesion, as in our series. Sometimes leiomyoma can be found in the second layer (C2 type), and it is often very difficult to differentiate lesions in the fourth layer. However, this discrimination is important because GISTs are potentially malignant lesions that require at least follow-up or surgical treatment. Diagnostic accuracy of endosonographers for SELs has not been clearly determined. According to studies, it ranges between 43% and 82%,[11,12] and most incorrect diagnoses based on findings from EUS were made for lesions in the third or fourth layer.
A definite diagnosis can be difficult to make based on endosonographic features alone in most hypoechoic lesions of the fourth layer.[13,14] The main problem is differentiating GISTs (potentially malignant lesions) from other SELs. A GIST is frequently found in the stomach, less often in the duodenum, and rare in the esophagus; however, the location is obviously not a sufficient argument for making a diagnosis. In a multicenter, prospective cohort study, Rösch et al.[6] investigated endosonographic criteria such as lesion size (>30 mm), irregular margins, inhomogeneous pattern or lymph nodes (size >10 mm). At least two criteria were required to obtain a specificity of 80% and a relatively low sensitivity of 64%. Further, SELs do not all show clear, obvious signs of malignancy, and histology remains the gold standard for characterization.
By definition, forceps biopsies are poor for making a histologic diagnosis of SELs, and biopsy sampling often remains difficult. Some authors have proposed more invasive procedures, such as deep biopsies (bite-on-bite) or endoscopic mucosal resection and submucosal resection for lesions arising from the second or third layer. These resections, which are mostly performed for asymptomatic lesions, can result in complications, such as bleeding or perforation. Further, the problem remains for SELs arising from the fourth layer because endoscopic resection has a high risk of perforation.[15,16] Therefore, EUS-FNA could play an important role in diagnosis. It has been recommended to use a 19-gauge needle for obtaining histological samples, the mitotic index, and immunostaining (CD-117, CD-34, etc.). A study of 53 patients[17] showed that the diagnostic rate of adequate specimen was in relation to the lesion size: 71% if lesions measured <20 mm, 86% if 20–40 mm, and 100% if >40 mm; the authors observed no complications. EUS-FNA provides adequate specimens with a diagnostic rate between 78% and 86%; however the repeated needle passes needed to obtain sufficient tissue can result in complications.[17,18,19,20,21,22,23,24,25]
For small SELs (<2 cm), EUS-FNA usually provides only a cytologic specimen and fails to obtain a histological specimen, mainly because of insufficient material.[7] EUS-FNA or deep needle biopsy have shown only moderate diagnostic yield in several studies.[26,27,28] Finally, for large SELs (>3 cm), which are often symptomatic or have suspicious features (e.g., irregular borders, inhomogeneous lesion, cystic spaces, or echogenic foci), surgery is quickly proposed regardless of the histologic findings. However, in others cases, the clinical management of smaller SELs (<2 or 3 cm) is not always as easy; especially, in our study, we found that EUS with or without FNA is often insufficient to provide an accurate diagnosis of hypoechoic intramural lesions in the fourth layer.
Some consider that follow-up EUS could be informative, and any tumor growth could be considered as an indicator of malignancy. Follow-up EUS should be performed for small SELs (<2 cm or <3 cm) if a GIST is suspected. If the SEL grows, it seems more prudent to suggest surgery because it is likely to be a GIST. However, what percentage of growth should be considered as suggestive of malignancy, for example, 1 mm/month, as evaluated in a retrospective study?[29] Several studies have investigated the results of prospective series, but none has validated the findings.[6] An additional drawback of follow-up EUS is the risk of losing patients to follow-up because of poor compliance.[13]
For all the aforementioned reasons, we consider that CE-EUS could be an alternative and an additional useful tool. It is a safe method without risk because ultrasound contrast agents are generally well tolerated by patients. It is also a fast, easy technique, as it lengthens the examination time by only 2 or 3 minutes. Diagnostic accuracy was independent from patient characteristics, such as age, sex, and location or size of the SEL. The only exception was a large leiomyoma >50 mm, which may be explained be atypical enhancement. This patient was also the first patient enrolled in the study so this result may be an effect of the learning curve.
Sakamoto et al.[30] assessed microvessel patterns by CE-EUS, and perfusion images enabled the physicians to classify the GISTs into two types: type I with regular microvessels and homogeneous enhancement, and type II with irregular microvessels and heterogeneous enhancement. A good relationship was shown between the images of CE-EUS in the perfusion phase and the histogram distribution patterns. The superiority of CE-EUS to the other modalities was remarkable even for evaluation of small GISTs. These results suggested that compared with power Doppler EUS and contrast-enhanced CT, CE-EUS was more sensitive for detecting intratumoral vessels with slow flow, and superior with regard to the evaluation of small GISTs.
Using the software of the ultrasound processor (Hitachi Preirus, Hitachi Medical Corp.), Kannengiesser et al.[31] analyzed arrival time, time to peak, and maximum intensity gain. CE-EUS findings were classified into groups according to their perfusion characteristics, such as hyperenhanced, isoenhanced, or hypoenhanced, and compared with the surrounding normal tissue. Hypoenhanced lesions were lipomas or leiomyomas (without significant difference between contrast agent enhancement). All hyperenhanced lesions were identified as GISTs. Only 1 GIST showed a slightly lower signal intensity; histologically, the proliferation index was very low in this case. The authors[31] concluded that sensitivity and specificity of 100% were required to correctly discriminate between GISTs and benign lesions.
Additionally, Kannengiesser et al.[31] studied more complex features, such as time to peak and area under the curve. Results differed significantly without any concordance to benignity or malignancy. Especially, hypoenhanced lesions could not be determined at times because of very low intensity and the lack of peak development. Hence, there may be no need to interpret a very complex data analysis and the first enhancement; lack of contrast enhancement may be sufficient to predict a GIST (potentially malignant) vs. a non-GIST (many benign lesions). Therefore, histologic findings and other criteria, such as lesion size, an irregular extraluminal border, necrotic appearance, echogenic foci, and cystic spaces, can be considered in the therapeutic approach.
Sakamoto et al.[30] reported that even small GISTs have malignant potential and can be detected by CE-EUS. Indeed, in their series, there was no significant difference in tumor size between low-grade and high-grade malignant GISTs (although high-grade GISTs tended to be >3 cm), suggesting that tumor size is not closely correlated with the lesion's malignant potential. Moreover, irregular vessels observed by CE-EUS were more sensitive than an irregular extraluminal border and necrotic appearance observed by standard EUS for the evaluation of high-grade malignant GISTs, especially for small GISTs (without lobulation or a heterogeneous appearance). The authors[30] concluded that the classification of vascular patterns by CE-EUS is complementary to the tumor size and other endosonographic features in the evaluation of the malignant potential of GISTs. Central necrosis in GISTs observed as necrotic areas (avascular solid tumor growth) detected by CE-EUS may be an additional characteristic to use in predicting the risk of malignancy.
Nevertheless, our results differ from other studies. For Sakomato et al.,[30] CE-EUS could not be used to distinguish leiomyomas or schwannomas from benign GISTs because all lesions were derived from spindle cells and had a regular vessel pattern (i.e., fine vessels flowing in the lesion). Conversely, in our series, leiomyomas (except one large one) and schwannomas had no enhancement, whereas all GISTs (without suspicion of malignancy) had enhancement. In all cases, EUS-FNA remains necessary for histological differentiation if there is doubt about malignancy.
CONCLUSIONS
In case of SELs of the stomach or esophagus, CE-EUS could be a complementary tool to EUS to differentiate GISTs (early and clear enhancement) from other SELs (few or no enhancement), such as leiomyomas or pancreatic rest. This diagnostic tool is easy and quick to use. Although this study was a retrospective analysis, the results are mostly similar to those of the few studies published on this topic. Obviously, further prospective studies with more patients are needed to assess CE-EUS in the evaluation of submucosal lesions of the GI tract.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fusaroli P, D’Ercole MC, De Giorgio R, et al. Contrast harmonic endoscopic ultrasonography in the characterization of pancreatic metastases (with video) Pancreas. 2014;43:584–7. doi: 10.1097/MPA.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 2.Hocke M, Menges M, Topalidis T, et al. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473–80. doi: 10.1007/s00432-007-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15:1293–301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817–72. [PubMed] [Google Scholar]
- 5.Hwang JH, Saunders MD, Rulyak SJ, et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–8. doi: 10.1016/s0016-5107(05)01567-1. [DOI] [PubMed] [Google Scholar]
- 6.Rösch T, Kapfer B, Will U, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: A prospective multicenter study. Scand J Gastroenterol. 2002;37:856–62. [PubMed] [Google Scholar]
- 7.Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–5. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: What is available, how do they work, and are they effective? Gastrointest Endosc. 2009;69:S71–7. doi: 10.1016/j.gie.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Reddymasu SC, Oropeza-Vail M, Pakseresht K, et al. Are endoscopic ultrasonography imaging characteristics reliable for the diagnosis of small upper gastrointestinal subepithelial lesions? J Clin Gastroenterol. 2012;46:42–5. doi: 10.1097/MCG.0b013e318226af8e. [DOI] [PubMed] [Google Scholar]
- 10.Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722–7. doi: 10.1016/j.gie.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54. doi: 10.1016/j.giec.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Ji F, Wang ZW, Wang LJ, et al. Clinicopathological characteristics of gastrointestinal mesenchymal tumors and diagnostic value of endoscopic ultrasonography. J Gastroenterol Hepatol. 2008;23:e318–24. doi: 10.1111/j.1440-1746.2008.05322.x. [DOI] [PubMed] [Google Scholar]
- 13.Nickl N. Endoscopic approach to gastrointestinal stromal tumors. Gastrointest Endosc Clin N Am. 2005;15:455–66. doi: 10.1016/j.giec.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002;56:S43–8. doi: 10.1016/s0016-5107(02)70085-0. [DOI] [PubMed] [Google Scholar]
- 15.Cantor MJ, Davila RE, Faigel DO. Yield of tissue sampling for subepithelial lesions evaluated by EUS: Acomparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc. 2006;64:29–34. doi: 10.1016/j.gie.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Hunt GC, Smith PP, Faigel DO. Yield of tissue sampling for submucosal lesions evaluated by EUS. Gastrointest Endosc. 2003;57:68–72. doi: 10.1067/mge.2003.34. [DOI] [PubMed] [Google Scholar]
- 17.Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound - Guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–82. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 19.Sepe PS, Moparty B, Pitman MB, et al. EUS-guided FNA for the diagnosis of GI stromal cell tumors: Sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254–61. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Lai R, Stanley MW, Bardales R, et al. Endoscopic ultrasound-guided pancreatic duct aspiration: Diagnostic yield and safety. Endoscopy. 2002;34:715–20. doi: 10.1055/s-2002-33443. [DOI] [PubMed] [Google Scholar]
- 21.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fineneedle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:63545. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 22.Brand B, Oesterhelweg L, Binmoeller KF, et al. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig Liver Dis. 2002;34:290–7. doi: 10.1016/s1590-8658(02)80150-5. [DOI] [PubMed] [Google Scholar]
- 23.Jeon SW, Park YD, Chung YJ, et al. Gastrointestinal stromal tumors of the stomach: Endosonographic differentiation in relation to histological risk. J Gastroenterol Hepatol. 2007;22:2069–75. doi: 10.1111/j.1440-1746.2006.04767.x. [DOI] [PubMed] [Google Scholar]
- 24.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EU-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–6. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 25.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 26.Polkowski M, Gerke W, Jarosz D, et al. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: Aprospective study. Endoscopy. 2009;41:329–34. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: Arandomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 28.Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–23. doi: 10.1016/j.gie.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Lachter J, Bishara N, Rahimi E, et al. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008;55:1653–6. [PubMed] [Google Scholar]
- 30.Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrastenhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–37. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515–20. doi: 10.3109/00365521.2012.729082. [DOI] [PubMed] [Google Scholar]


