Abstract
Purpose
The aim of this study was to assess the efficacy and safety of concurrent apatinib and docetaxel therapy vs apatinib monotherapy as third- or subsequent-line treatment for advanced gastric adenocarcinoma (GAC).
Methods
Patients, who had received apatinib with or without docetaxel as third or more line therapy for advanced GAC, were retrospectively reviewed. Propensity score matching (PSM) analysis was performed to minimize the potential confounding bias. Kaplan–Meier curve and log-rank test were used to analyze the survival. Prognostic factors were estimated by Cox regression. Adverse events (AEs) were evaluated using CTCAE 4.0.
Results
Thirty-four patients received concurrent therapy, whereas 31 received monotherapy. The median progression-free survival (PFS) and overall survival (OS) in monotherapy and con-therapy groups were 2.5 and 4 months (P=0.002), 3.3 and 6 months (P=0.004), respectively. After PSM, the median PFS and OS in the con-therapy group were also superior to the monotherapy group (P=0.004 and P=0.017). Cox regression suggested that Eastern Cooperative Oncology Group performance status (ECOG PS; HR =2.437, 95% CI: 1.349–4.404, P=0.003), CA199 (HR =1.001, 95% CI: 1.000–1.002, P=0.016), and treatment options (HR =0.388, 95% CI: 0.222–0.679, P=0.001) had significant effects on OS. Grade 3/4 toxicities in the monotherapy and con-therapy groups were as follows: leukopenia (0% vs 8.8%), neutropenia (3.2% vs 2.9%), anemia (9.8% vs 8.8%), thrombocytopenia (6.4% vs 2.9%), proteinuria (3.2% vs 2.9%), aminotransferase (0% vs 11.8%), hyperbilirubinemia (9.8% vs 5.9%), hypertension (9.8% vs5.9%), hand–foot syndrome (3.2% vs 8.8%), nausea and vomiting (0% vs 11.8%), diarrhea (0% vs 5.9%), and fatigue (6.5% vs 2.9%).
Conclusion
Patients with advanced GAC benefit more from concurrent apatinib and docetaxel therapy than apatinib monotherapy.
Keywords: propensity score matching, progression-free survival, overall survival
Introduction
Gastric carcinoma is one of the most common neoplasms and the second leading cause of cancer-related mortality both in China and worldwide.1 Among the histological types, adenocarcinoma is predominant. Surgery is recognized as the only radical treatment option for early gastric adenocarcinoma (GAC).2 However, recurrence after surgery occurs frequently,3 and approximately 80% of the patients with GAC are diagnosed at advanced stage.2 For these patients, systemic chemotherapy is indispensable and various chemotherapeutic regimens have been trialed. The first-line therapy includes platinum compound combined with a fluoropyrimidine, with additional trastuzumab necessary if HER2 positive.4 However, failure or relapse frequently occurred in quite a few patients, even with the second-line chemotherapy (ramucirumab and paclitaxel single or in combination or irinotecan or docetaxel single agent), resulting in a dismal outcome. The third-line treatment options commonly include agents recommended for second-line that were not used previously as well as pembrolizumab for PD-L1 positive according to the NCCN guidelines.5 Moreover, docetaxel, a second-generation taxane, had been reported to be feasible as a third-line therapy regimen for advanced GAC after m-FOLFIRI and m-FOLFOX-4 regimens.6
Angiogenesis, regulated by angiogenesis and anti-angiogenesis factors, is one of the landmarks of cancer.7 Among the factors, vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2)-mediated signaling play a crucial role in gastric cancer pathogenesis.8 Anti-angiogenesis targeted to VEGFR-2 contributes to improve the outcome for patients with advanced gastric cancer. Apatinib, a selectively small-molecule tyrosine kinase inhibitor (TKI), binds to VEGFR-2 and inhibits its phosphorylation to block angiogenesis via a series of cascade reactions, showing a promising outcome in multifarious tumors including advanced gastric carcinoma.2,9–11 Clinical trials9,10 have recently suggested that patients with advanced GAC in third-line therapy benefit from apatinib compared with placebo. Apatinib has therefore been recommended to treat advanced gastric carcinoma by Chinese guidelines.12 However, it is important to note that although the disease control rate (DCR) of apatinib monotherapy has reached 58.3%, the objective response rate (ORR) is still poor in the real world.2 Furthermore, the synergistic effects of the combination of apatinib and cytotoxic chemotherapeutic agents (paclitaxel and 5-fluorouracil) in gastric cancer cells and xenograft model have been reported.13 Nevertheless, there is currently no report that addresses the combined use of apatinib and cytotoxic agents in clinical practice.
Thus, in this study, we retrospectively analyze the toxicity profiles and survival benefit between the combination of apatinib and docetaxel and apatinib monotherapy as third or more line treatment for patients with advanced GAC.
Patients and methods
Patient selection
The study algorithm is presented in Figure 1. From November 17, 2015, to April 4, 2017, a total of 71 patients took apatinib with or without docetaxel as third- or subsequent-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma at our institutes. Among them, 65 patients took apatinib equal to or greater than one cycle. These were the patients who were retrospectively analyzed. The details eligible for docetaxel and/or apatinib in GAC are as follows: 1) patients with advanced GAC or GEJ adenocarcinoma confirmed by histopathology; 2) failure after undergoing second-line therapy; 3) with at least one measurable or evaluable disease; 4) adequate organ function, including an absolute neutrophil count of at least 1,800/µL, platelet count of at least 100,000/µL, serum bilirubin less than 34 mol/L, serum albumin of more than 3.2 g/L, serum aspartate aminotransferase and alanine aminotransferase less than three times the upper limit of normal for the institution, and creatinine no more than three times the upper limit of normal for the institution or creatinine clearance of at least 60 mL/min; and 5) treated with apatinib at least one cycle. The exclusion criteria are as follows: 1) Eastern Cooperative Oncology Group performance status (ECOG PS) equal or greater than 3; 2) received apatinib less than one cycle; 3) or serious heart, lung, liver, and kidney diseases.
Figure 1.

Study algorithm.
Abbreviation: GAC, gastric adenocarcinoma.
Treatment
Patients were treated with apatinib at a dosage of 250–750 mg by oral administration once a day, for 4 weeks as a cycle. Whether additional docetaxel was added during the apatinib treatment was determined by multidisciplinary team (MDT) or a senior oncology physician at one of our institutes. The dosage of docetaxel was 75–100 mg/m2 on day 1 every 3 weeks. After failure of treatment, patients were managed according to MDT discretion.
Assessment of efficacy and adverse events (AEs)
Patients were followed up every 3–12 weeks during treatment. The follow-up consisted of physical examinations, complete blood counts, biochemical profiles, carcinoembryonic antigen (CEA), Carbohydrate antigen 199 (CA199), and either dynamic contrast enhancement computed tomography (CT) or magnetic resonance image studies. Overall survival (OS) and progression-free survival (PFS) were then estimated from the date of the start of apatinib administration until the date of a patient’s death, the last follow-up examination or the date of tumor recurrence.
All AEs were either evaluated on the basis of patients’ medical history and laboratory examination results or in accordance with communication tools according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE4.0).
Statistical analyses
Categorical variables were compared using the Pearson or Continuity Correction chi-squared test or Fisher’s exact test. Continuous variables were compared by Student’s t-test. Propensity score matching (PSM) analysis with the match tolerance of 0.2 was performed for the minimization of potential confounding bias. The OS and PFS were calculated using the Kaplan–Meier method, and statistical significance between groups was compared using the log-rank test. Univariate Cox proportional hazards model regression was then performed, and variables were entered into multivariate analysis when the P-value was less than 0.1 in the univariate analysis. The clinical variables covered age, gender, ECOG PS, tumor location, histology, differential degree, American Joint Committee on Cancer (AJCC) stage (Version 7), initial CEA, initial CA199, and surgery history. All statistical analyses were performed with the SPSS statistical package (version24.0; IBM Corporation, Armonk, NY, USA). The survival curves were plotted with GraphPad Prism 7.00. A P-value of <0.05 was considered to be statistically significant.
Ethics
This study was approved by the ethics committee of both the Shandong Cancer Hospital Affiliated to Shandong University and the Shandong Provincial Hospital Affiliated to Shandong University. The patient data used in the study were de-identified. All processes were in agreement with the Declaration of Helsinki. The informed consent form was waived as this is a retrospective study.
Result
Patient characteristics
From November, 2015, to March, 2017, 31 patients received apatinib monotherapy (monotherapy group), whereas 34 patients received concurrent therapy of docetaxel and apatinib (con-therapy group). The characteristics of patients are summarized in Table 1. All patients received at least the failure of second-line therapy. 80% of them were male, and the median age was 58.6 years. More than half of the patients had an ECOG PS of 0 (53.8%), and 70.8% of the tumors were found to be located in stomach. Most of the patients had AJCC stage IV (Version 7) (72.3%), 38.4% of the patients had received surgery, and 15.4% had been treated with radiotherapy. There were no statistically significant differences in gender, age, ECOG PS, tumor location, tumor differential degree, AJCC stages, initial CEA, initial CA199, surgery history, radiotherapy, and treatment lines.
Table 1.
Characteristics of patients
| Variables | All patients
|
Propensity score-matched patients
|
||||
|---|---|---|---|---|---|---|
| Monotherapy group (N=31) | Con-therapy group (N=34) | P-value | Monotherapy group (N=24) | Con-therapy group (N=24) | P-value | |
|
| ||||||
| Gender | 0.264 | 1.000 | ||||
| Male | 23 | 29 | 20 | 20 | ||
| Female | 8 | 5 | 4 | 4 | ||
| Age | 0.878 | 0.964 | ||||
| Median | 58.4 | 58.8 | 59.3 | 59.1 | ||
| Range | 35–78 | 29–81 | 37–78 | 29–83 | ||
| ECOG PS | 0.988 | 0.771 | ||||
| 0 | 17 | 18 | 14 | 13 | ||
| 1–2 | 14 | 16 | 10 | 11 | ||
| Tumor location | 0.608 | 0.745 | ||||
| Stomach | 21 | 25 | 18 | 17 | ||
| GEJ | 10 | 9 | 6 | 7 | ||
| Differential degree | 0.415 | 0.655 | ||||
| Poor/undifferentiated | 14 | 10 | 10 | 7 | ||
| Moderate | 9 | 12 | 7 | 8 | ||
| Well | 8 | 12 | 7 | 9 | ||
| AJCC stage | 0.151 | 0.505 | ||||
| III | 6 | 12 | 5 | 7 | ||
| IV | 25 | 22 | 19 | 17 | ||
| Initial CEA (ng/mL) | 0.158 | 0.308 | ||||
| Median | 69.5 | 18.4 | 40.9 | 21 | ||
| Range | 0.4–441.9 | 0.5–225.6 | 0.4–441.9 | 0.5–225.6 | ||
| Initial CA199 (U/mL) | 0.991 | 0.623 | ||||
| Median | 145.7 | 144.0 | 159.1 | 117.8 | ||
| Range | 1.3–1,600.0 | 0.6–1,571.0 | 1.3–1,600.0 | 0.8–1,000 | ||
| Surgery history | 0.638 | 0.763 | ||||
| No | 20 | 20 | 15 | 16 | ||
| Yes | 11 | 14 | 9 | 8 | ||
| Radiotherapy history | 0.615 | 0.416 | ||||
| No | 25 | 30 | 19 | 22 | ||
| Yes | 6 | 4 | 5 | 2 | ||
| Apatinib dosage (mg) | 0.796 | 0.350 | ||||
| <500 | 10 | 12 | 6 | 9 | ||
| ≥500 | 21 | 22 | 18 | 15 | ||
| Treatment lines | 0.531 | 1.000 | ||||
| Third-line | 7 | 10 | 6 | 6 | ||
| Subsequent-line | 24 | 24 | 18 | 18 | ||
Abbreviations: AJCC, American Joint Committee on Cancer; CA199, carbohydrate antigen 199; CEA, carcinoembryonic antigen; ECOG PS, Eastern Cooperative Oncology Group performance status; GEJ, gastroesophageal junction.
Although no significant difference in baseline characteristics exists between the two groups, PSM with matched tolerance 0.2 was also performed to minimize the potential confounding bias. After PSM, 24 matched pairs of the monotherapy group vs the con-therapy group were created. In the matched cohort, the baseline characteristics were found to be distributed more symmetrically between the two groups (Table 1).
Survival
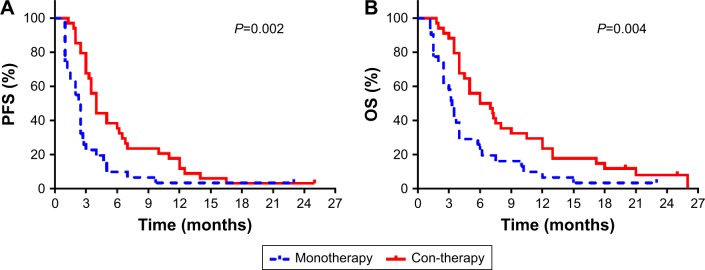
Before PSM was performed, the median PFS (Figure 2A) was found to be 2.5 months (95% CI, 1.99–3.01) in the monotherapy group and 4 months (95% CI, 3.29–4.71) (P=0.002) in the con-therapy group, with median OS (Figure 2B) being 3.3 months (95% CI, 2.76–3.84) and 6 months (95% CI,2.86–9.14) (P=0.004), respectively. After PSM, the median PFS (Figure 3A) was 2.3 months (95% CI, 1.77–2.83) and 4 months (95% CI, 2.37–5.63) (P=0.004), with median OS (Figure 3B) being 3.2 months (95% CI, 2.24–4.16) and 7 months (95% CI, 4.00–10.00) (P=0.017) in the mono-therapy group and the con-therapy group, respectively.
Figure 2.
Kaplan–Meier survival curves of PFS (A) and OS rates (B) before propensity score matching analysis.
Abbreviations: OS, overall survival; PFS, progression-free survival.
Figure 3.
Kaplan–Meier survival curves of PFS (A) and OS rates (B) after propensity score matching analysis.
Abbreviations: OS, overall survival; PFS, progression-free survival.
Independent prognostic factors for OS
Both the univariate and multivariate Cox regressions for independent prognostic factors for OS in all patients are summarized in Table 2. In the univariate analysis, gender, age, ECOG PS, initial CEA, initial CA199, and treatment options (Monotherapy vs Con-therapy) were deemed as significant factors for OS. Meanwhile, multivariate analyses demonstrated that the ECOG PS (HR =2.437, 95% CI: 1.349–4.404, P=0.003), initial CA199 (HR =1.001, 95% CI: 1.000–1.002, P=0.016), and treatment options (HR =0.388, 95% CI: 0.222–0.679, P=0.001) had significant effects on OS.
Table 2.
Univariate and multivariate Cox regression analyses of prognostic parameters for overall survival in all patients
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Gender | 0.034 | 1.975 | 1.052–3.709 | 0.735 | 1.142 | 0.530–2.460 |
| Age | 0.033 | 0.974 | 0.950–0.998 | 0.153 | 0.980 | 0.953–1.008 |
| ECOG PS | 0.004 | 2.137 | 1.267–3.605 | 0.003 | 2.437 | 1.349–4.404 |
| Tumor location | 0.186 | 0.682 | 0.387–1.202 | – | – | – |
| Differential degree | 0.880 | 0.975 | 0.699–1.360 | – | – | – |
| AJCC stage | 0.155 | 1.518 | 0.854–2.697 | – | – | – |
| Initial CEA | 0.001 | 1.004 | 1.001–1.006 | 0.230 | 1.002 | 0.009–1.004 |
| Initial CA199 | 0.001 | 1.001 | 1.001–1.002 | 0.016 | 1.001 | 1.000–1.002 |
| Surgery history | 0.143 | 0.671 | 0.394–1.143 | – | – | – |
| Radiotherapy history | 0.814 | 1.085 | 0.548–2.151 | – | – | – |
| Apatinib dosage | 0.955 | 1.015 | 0.597–1.727 | – | – | – |
| Treatment lines | 0.966 | 0.988 | 0.557–1.751 | – | – | – |
| Treatment options | 0.006 | 0.490 | 0.294–0.816 | 0.001 | 0.388 | 0.222–0.679 |
Abbreviations: AJCC, American Joint Committee on Cancer; CA199, carbohydrate antigen 199; CEA, carcinoembryonic antigen; ECOG PS, Eastern Cooperative Oncology Group performance status; GEJ, gastroesophageal junction.
For the PSM cohorts, their univariate and multivariate analyses are presented in Table 3. Both Cox regression analyses indicated that the ECOG PS (HR =2.480, 95% CI: 1.259–4.886, P=0.009), initial CA199 (HR =1.001, 95% CI: 1.000–1.002, P=0.021), and treatment options (HR =0.377, 95% CI: 0.198–0.720, P=0.003) were the independent prognostic factors.
Table 3.
Univariate and multivariate Cox regression analyses of prognostic parameters for overall survival in propensity score matching cohorts
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Gender | 0.132 | 1.816 | 0.835–3.949 | – | – | – |
| Age | 0.249 | 0.983 | 0.956–1.012 | – | – | – |
| ECOG PS | 0.033 | 1.935 | 1.055–3.552 | 0.009 | 2.480 | 1.259–4.886 |
| Tumor location | 0.319 | 0.711 | 0.364–1.390 | – | – | – |
| Differential degree | 0.638 | 0.915 | 0.632–1.325 | – | – | – |
| AJCC stage | 0.116 | 1.738 | 0.872–3.463 | – | – | – |
| Initial CEA | 0.187 | 1.002 | 0.999–1.006 | – | – | – |
| Initial CA199 | 0.009 | 1.001 | 1.000–1.002 | 0.021 | 1.001 | 1.000–1.002 |
| Surgery history | 0.372 | 0.756 | 0.410–1.396 | – | – | – |
| Radiotherapy history | 0.647 | 1.209 | 0.536–2.728 | – | – | – |
| Apatinib dosage | 0.461 | 1.269 | 0.674–2.390 | – | – | – |
| Treatment lines | 0.336 | 0.720 | 0.368–1.407 | – | – | – |
| Treatment options | 0.022 | 0.504 | 0.280–0.907 | 0.003 | 0.377 | 0.198–0.720 |
Abbreviations: AJCC, American Joint Committee on Cancer; CA199, carbohydrate antigen 199; CEA, carcinoembryonic antigen; ECOG PS, Eastern Cooperative Oncology Group performance status; GEJ, gastroesophageal junction.
Toxicity Profile
The main toxicity profiles in all patients are listed in Table 4. In summation, the grade 3/4 toxicities in the monotherapy group were neutropenia (1/31, 3.2%), anemia (3/31, 9.8%), thrombocytopenia (2/31, 6.4%), proteinuria (1/31, 3.2%), hyperbilirubinemia (3/31, 9.8%), hypertension (3/31, 9.8%), hand–foot syndromes (1/31, 3.2%), fatigue (2/31, 6.5%), in the con-therapy group, and the severe toxicities were leukopenia (3/34, 8.8%), neutropenia (1/34, 2.9%), anemia (3/34,8.8%), thrombocytopenia (1/34, 2.9%), proteinuria (1/34,2.9%), aminotransferase (4/34, 11.8%), hyperbilirubinemia (2/34, 5.9%), hypertension (2/34, 5.9%), hand–foot syndrome (3/34, 8.8%), nausea or vomiting (4/34, 11.8%), diarrhea (2/34, 5.9%), and fatigue (1/34, 2.9%). No treatment-related death was observed during the administration of the drugs.
Table 4.
Toxic profiles of patients
| Toxicities | Monotherapy group (N=31), n (%) | Con-therapy group (N=34), n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G3 | G4 | G0 | G1 | G2 | G3 | G4 | |
| Leukopenia | 20 (64.5) | 8 (25.8) | 3 (9.7) | 0 (0) | 0 (0) | 14 (41.2) | 8 (23.5) | 9 (26.5) | 3 (8.8) | 0 (0) |
| Neutropenia | 23 (74.2) | 4 (12.9) | 3 (9.7) | 1 (3.2) | 0 (0) | 16 (47.1) | 15 (44.1) | 3 (8.8) | 1 (2.9) | 0 (0) |
| Anemia | 17 (54.8) | 10 (32.3) | 1 (3.2) | 2 (6.5) | 1 (3.2) | 18 (52.9) | 9 (26.5) | 4 (11.8) | 2 (5.9) | 1 (2.9) |
| Thrombocytopenia | 24 (77.4) | 3 (9.7) | 2 (6.5) | 0 (0) | 2 (6.5) | 27 (79.4) | 4 (11.8) | 2 (5.9) | 1 (2.9) | 0 (0) |
| Proteinuria | 24 (77.4) | 4 (12.9) | 2 (6.5) | 1 (3.2) | 0 (0) | 27 (79.4) | 4 (11.8) | 2 (5.9) | 1 (2.9) | 0 (0) |
| Aminotransferase | 21 (67.7) | 10 (32.3) | 0 (0) | 0 (0) | 0 (0) | 22 (64.7) | 7 (20.6) | 1 (2.9) | 4 (11.8) | 0 (0) |
| Hyperbilirubinemia | 19 (61.3) | 8 (25.8) | 1 (3.2) | 0 (0) | 3 (9.7) | 14 (41.2) | 7 (20.6) | 1 (2.9) | 2 (5.9) | 0 (0) |
| Hypertension | 18 (58.1) | 9 (29.0) | 1 (3.2) | 3 (9.7) | 0 (0) | 18 (52.9) | 9 (26.5) | 5 (14.7) | 2 (5.9) | 0 (0) |
| Hand–foot syndrome | 22 (71.0) | 6 (19.4) | 2 (6.5) | 1 (3.2) | 0 (0) | 13 (38.2) | 9 (26.5) | 9 (26.5) | 3 (8.8) | 0 (0) |
| Nausea/vomiting | 15 (48.4) | 13 (41.9) | 3 (9.7) | 0 (0) | 0 (0) | 4 (11.8) | 6 (17.6) | 16 (47.0) | 2 (5.9) | 2 (5.9) |
| Diarrhea | 27 (87.1) | 3 (9.7) | 1 (3.2) | 0 (0) | 0 (0) | 27 (79.4) | 5 (14.7) | 0 (0) | 2 (5.9) | 0 (0) |
| Fatigue | 21 (67.7) | 6 (19.4) | 2 (6.5) | 2 (6.5) | 0 (0) | 20 (64.5) | 11 (32.4) | 2 (5.9) | 1 (2.9) | 0 (0) |
Abbreviations: G0, grade 0; G1, grade 1; G2, grade 2; G3, grade 3; G4, grade 4.
Discussion
Cytotoxic agents available for advanced gastric carcinoma have been expanded in first-line and second-line treatments.14,15 However, there still exist no standard treatment options for advanced GAC after the failure of second-line therapy, with poor prognosis, as patients rarely survive more than 12 months, this holding true even in the most recent studies.16 Lee et al6 reported that docetaxel has the potential to serve as a third-line therapy for patients with relapsed gastric cancer. Using this treatment, it was found that the median time to progression was 2.1 months and that of OS was 4.7 months with grade 3/4 neutropenia, nausea, and vomiting being common. Kang et al17 have reported the efficacy of the combination treatment of irinotecan, 5-fluorouracil, and leucovorin as a third-line chemotherapy option for advanced gastric carcinoma with the median PFS being 2.1 months and OS being 5.6 months and the main grade 3/4 toxicity being myelosuppression accounting for 36.7% of the patients. Pasquini et al16 have reported the FOLFIRI regime as a third-line therapy option with a favorable safety profile for metastatic gastric cancer; its median PFS and OS were 3.3 and 7.5 months, respectively. Therefore, the NCCN guidelines recommended the second-line agents that had not been used previously as the third-line treatment.5 However, all these treatment options remained far from expectations.
Targeting agents either alone or combined with chemotherapy in first or subsequent line therapies have recently become available for these patients with advanced gastric cancer.18 For example, trastuzumab administered alongside chemotherapy has become the standard option for first-line therapy of HER2-positive advanced gastric cancer.19 However, an international randomized, open-label, adaptive, Phase II/III study indicated that patient with HER2-positive advanced gastric cancer that have been previously treated with trastuzumab did not benefit from trastuzumab compared with taxane.19 A Phase II trial showed that a selective c-Met inhibitor tivantinib monotherapy as a second- or third-line therapy has modest efficacy in the patients with metastatic gastric cancer.20 Moreover, in addition to trastuzumab and tivantinib therapy, anti-VEGFR-2 had also been reported in the treatment of gastric cancer. Likewise, two Phase III trials that used ramucirumab – a human IgG1 monoclonal antibody that targets VEGFR-2 either when alone or combined with paclitaxel – have demonstrated that second-line therapy has the potential to improve the survival of patients with GAC. However, it is important to note that a randomized, double-blind, multicenter Phase II trial demonstrated that the addition of ramucirumab to front-line mFOLFOX6 did not improve PFS in the intent-to-treat population.21
Apatinib, a novel selective VEGFR-2 inhibitor, has shown promising survival benefits in advanced GAC. One Phase II clinical trial10 has indicated that the median OS of apatinib in the treatment of chemotherapy-refractory advanced metastatic gastric cancer was 2.5 months in the placebo group, 4.83 months in the once daily 850 mg group, and 4.27 months in the 425 mg twice daily group, with PFS being 1.40, 3.67, and 3.20 months, respectively. Furthermore, it was found that apatinib also improved OS (6.5 vs 4.7 months) and PFS (2.6 vs 1.8 months) in Phase III clinical trials9 for chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or GEJ, compared with the placebo. In addition, Zhang et al2 have reported that treatment of advanced gastric cancer with apatinib resulted in a median PFS of 2.65 months (95% CI 1.66–3.54) and a median OS of 5.8 months (95% CI 4.77–6.83) in the real-world study. All these results indicate that apatinib monotherapy ameliorates prognosis of patients with advanced GAC.
In the present study, the median PFS was 2.5 months in the monotherapy group, similar to Phase III clinical study9 and the real world study.2 It was found that additional docetaxel significantly prolonged the PFS. The results of both all patients and PSM suggested that the median OS was also significantly improved with alliance treatment of apatinib and docetaxel, compared with apatinib monotherapy (3.3 vs 6 months, P=0.004 and 3.2 vs 7 months, P=0.017). To further confirm possible prognostic factors for OS, univariate and multivariate Cox hazard regression analyses were performed. The results of both all patients (HR =0.388; 95% CI, 0.222–0.679; P=0.001) and PSM analysis (HR =0.377; 95% CI, 0.198–0.720; P=0.003) indeed demonstrated that adding docetaxel to apatinib as third or more line treatment for advanced GAC could reduce the risk of death.
In terms of treatment safety, no treatment-related death occurred. Furthermore, all toxicities in the concurrent apatinib and docetaxel group as well as the apatinib monotherapy group were less than 12%, which is acceptable. Grade 3/4 toxicities of neutropenia, thrombocytopenia, proteinuria, hyperbilirubinemia, hypertension, and fatigue were found to be less prevalent in the con-therapy group than the mono-therapy group, although this may be due to small sample size. In addition, there are no guidelines for the combination of apatinib and docetaxel in the treatment of GAC. Thus, additional prevention treatment for reducing patient toxicity may be given by clinicians during treatment. Furthermore, whether concurrent apatinib and docetaxel reduces the side effects still remains unreported.
However, there are several limitations in our present study. First, the sample size was relatively small. Second, for the reduction of intrinsic selection bias inevitable in retrospective study, PSM analysis was performed, causing sample size further deduction. Finally, all the analyses were focused on survival and toxicities with the exception of quality of life.
Although there are limitations, our data have demonstrated that patients with advanced GAC obtain some survival benefit from the combination therapy of apatinib and docetaxel, particularly when compared with apatinib monotherapy. The concurrent therapy of apatinib and docetaxel may be a treatment option for advanced GAC patients with previous failed treatments. We hope in the future that a larger prospective trial is warranted to further confirm our observations.
Conclusion
Patients with advanced GAC benefit more from concurrent apatinib and docetaxel therapy than apatinib monotherapy.
Acknowledgments
This work was supported in part by the Natural Science Foundation of Shandong Province (No ZR2017MH115), the Science and Technology Development Plans of Shandong Province (No 2014GSF118157), and the Scientific Research Foundation of Shandong Province of Outstanding Young Scientists (No BS2013YY058).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Han C, Li J, et al. Efficacy and safety for apatinib treatment in advanced gastric cancer: a real world study. Sci Rep. 2017;7(1):13208. doi: 10.1038/s41598-017-13192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 4.Mizrak Kaya D, Harada K, Shimodaira Y, Amlashi FG, Lin Q, Ajani JA. Advanced gastric adenocarcinoma: optimizing therapy options. Expert Rev Clin Pharmacol. 2017;65(3):1–9. doi: 10.1080/17512433.2017.1279969. [DOI] [PubMed] [Google Scholar]
- 5.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric cancer, version 3. 2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(10):1286–1312. doi: 10.6004/jnccn.2016.0137. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Kim SH, Oh SY, et al. Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer. Korean J Intern Med. 2013;28(3):314–321. doi: 10.3904/kjim.2013.28.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs CS, Tabernero J, Tomášek J, et al. Biomarker analyses in REGARD gastric/GEJ carcinoma patients treated with VEGFR2-targeted antibody ramucirumab. Br J Cancer. 2016;115(8):974–982. doi: 10.1038/bjc.2016.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with Chemotherapy-Refractory advanced or metastatic adenocarcinoma of the stomach or gastroesopha-geal junction. J Clin Oncol. 2016;34(13):1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31(26):3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Wei Y, Shen S, et al. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget. 2017;8(17):29346–29354. doi: 10.18632/oncotarget.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs. 2018;78(7):747–758. doi: 10.1007/s40265-018-0903-9. [DOI] [PubMed] [Google Scholar]
- 13.Feng J, Qin S. The synergistic effects of apatinib combined with cytotoxic chemotherapeutic agents on gastric cancer cells and in a fluorescence imaging gastric cancer xenograft model. Onco Targets Ther. 2018;11:3047–3057. doi: 10.2147/OTT.S159935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maron SB, Catenacci DVT. Update on gastroesophageal adenocarcinoma targeted therapies. Hematol Oncol Clin North Am. 2017;31(3):511–527. doi: 10.1016/j.hoc.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1760–1769. doi: 10.1200/JCO.2014.60.1799. [DOI] [PubMed] [Google Scholar]
- 16.Pasquini G, Vasile E, Caparello C, et al. Third-line chemotherapy with irinotecan plus 5-fluorouracil in Caucasian metastatic gastric cancer patients. Oncology. 2016;91(6):311–316. doi: 10.1159/000443962. [DOI] [PubMed] [Google Scholar]
- 17.Kang EJ, Im SA, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival. Gastric Cancer. 2013;16(4):581–589. doi: 10.1007/s10120-012-0227-5. [DOI] [PubMed] [Google Scholar]
- 18.Pasini F, Fraccon AP, Modena Y, et al. Targeted therapies for advanced and metastatic adenocarcinoma of the gastroesophageal junction: is there something new? Gastric Cancer. 2017;20(1):31–42. doi: 10.1007/s10120-016-0626-0. [DOI] [PubMed] [Google Scholar]
- 19.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18(5):640–653. doi: 10.1016/S1470-2045(17)30111-0. [DOI] [PubMed] [Google Scholar]
- 20.Kang YK, Muro K, Ryu MH, et al. A phase II trial of a selective c-Met inhibitor tivantinib (ARQ 197) monotherapy as a second- or third-line therapy in the patients with metastatic gastric cancer. Invest New Drugs. 2014;32(2):355–361. doi: 10.1007/s10637-013-0057-2. [DOI] [PubMed] [Google Scholar]
- 21.Yoon HH, Bendell JC, Braiteh FS, et al. Ramucirumab combined with FOLFOX as front-line therapy for advanced esophageal, gastroesophageal junction, or gastric adenocarcinoma: a randomized, double-blind, multicenter phase II trial. Ann Oncol. 2016;27(12):2196–2203. doi: 10.1093/annonc/mdw423. [DOI] [PMC free article] [PubMed] [Google Scholar]