Abstract
Background and Objectives:
Since the introduction of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), most pulmonary centers use this technique exclusively for mediastinal lymph node (LN) sampling. Conventional “blind” TBNA (cTBNA), however, is cheaper, more accessible, provides more tissue, and requires less training. We evaluated whether sampling of mediastinal LN using EBUS-TBNA or cTBNA according to a predefined set of criteria provides acceptable diagnostic yield.
Materials and Methods:
Sampling method was determined prospectively according to a predefined set of criteria based on LN station, LN size, and presumed diagnosis. Sensitivity, specificity, positive, and negative predictive value were evaluated for each modality.
Results:
One hundred and eighty-six biopsies were carried out over a 3-year period (86 cTBNA, 100 EBUS-TBNA). Seventy-seven percent of LN biopsied by EBUS-TBNA were <20 mm, while 83% of cTBNA biopsies were ≥20 mm. Most common sites of cTBNA sampling were station 7, 4R, and 11R as opposed to 7, 11R, 4R, and 4 L in the case of EBUS-TBNA. Most common EBUS-TBNA diagnosis was malignancy versus sarcoidosis in cTBNA. EBUS-TBNA and cTBNA both had a true positive yield of 65%, but EBUS-TBNA had a higher true negative rate (21% vs. 2% for cTBNA) and a lower false negative rate (7% vs. 28%). Sensitivity, specificity, positive predictive value, and negative predictive value for EBUS-TBNA were 90%, 100%, 100%, and 75%, respectively, and for cTBNA were 68%, 100%, 100%, and 7%, respectively.
Conclusion:
A stepwise approach based on LN size, station, and presumed diagnosis may be a reasonable, cost-effective approach in choosing between cTBNA and EBUS-TBNA.
Keywords: Biopsy, conventional transbronchial needle aspiration, endobronchial ultrasound-guided transbronchial needle aspiration, lung cancer, lymphadenopathy
INTRODUCTION
Convex “real-time” endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has become increasingly popular and widespread since its introduction. Many consider it the preferred modality for the diagnosis and staging of mediastinal lymph nodes (LNs) or masses.[1,2] EBUS-TBNA has gradually replaced conventional “blind” TBNA (cTBNA) due to its higher diagnostic yield. It has superior sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV),[3] and it is expected to continue to reduce the need for mediastinoscopy for evaluation and staging of abnormal mediastinal LNs.[4] However, along with its distinct advantages, this technique bears some limitations. Samples obtained by EBUS-TBNA have often been claimed to be insufficient for the diagnosis of diseases where histological samples are required as with lymphomas (in particular Hodgkin's disease).[2,5] EBUS-TBNA is also considered more costly and entails additional, specialized training and expertise.[6,7] These drawbacks make EBUS-TBNA less accessible than cTBNA, particularly in small centers[6] with limitations in resources. In this study, we aimed to create an integrative approach utilizing both cTBNA and EBUS-TBNA focusing on the most appropriate individual advantages of each technique. We hypothesized that applying a stepwise approach, based on node characteristics and presumed diagnosis, may avert the default for EBUS-TBNA and suggest the appropriate modality for diagnostic, while providing a welcome, cost-saving strategy.
MATERIALS AND METHODS
Patients
This study was approved by the Ethics Committee of the Hadassah-Hebrew University Medical Center. One hundred and eighty-six patients participated in the study. We included all patients over 18 years of age, who underwent diagnostic LN sampling through fiberoptic bronchoscopy over a period of 3 years (January 2011–December 2013).
Study design
All patients referred for mediastinal LN sampling were assigned to undergo either an EBUS-TBNA or a cTBNA procedure based on a proposed predefined set of criteria [Figure 1]. These criteria were put forth based on (1) the relatively easy accessibility of LNs at station 4R, 7, and 11R by “blind” cTBNA;[8,9] (2) LN size cutoff of 15–20 mm;[10] and (3) disease characteristics. We used cTBNA for LN sampling at stations 7, 4R, or 11R which were >20 mm (short axis diameter) and in cases of a presumed diagnosis of sarcoidosis or lymphoma (based on the combination of clinical presentation, imaging characteristics, and laboratory findings) for sampling smaller LNs 15–20 mm at the same stations. In all other cases, sampling was carried out by EBUS-TBNA. LN aspirates and biopsies were sent for pathologic diagnosis.
Figure 1.
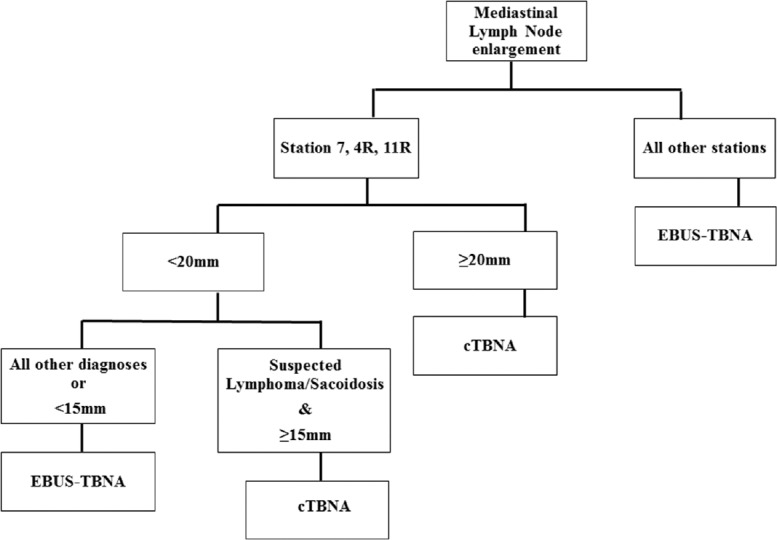
Clinical diagnostic algorithm for mediastinal lymph node sampling. Diagnostic approach is based on lymph node station, lymph node size and presumed diagnosis. cTBNA: Conventional transbronchial needle aspiration, EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration
Diagnostic procedure
For all patients, flexible bronchoscopy was performed using intravenous sedation (midazolam 1–10 mg and dolestine 25–50 mg) and local anesthesia with 2% lidocaine delivered as required through the bronchoscope. Pulse rate, blood pressure, electrocardiogram, and oxygen saturation were monitored, and clinically, significant adverse events were captured. Following a complete inspection of the bronchial tree, for each LN station biopsied, a minimum of four aspirates were performed and sent for pathological analysis. In the cTBNA group, 19-gauge WANG needle (Cook Medical Inc., Bloomington, IN, USA) was used. EBUS-TBNA was performed using an Olympus endobronchial ultrasonography bronchoscope and NA-411 D-1521 21-gauge needles (Olympus Medical Systems Corporation, Tokyo, Japan). Patients were observed for at least 3 h after endoscopy.
Statistical analysis
The sensitivity, specificity, accuracy, PPV, and NPV (including 95% confidence interval) were calculated for each technique using the GraphPad InStat 3.05 software (GraphPad Software, San Diego, California, United States). The level of significance was set at P < 0.05.
RESULTS
The characteristics of our study populations are shown in Table 1. One hundred and eighty-six procedures perfor med in 180 patients were included in the study. Of these, 100 procedures were carried out in 96 patients using EBUS-TBNA, and 86 procedures were performed by cTBNA in 84 patients (EBUS-TBNA was repeated in four patients as the first attempt was not diagnostic, and cTBNA was repeated in two patients for the same reason). Mean age was 55 years (61 in EBUS-TBNA vs. 48 in cTBNA). For both groups, patients were predominantly male (60% in EBUS-TBNA group vs. 62% in cTBNA).
Table 1.
Basic clinical characteristics
| cTBNA | EBUS-TBNA | |
|---|---|---|
| Age, years (range) | 48 (18-88) | 61 (21-84) |
| Male:female | 52:32 | 58:38 |
| Number of patients | 84 | 96 |
| Number of procedures | 86 | 100 |
cTBNA: Conventional transbronchial needle aspiration, EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration
Lymph node distribution by procedure
As demonstrated graphically in Figure 2, cTBNA was used to sample most of the LNs of size ≥20 mm (83%), whereas most of the LNs of size <20 mm were sampled by EBUS-TBNA (77%). cTBNA was mostly utilized at stations 7 (61%), 4R (13%), and 11R (10%), whereas EBUS-TBNA was most frequently utilized for LNs at stations 7 (28%), 11R (26%), 4R (16%), and 4 L (11%) [Table 2]. Subcarinal LNs at station 7 were the only instance where cTBNA was used more frequently than EBUS-TBNA (69% vs. 31%).
Figure 2.
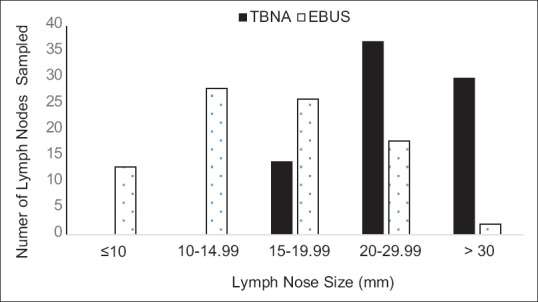
Rates of mediastinal lymph node sampling by cTBNA vs. EBUS-TBNA sorted by lymph node size. cTBNA: Conventional transbronchial needle aspiration, EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration
Table 2.
Lymph node sampling method by station
| Nodal station | cTBNA (%) | EBUS-TBNA (%) |
|---|---|---|
| 2R | 3 (3.3) | 5 (5.0) |
| 4R | 13 (14.4) | 16 (15.8) |
| 4L | 0 | 11 (10.9) |
| 7 | 61 (67.8) | 28 (27.7) |
| 10R | 1 (1.1) | 3 (3.0) |
| 10L | 1 (1.1) | 1 (1.0) |
| 11R | 10 (11.1) | 26 (25.7) |
| 11L | 1 (1.1) | 8 (7.9) |
| Stump | 0 | 3 (3.0) |
| Total | 90 (100) | 101 (100) |
cTBNA: Conventional transbronchial needle aspiration, EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration
Diagnoses by procedure
Sarcoidosis was the most common diagnosis made by cTBNA (50%), while lung cancer was most commonly detected by the EBUS-TBNA group (58%). Other diagnoses made by cTBNA, in decreasing order, were lung cancer 31% (87% nonsmall cell lung cancer, 13% small cell lung cancer), lymphoma 9%, breast cancer 4%, melanoma 2%, colon cancer 2%, and tuberculosis 2%. Diagnoses made by EBUS-TBNA include 49% non small cell lung cancer and 9% small cell lung cancer, as well as breast cancer 11%, sarcoidosis 8%, mediastinal cysts 5%, tuberculosis 3%, melanoma 3%, renal cell carcinoma 3%, and other malignancies (carcinoid, thyroid carcinoma, rectal carcinoma, pancreatic carcinoma and neuroblastoma) accounting for 15% of cases [Figure 3].
Figure 3.
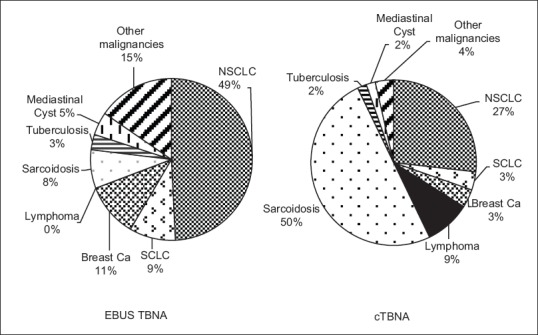
Distribution of mediastinal lymph node diagnoses. cTBNA: Conventional transbronchial needle aspiration, EBUS-TBNA: Endobronchial ultrasound-guided transbronchial needle aspiration, SCLC: Small Cell Lung Cancer, NSCLC: Non-Small Cell Lung Cancer
Statistical analysis
Both cTBNA and EBUS-TBNA had a true positive yield of 65%. The true negative rate was higher for EBUS-TBNA (21%) compared with cTBNA (2%), whereas the false negative rate was lower for EBUS-TBNA (7%) compared to cTBNA (28%). Sensitivity, specificity, PPV, and NPV for cTBNA were 68%, 100%, 100%, and 7%, respectively, and for EBUS-TBNA were 90%, 100%, 100%, and 75%, respectively.
DISCUSSION
Despite the overall superiority of EBUS-TBNA in terms of diagnostic yield, cTBNA remains a reasonable option for sampling LNs at stations 4R, 7, 11R, in particular in the case of presumed sarcoidosis or lymphoma when pathology, rather than cytology, is essential for diagnosis. Considering its simplicity, availability, affordability, safety, and several unique indications, C-TBNA continues to contribute to the welfare of patients worldwide.[11] Alternatively, EBUS-TBNA provides direct visualization of the biopsy site, which makes it a more accurate tool than cTBNA.[12,13,14,15] However, the initial capital cost of the equipment, the disposable aspiration needles, and the maintenance and repair costs are significantly higher.[6,16] The suggested approach aims to strike a balance between the accuracy advantage of EBUS-TBNA and the better accessibility and cost advantages of cTBNA.
According to our findings, cTBNA has an acceptable diagnostic yield when applied for nodes ≥20 mm at stations 7, 4R, and 11R or smaller size when sarcoidosis or lymphoma is the presumed diagnosis. Our findings are on par with previous data published showing EBUS-TBNA and cTBNA to have close diagnostic yield when assessing LNs at stations 4R, 7, and 11R.[9] LNs at stations 4R, 7, and 11R are relatively easy to navigate. This facilitates localization of the point of interest with the needle, making it relatively easy to sample by cTBNA despite the lack of direct visualization through the bronchoscope.[9,17] In this scenario, the direct visualization advantage of EBUS-TBNA may be less profound. On the other hand, given the low NPV of cTBNA, a negative biopsy result in the setup of high clinical suspicion should prompt further investigation.
cTBNA maintains a valuable role in the diagnosis of sarcoidosis[9,18,19] and lymphoma where cytology or histology alone is often insufficient to make a diagnosis, and pathologic findings serve as means to confirm the diagnosis and exclude other pathologies.[20] In fact, it has been claimed that a lack of operator skills and adequate experience is what mainly limits further cTBNA use for diagnosis of sarcoidosis.[9,21] Furthermore, the cTBNA 19-gauge needle has the advantage of a larger sample of core tissue over the usual small 22-gauge EBUS-TBNA needle.[20,22] This difference in sample size may potentially influence the diagnosis of specific lymphoma subtypes as demonstrated in marginal zone and follicular lymphoma.[5] Consequently, diagnosis of lymphoma with EBUS-TBNA may be trickier, requiring a combination of cytology, immunophenotyping, and histology to confirm the subtype.[20,23] The controversy regarding the role of EBUS-TBNA in diagnosing lymphoma has been expressed by the British Thoracic Society guidelines, which suggest that EBUS-TBNA is not necessarily the preferred diagnostic modality when lymphoma is suspected.[24]
This study has several notable limitations. We depict the experience of a single institution, while selection criteria, bronchoscopy technique, and sample processing may potentially vary between organizations. The reproducibility of our results remains to be further investigated. Furthermore, we did not perform a direct comparison between the pair diagnostic procedures (i.e., sampling each node by both cTBNA and EBUS-TBNA). In our view, applying both techniques for every sampled LN would pose an unnecessary risk that would be hard to justify. In spite of this limitation, our findings are consistent with previously published sensitivity, specificity, PPV, NPV, and diagnostic yield. Finally, our work did not attempt to examine the cost-effectiveness of the proposed approach, but we do believe that integration of cTBNA will have potential cost-effectiveness benefit.
CONCLUSION
While EBUS-TBNA is considered a more accurate tool than cTBNA, we have shown that applying an integrative, stepwise approach, based on predefined clinical criteria, would maintain an acceptable diagnostic yield in real-world practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We wish to thank Jean Pnini for her editorial contribution to the preparation of this manuscript.
REFERENCES
- 1.Zhu T, Zhang X, Xu J, et al. Endobronchial ultrasound guided-transbronchial needle aspiration vs. conventional transbronchial needle aspiration in the diagnosis of mediastinal masses: A meta-analysis. Mol Clin Oncol. 2014;2:151–5. doi: 10.3892/mco.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakajima T, Yasufuku K, Fujiwara T, et al. Recent advances in endobronchial ultrasound-guided transbronchial needle aspiration. Respir Investig. 2016;54:230–6. doi: 10.1016/j.resinv.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006;130:710–8. doi: 10.1378/chest.130.3.710. [DOI] [PubMed] [Google Scholar]
- 4.Czarnecka K, Yasufuku K. The role of endobronchial ultrasound/esophageal ultrasound for evaluation of the mediastinum in lung cancer. Expert Rev Respir Med. 2014;8:763–76. doi: 10.1586/17476348.2014.985210. [DOI] [PubMed] [Google Scholar]
- 5.Farmer PL, Bailey DJ, Burns BF, et al. The reliability of lymphoma diagnosis in small tissue samples is heavily influenced by lymphoma subtype. Am J Clin Pathol. 2007;128:474–80. doi: 10.1309/J7Y74D9DXEAJ9YUY. [DOI] [PubMed] [Google Scholar]
- 6.Medford AR, Agrawal S, Free CM, et al. A prospective study of conventional transbronchial needle aspiration: Performance and cost utility. Respiration. 2010;79:482–9. doi: 10.1159/000277931. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger CR, Chatterjee AB, Adair N, et al. Training in and experience with endobronchial ultrasound. Respiration. 2014;88:478–83. doi: 10.1159/000368366. [DOI] [PubMed] [Google Scholar]
- 8.Arslan Z, Ilgazli A, Bakir M, et al. Conventional vs. endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymphadenopathies. Tuberk Toraks. 2011;59:153–7. doi: 10.5578/tt.2403. [DOI] [PubMed] [Google Scholar]
- 9.Oki M, Saka H, Kitagawa C, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology. 2007;12:863–8. doi: 10.1111/j.1440-1843.2007.01145.x. [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: An experience in 242 patients. Chest. 2003;123:604–7. doi: 10.1378/chest.123.2.604. [DOI] [PubMed] [Google Scholar]
- 11.Mehta AC, Wang KP. Teaching conventional transbronchial needle aspiration. A continuum. Ann Am Thorac Soc. 2013;10:685–9. doi: 10.1513/AnnalsATS.201308-272ED. [DOI] [PubMed] [Google Scholar]
- 12.Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: Systematic review and meta-analysis. Thorax. 2009;64:757–62. doi: 10.1136/thx.2008.109868. [DOI] [PubMed] [Google Scholar]
- 13.Stather DR, Chee A, MacEachern P, et al. Endobronchial ultrasound learning curve in interventional pulmonary fellows. Respirology. 2015;20:333–9. doi: 10.1111/resp.12450. [DOI] [PubMed] [Google Scholar]
- 14.Toloza EM, Harpole L, Detterbeck F, et al. Invasive staging of non-small cell lung cancer: A review of the current evidence. Chest. 2003;123(1 Suppl):157S–66S. doi: 10.1378/chest.123.1_suppl.157s. [DOI] [PubMed] [Google Scholar]
- 15.Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: A systematic review. Eur Respir J. 2009;33:1156–64. doi: 10.1183/09031936.00097908. [DOI] [PubMed] [Google Scholar]
- 16.Hergott CA, Maceachern P, Stather DR, et al. Repair costs for endobronchial ultrasound bronchoscopes. J Bronchology Interv Pulmonol. 2010;17:223–7. doi: 10.1097/LBR.0b013e3181e77280. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thora×. 2008;63:360–5. doi: 10.1136/thx.2007.084079. [DOI] [PubMed] [Google Scholar]
- 18.Morales CF, Patefield AJ, Strollo PJ, Jr, et al. Flexible transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 1994;106:709–11. doi: 10.1378/chest.106.3.709. [DOI] [PubMed] [Google Scholar]
- 19.Trisolini R, Lazzari Agli L, Cancellieri A, et al. Transbronchial needle aspiration improves the diagnostic yield of bronchoscopy in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:147–51. [PubMed] [Google Scholar]
- 20.Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol. 2010;5:804–9. doi: 10.1097/jto.0b013e3181d873be. [DOI] [PubMed] [Google Scholar]
- 21.Wong M, Yasufuku K, Nakajima T, et al. Endobronchial ultrasound: New insight for the diagnosis of sarcoidosis. Eur Respir J. 2007;29:1182–6. doi: 10.1183/09031936.00028706. [DOI] [PubMed] [Google Scholar]
- 22.Medford AR, Bennett JA, Free CM, et al. Endobronchial ultrasound guided transbronchial needle aspiration. Postgrad Med J. 2010;86:106–15. doi: 10.1136/pgmj.2009.089391. [DOI] [PubMed] [Google Scholar]
- 23.Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med. 2013;188:1216–23. doi: 10.1164/rccm.201303-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax. 2011;66(Suppl 3):iii1–21. doi: 10.1136/thoraxjnl-2011-200713. [DOI] [PubMed] [Google Scholar]


