Abstract
Purpose
The present study evaluated the modulation effect of a painless acupuncture technique, ankle acupuncture (AA), on resting-state functional change in patients with chronic low back pain (CLBP).
Patients and methods
Fourteen participants diagnosed with CLBP received AA and underwent one brain functional image scan after tactile stimulation and another one following the insertion of the needle. The needling sensations and clinical pain intensities were evaluated after the end of each functional image scan. The significance levels of Visual Analog Scales/Scores (VASs) before and after acupuncture were determined using paired t-test. The brain areas showing differences in the amplitude of low-frequency fluctuation (ALFF) and fractional ALFF (fALFF) between the two scans were identified. We also explored the relationship between mean ALFF values in brain areas identified and VAS scores based on Pearson correlation coefficient.
Results
A complete-case analysis was performed on 12 participants. Neither different needling sensations nor any local sensations during the two scans was found. The clinical findings indicated that the scores of VAS scores were significantly lower after AA intervention (P<0.001). Compared with those after tactile stimulation, ALFF decreased in the left insular and increased in the left precuneus and right precentral gyrus, and fALFF decreased in the left insular, during retaining of AA (corrected). Moreover, there was a positive correlation found between mean ALFF change in the left insular and that of VAS values (P<0.05).
Conclusion
The present study demonstrated the low-frequency BOLD signal oscillation response in the left insular in brain activity was associated with an immediate analgesia of AA in patients with CLBP, which provides new insights into intrinsic connections between low-frequency brain signals and analgesic effects of acupuncture.
Keywords: ankle acupuncture, low-frequency fluctuation, insular, chronic pain
Introduction
Acupuncture, an alternative and complementary therapeutic intervention, has been commonly used for the treatment of various kinds of pain. A growing body of evidence demonstrates the effectiveness of acupuncture in the treatment of acute and chronic pains, such as chronic low back pain (CLBP) and osteoarthritis.1–4 Nevertheless, the neural mechanisms underlying the analgesic effects of acupuncture treatment are still not fully understood. The development of imaging techniques such as positron resonance imaging and functional magnetic resonance imaging (fMRI) has provided tools to investigate the anatomical and physiological mechanisms targeted by acupuncture in humans and animals noninvasively.5,6 Recent advances in brain imaging have contributed to our understanding of the neural activity associated with acupuncture analgesia.7
Traditionally, acupuncture needles are inserted into the body, and sufficient needling manipulation typically elicits the characteristic needle-manipulation sensation termed deqi. Deqi manifests as numbness, heaviness, distention, and soreness, and is believed to reflect a characteristic effect.8,9 It is noteworthy that deqi is a strong stimulation and can evoke widespread brain activity changes.10,11 Thus, it is hard to distinguish widespread brain activation of the brain induced by acupuncture from the brain activity resulting from its therapeutic effect. Indeed, the results of many fMRI studies have suggested differences depending on stimulation intensity (mild vs strong) correlated differences and temporal variability (immediate vs cumulative effects of acupuncture treatment elicit different temporal neural responses) in a wide range of brain networks following acupuncture.12,13 And the emergence or disappearance of needling sensations (including deqi and sharp pain) is always involved in the above mentioned differential brain functional responses. In addition, somatosensory/tactile stimulation is one of the primary components during acupuncture processing. Tactile sensation on its own can induce brain responses in the areas similar to these activated by acupuncture.11 Above all, the widespread response induced by acupuncture represents a major confounding variable for the assessment of the specific brain activation patterns associated with acupuncture effectiveness.
The aim of our study was conducted to assess the modulatory effect of a painless acupuncture technique, ankle acupuncture (AA), on resting-state functional change in patients with CLBP. AA is a type of subcutaneous acupuncture that has been developed in the 1970s.14 It requires only the insertion of a single needle with no further manual manipulation. The needle is inserted through the skin shallowly in the subcutaneous tissue above the ankle, with no characteristic needle-manipulation sensation. AA stimulation is mild and mimics tactile sensation in the skin. Interestingly, AA exerts therapeutic effects similar to those induced by traditional acupuncture in the treatment of pain.15,16 This raises the possibility that the analysis of AA might be helpful in determining brain responses specifically associated with acupuncture analgesia by excluding or minimizing the interference from general stimulation induced by deqi needle manipulation of traditional acupuncture. Such findings indicate that studying AA may help to address the efficacy and mechanism of the action of acupuncture treatment.
Materials and methods
Participants
This was a pilot study registered in the Chinese Clinical Trial Register (ChiCTR-IPR-15007127). Fourteen right-handed patients, diagnosed with CLBP, originally recruited from Hangzhou Red Cross Hospital, participated in this study. Written informed consent was obtained from all patients. The protocol applied was in accordance with the Declaration of Helsinki and approved by the ethical committee of the Center of Cognition and Brain Disorders of Hangzhou Normal University (20151208).
The inclusion criteria were that all patients 1) would be aged between 18 and 65 years, 2) had a disease duration of ≥6 months, and 3) were experiencing pain intensity of at least 3 (Visual Analog Scale/Score (VAS)=0–10). Patients were excluded if they 1) were accompanied with pain unrelated to the present diagnosis, or were suffering from psychiatric, or neurological disorders, or serious infections; 2) had MRI contraindications, such as claustrophobia; 3) showed contraindications for acupuncture, such as bleeding tendency and pregnancy; 4) had received any therapies within the past 5 days; 5) consumed alcohol within 10 hours preceding the first scan; 6) had head motion that exceeded 2 mm in translation or 2° in rotation during scans; or 7) reported a different level of local sensation between the two interventions.
Study paradigm
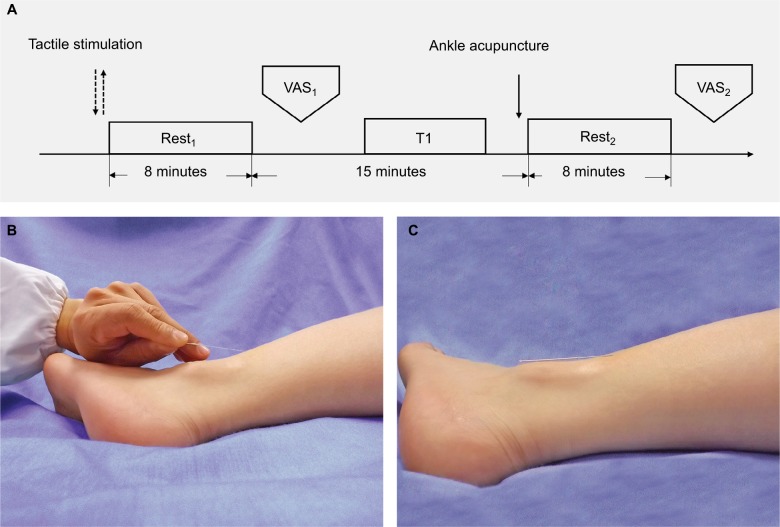
As shown in Figure 1A, this was a single-blinded and tactile-controlled study. Tactile stimulation was designed with the aim to control the effect of momentary sensation, as a potential confounder, upon the insertion of the needle during acupuncture manipulation. Similar processes were performed in the previous studies of acupuncture.17–19 Two fMRI scanning sessions, that is, rest1 after the tactile stimulation and rest2 after the insertion of the acupuncture needle, were completed. The present study focused on the low frequency blood oxygen level-dependent (BOLD) signals. All the individuals were required to provide an evaluation of the pain intensity after rest1 and rest2, respectively. Data collection was completed in the Center for Cognition and Brain Disorders of Hangzhou Normal University.
Figure 1.
Design of the present study. (A) The paradigm of interventions (tactile stimulation and acupuncture), functional-image scans (rest1 and rest2), structural-image scan (T1) and evaluations (VAS1 and VAS2) of pain intensity; (B) The tactile stimulation/needle insertion time point; (C) The needle retaining of ankle acupuncture.
Tactile stimulation
All participants underwent a tactile stimulation before the acupuncture manipulation. The tactile sensation was released by consistently pricking the skin without penetration at the needle-insertion point for around 3 seconds. The type of the needle and its angle (ie, 30°) to the skin surface were the same as those depicted in the following section.
Acupuncture manipulation
AA was performed by an experienced acupuncturist (J. Rong) with over 20 years of clinical practice. The needle-insertion point was the ankle zone 5 of the left leg, located at the cross-point of three-finger width above the lateral malleolus and posterior margin. The disposable silver acupuncture needles (0.35×40 mm; Taihe Brand, Beijing, China) were applied in the present study. After sterilizing the skin at the selected point, the acupuncturist held the needle and swiftly inserted it into the subcutis with an angle of about 30° to the surface (Figure 1B), then placed the needle down to the skin and pushed it toward the knee direction until half of the needle was inserted into the subcutaneous tissue (Figure 1C). The participants would not feel any sensation while retaining the needles if the AA was successfully performed.
Pain evaluation
A VAS was used to evaluate the intensity of the pain experienced by the participants. The scale comprised a 10-cm horizontal line with a “0” (indicating no pain) at the start point and a “10” (indicating the strongest pain imaginable) at the end. Each participant completed the evaluation by marking a cross on the line. The length (in centimeters) from “0” to point marked at VAS was measured using a ruler to represent the score of pain intensity score. The evaluations were performed immediately following rest1 and rest2, respectively.
Local needling sensation evaluation
All the participants reported their local needling sensations attributed to tactile stimulation and AA manipulation, respectively, in addition to sensations during each scan period as well, using a numerical rate scale (NRS) of 0=no sensation; 1=mild sensation; 2=moderate sensation; and 3=strong sensation. The individuals who reported a different level of local sensation between the two interventions, or any local sensations during rest1 and rest2, were excluded to ensure the validity of both AA and tactile control.
Imaging data acquisition
MRI data were acquired using a 3.0 Tesla GE MR750 scanner. All participants were fitted with foam padding to minimize head movement, and the participants were asked to keep their eyes closed and not think of anything in particular. Headphones were provided as protection from scanner noise. The 3 D T1-weighted structural images were acquired between two functional image scans, that is, rest1 and rest2. Functional images were acquired using the following parameters: 43 contiguous axial slices, repetition time (TR) of 2,000 ms, echo time (TE) of 30 ms, slice thickness of 1.33 mm, flip angle (FA) of 90°, matrix size of 64×64, field of view (FOV) of 200×200 mm2, total scan time of 8′00″. 3D T1-weighted structural images were acquired using the following parameters: 128 slices, TR of 8,100 ms, TE of 3.1 ms, slice thickness of 1 mm, FA of 8°, matrix size of 256×256, FOV of 256×256 mm2, total scan time of 5′05″. The time interval between rest1 and rest2 was 15 minutes.
fMRI preprocessing
The resting-state fMRI data were processed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and RESTplus V1.2 (http://www.restfmri.net/forum/RESTplusV1.2). The first 10 time points were discarded as adaptation of the participant to the scanner noise. The data preprocessing steps included slice timing, realignment, and spatial normalization. First, an individual T1-weighted image was co-registered to the mean functional image and then the T1-weighted image was segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Using y_*.nii, all EPI images were spatially normalized to the Montreal Neurological Institute (MNI) space and voxel size was resampled to 3×3×3 mm3. Smoothing was performed with a 6 mm full width-half maximum (FWHM) Gaussian kernel. After removing the linear trend, we regressed out of covariates, which consisted of Friston-24 head motion parameters,20 WM, and CSF.
ALFF and fractional ALFF (fALFF) computing
We performed amplitude of low-frequency fluctuation (ALFF) and fALFF analysis for each scan. ALFF calculation was based on fast Fourier transform (FFT).21 Using FFT, each time course was converted to frequency domain. Then, the square root of the power spectrum at each frequency was averaged across the filtered band (0.01–0.08 Hz). This averaged square root was taken as ALFF. Then we calculated fALFF by obtaining the ratio of the power spectrum of low frequency (0.01–0.08 Hz) to that of the entire frequency range (0–0.25 Hz, with TR=2 seconds).22
Statistical analyses
Scales analysis
The VAS scores before and after AA, and NRS scores on the two manipulations were analyzed with SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA) using paired t-test, or nonparametric t-test only when data were not subjected to normal distribution. An alpha level of 0.05 or less was accepted as statistically significant.
ALFF and fALFF analysis
RESTplus V1.2 was used for statistical analysis on ALFF and fALFF. Paired t-test was used to compare the values between rest1 and rest2. Multiple comparison correction was performed based on Gaussian random field theory (voxel-wise P<0.05, cluster-wise P<0.05, and two-tailed test) and a threshold of a minimum cluster size of 10 and connection criteria (rmm)=5 (edge connected).
Correlation analysis
To investigate the relationship between the immediate analgesic effect of AA and brain functional alterations, we explored the potential correlation between mean values of ALFF and fALFF in characterized brain areas and VAS scores based on Pearson correlation coefficient. Signals within the clusters of statistically significant ALFF and fALFF maps and overlap clusters of statistically significant ALFF and fALFF maps were extracted for correlation analysis. To determine the potential role of pain duration in analgesic effect of AA, we calculated the Pearson correlation coefficient between the amplitude of VAS change and pain duration.
Results
Subjects information
Fourteen right-handed participants completed the experiment. The data of two participants were excluded in subsequent analyses because of significant head motion (>2 mm/2°). Twelve participants (five females, seven males), with a mean age of 44.42±6.99 years and a pain duration of 7.58±6.60 years, were included in the subsequent data analysis. All the participants were Han Chinese. There was no significant difference of local needling sensations reported between the two interventions.
Clinical results
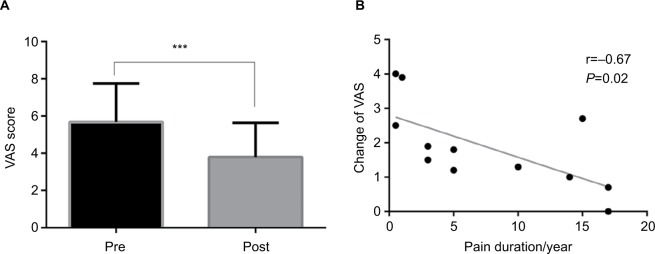
As shown in Figure 2A, the group-level results indicated that the VAS score of pain intensity significantly decreased (T=5.38, P<0.001) after the 8-minute AA (3.79±1.84) compared to that before the acupuncture intervention (5.68±2.07). Additionally, as shown in Figure 2B, there was a significantly negative correlation found between the change of VAS score (ie, VAS1–VAS2) and the duration of pain (r=−0.67, P=0.02).
Figure 2.
Results of investigation of the pain intensity. (A) The VAS scores of the pain intensity pre (VAS1) and post (VAS2) acupuncture (paired t-test, ***P=0.001); (B) The negative correlation between the change in VAS (ie, VAS1–VAS2) and pain duration (Pearson correlation, P=0.02).
ALFF result
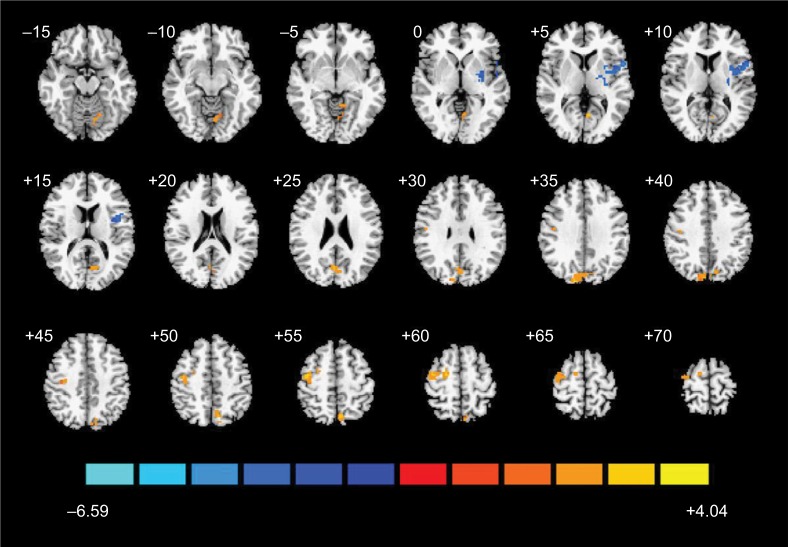
As shown in Figure 3 and Table 1, the ALFF values of four clusters significantly altered during the scan after the insertion of the needle (ie, rest1), in contrast to those obtained during the scan after tactile stimulation (ie, rest2). The ALFF value decreased in the left insular (T-value=−4.11) and increased in the right cuneus (T-value=3.57) and precentral gyrus (PG, T-value=3.66 and 2.40, respectively). The distributions of mean ALFF values within the areas stated above at each scan passed the Kolmogorov–Smirnov normality test (P>0.1). The individual and group ALFF values are presented in Figure S1.
Figure 3.
Map of brain functional changes before and after AA.
Notes: Brain areas showing increased (red and yellow) and decreased (blue) ALFF during ankle acupuncture (rest2) contrast to those showing changes after tactile stimulation (rest1) with GRF threshold (voxel-wise P<0.05, cluster-wise P<0.05, and two-tailed testing).
Abbreviations: AA, ankle acupuncture; ALFF, amplitude of low-frequency fluctuation; GRF, Gaussian random field.
Table 1.
The areas of statistically different ALFF values during ankle acupuncture contrast to those after tactile stimulation
| Brain area | BA | L/R | Cluster size (mm3) | MNI coordinates | T-value |
|---|---|---|---|---|---|
|
| |||||
| Insular | 13, 44 | L | 169 | −57, 12, 6 | −4.11 |
| Precuneus/OL/PL | 7, 19 | L | 222 | 15, −90, 36 | 3.57 |
| PG | 6, 4 | R | 107 | 21, −9, 60 | 3.66 |
| 6 | R | 14 | 42, −15, 39 | 2.40 | |
Abbreviations: ALFF, amplitude of low-frequency fluctuation; BA, Brodmann area; L, left; MNI, Montreal Neurological Institute; OL, occipital lobe; PG, precentral gyrus; PL, parietal lobe; R, right.
fALFF result
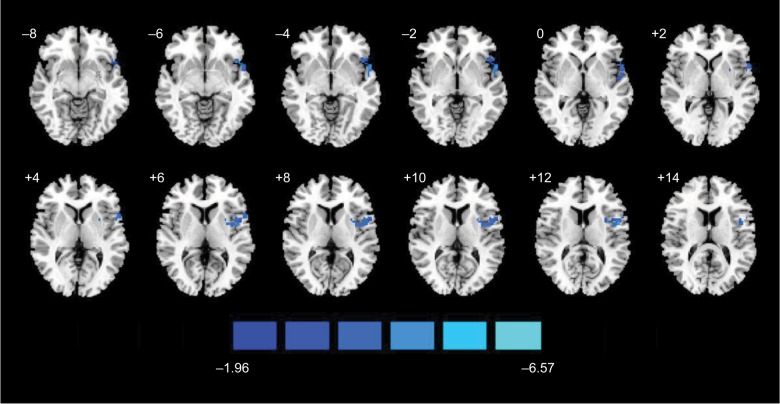
As shown in Figure 4 and Table 2, the group fALFF value of one cluster showed significant alterations during the scan after the insertion of the needle (ie, rest1), in contrast to those obtained during the scan after tactile stimulation (ie, rest2). The fALFF value decreased in the left insular (T-value=−4.11). The distributions of mean fALFF values within the left insular at each scan passed the Kolmogorov–Smirnov normality test (P>0.1). The individual and group fALFF values are presented in Figure S2.
Figure 4.
Map of brain functional changes before and after AA.
Notes: Brain areas showing increased (red and yellow) and decreased (blue) fALFF during ankle acupuncture (rest2) contrast to those showing changes after tactile stimulation (rest1) with GRF threshold (voxel-wise P<0.05, cluster-wise P<0.05, and two-tailed testing).
Abbreviations: AA, ankle acupuncture; fALFF, fractional amplitude of low-frequency fluctuation; GRF, Gaussian random field.
Table 2.
The areas of statistically different fALFF values during ankle acupuncture contrast to those after tactile stimulation
| Brain area | BA | L/R | Cluster size (mm3) | MNI coordinates | T-value |
|---|---|---|---|---|---|
|
| |||||
| Insular | 22, 44, 13 | L | 123 | −57, 9, −3 | −4.11 |
Abbreviations: BA, brodmann area; fALFF, fractional amplitude of low-frequency fluctuation; L, left; MNI, R, right.
Correlations between ALFF and VAS
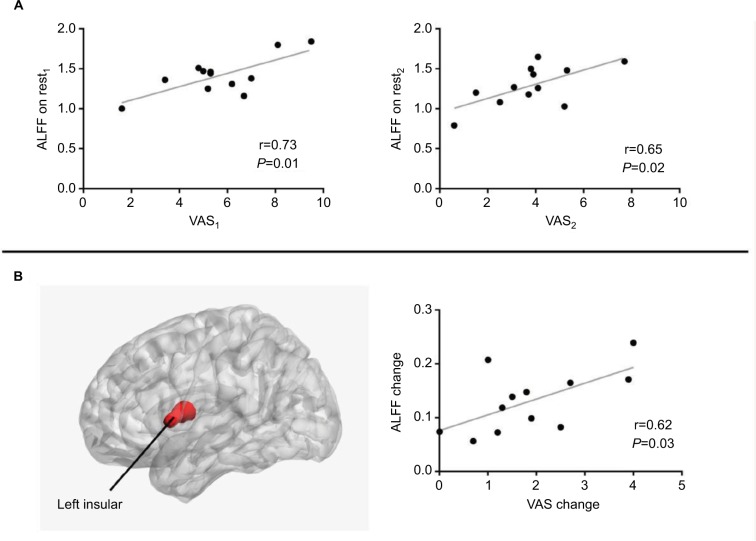
As shown in Figure 5A, the ALFF value in a spherical area (MNI central coordinate, −57, 12, 6; radium, 6 mm) in rest1 and rest2 was positively correlated with VAS1 score (r=0.73, P=0.01) and VAS2 score (r=0.65, P=0.02), respectively. Meanwhile, as shown in Figure 5B, one overlapping cluster of statistically significant ALFF and fALFF maps was located in the left insular. Moreover, a positive correlation was found between the change level of ALFF value in the left insular and the decrease level of VAS (r=0.62, P=0.03).
Figure 5.
Scatter plots of ALFF and VAS pain. (A) Significant correlation between VAS and ALFF in the left insular (Pearson correlation, P=0.01 on rest1, P=0.02 on rest2). (B) The left insular (overlapping area of statistically significant ALFF and fALFF maps) and significant correlation between ALFF change in the left insular and VAS change (Pearson correlation, P=0.03).
Abbreviations: ALFF, amplitude of low-frequency fluctuation; fALFF, fractional ALFF; VAS, Visual Analog Scale/Score.
Discussion
The present study has shown a significant decrease in VAS pain scores after AA intervention in patients with CLBP. Decreased low-frequency oscillation responses (ALFF and fALFF) in the left insular and an increased ALFF in the left precuneus and right precentral gyrus during AA were observed during needle retaining when compared with those obtained during tactile stimulation. There was a significant correlation between the low-frequency oscillation responses in the left insular and immediate analgesic effect of AA in patients with CLBP. Additionally, we found a negative correlation between the duration of pain and immediate analgesic effect of AA, consistent with the clinical observation that pain duration positively correlates with difficulties in achieving satisfactory pain relief by currently available treatments.23 Given that there was no significant difference in the local needling sensation induced by tactile stimulation vs AA, our results provided evidence for an intrinsic connection between low-frequency brain signals with “real” analgesic effect of acupuncture.
AA stimulation resembles tactile stimulation on the skin and does not induce any characteristic needle-manipulation sensation. Interestingly, AA has similar therapeutic effects as traditional acupuncture in the treatment of pain, but tactile stimulation (or non-penetrating sham acupuncture) does not.15,16 By excluding or minimizing the interference from widespread response in brain networks induced by deqi during traditional acupuncture, our present study detected low-frequency oscillation response in only a few brain areas during AA, when contrasted to the responses during tactile stimulation. It is suggested that AA is not only effective in the treatment of acute and chronic pain but also a useful tool to elucidate the specific brain activity associated with effectiveness of acupuncture. Clinically, in view of the controversial conclusions regarding the necessity of deqi in achieving a satisfactory therapeutic effect,24,25 such findings in our study indicated that AA may help address the efficacy and essential mechanism underlying the effects of acupuncture treatment.
In the present study, we evaluated the modulation effect of AA on resting-state functional change (ie, ALFF and fALFF) in patients with CLBP. Unlike block-designed fMRI used in previous acupuncture studies, the resting-state fMRI technique is practical to integrate with clinical practice in patients diagnosed with specific diseases because it is performed in task-free conditions.26 The low-frequency BOLD signals, reflecting predominant spontaneous brain activity physiologically, have been successfully characterized in human brains of specific pathological states.27 ALFF is a potentially significant and reliable algorithm for investigating intrinsic responses of the brain to acupuncture effect in clinical pain practice as well. A recent study revealed that the modulation of abnormal ALFF in a specific area (brainstem) correlated to an accumulating effect of acupuncture in migraine without aura patients.28 Then considering that fALFF is more effective than ALFF in some brain regions,29 our study combined the ALFF and fALFF algorithm. The results further revealed that altered ALFF in the left insular was associated with the immediate analgesic effect of AA.
One of the important findings in our present study is a significant correlation between the changes in the left insular and pain intensity. As we know, the insular is a key component within the pain matrix.30 It also tends to be one of the essential responsive brain areas in the chronic state of LBP. Patients with CLBP showed increased activation in the anterior insular as compared with patients with depression and healthy subjects, which to some extent indicates the significance of the insular as a dependent area in response to the sensory dimension of pain in CLBP.31 Another rest-state fMRI study measured the ALFF value in CLBP and indicated that increased ALFF value located in several brain areas, including the insular.32 The fact that the low-frequency oscillation in the insular cortex was disrupted in patients with CLBP was also reported elsewhere.33 The study by Chen et al34 reported that the connectivity between the right frontoparietal network and left insula was significantly associated with improvement in clinical pain after repeated manual acupuncture treatment in knee osteoarthritis patients. We further found that AA can suppress the increased ALFF values induced by CLBP. With regard to increased ALFF in the left precuneus and right precentral gyrus in our results, it was unrelated to the immediate analgesia of AA, and these areas may be considered as general responding areas to acupuncture stimulation compared with tactile stimulation.11
These results suggest that the low-frequency oscillation response in the insular may be an important neurobiological mechanism underlying analgesia following AA. Notably, several studies on healthy subjects revealed a significantly greater activation at insular after a real acupuncture when compared with tactile stimulation.19,35 Similar results were also found in Makary et al’s36 study targeting patients with CLBP. The discrepancy between the abovementioned study and the present work might be explained by differences in needling manipulation. Traditional acupuncture is a strong stimulation and can evoke widespread brain activity changes. The widespread response induced by acupuncture represents a major confounding variable for the assessment of specific patterns of brain activation associated with acupuncture effectiveness.
It has been suggested that the duration of pain positively correlates with the difficulties of achieving satisfactory pain relief using currently available treatments.23 Interestingly, the transient (>1 hour) analgesic effect of ketamine in chronic orofacial pain distributed equally between different pain durations.37 We found that a negative correlation between duration of pain and immediate (8 minutes following the insertion of the needle) analgesic effect was induced by AA. Furthermore, Wu et al38 reported diversity of brain areas responding to acupuncture in Bell’s palsy patients with different clinical durations. Together with the evidence on the correlation of structural plasticity in certain brain regions with the duration of CLBP and it being modulated by accumulated analgesia,39,40 the therapeutic effect by acupuncture may be mediated by central nervous system plasticity. Further investigations using a larger sample size and multiple acupuncture sessions are needed to support these conclusions. Besides, functional outcomes alongside the pain should also be considered in future studies.
The insular is also one of the major components in the default mode network (DMN) and salience network (SN).41,42 In addition, several studies have reported the disruptions of DMN and SN in chronic pain states.43,44 Moreover, fMRI studies have demonstrated the modulation in DMN by acupuncture in both healthy subjects and patients with CLBP.45,46 To further unravel the therapeutic mechanism of AA, network analysis techniques such as seed-based functional connectivity and independent component analysis may be helpful in future studies.
Conclusion
The present study demonstrated the low-frequency BOLD signal oscillation response in the left insular in brain activity was associated with an immediate analgesia of AA in patients with CLBP, which provides new insights into intrinsic connections between low-frequency brain signals and the analgesic effects of acupuncture.
Supplementary material
Individual and group ALFF values within significant clusters on each functional image scan.
Abbreviation: ALFF, amplitude of low-frequency fluctuation.
Individual and group fALFF values within the significant cluster on each functional image scan.
Abbreviation: fALFF, fractional amplitude of low-frequency fluctuation.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (NSFC: 81873379), the Shanghai Natural Science Foundation (13ZR1441900), the National Basic Research Program of China (973 Program: 2015CB554505), the Graduate Innovation Capacity Project of TCM (Y-201832) which supported the authors with conducting the experiment and paying the publication processing fee and the Three-Year development plan project for Traditional Chinese Medicine (ZY(2018–2020)- CCCX-2001–05).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shi Q, Langer G, Cohen J, Cleeland CS. People in pain: how do they seek relief? J Pain. 2007;8(8):624–636. doi: 10.1016/j.jpain.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Manyanga T, Froese M, Zarychanski R, et al. Pain management with acupuncture in osteoarthritis: a systematic review and meta-analysis. BMC Complement Altern Med. 2014;14:312. doi: 10.1186/1472-6882-14-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam M, Galvin R, Curry P. Effectiveness of acupuncture for nonspecific chronic low back pain: a systematic review and meta-analysis. Spine (Phila Pa 1976) 2013;38(24):2124–2138. doi: 10.1097/01.brs.0000435025.65564.b7. [DOI] [PubMed] [Google Scholar]
- 4.Xu M, Yan S, Yin X, et al. Acupuncture for chronic low back pain in long-term follow-up: a meta-analysis of 13 randomized controlled trials. Am J Chin Med. 2013;41(1):1–19. doi: 10.1142/S0192415X13500018. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh JC, Tu CH, Chen FP, et al. Activation of the hypothalamus characterizes the acupuncture stimulation at the analgesic point in human: a positron emission tomography study. Neurosci Lett. 2001;307(2):105–108. doi: 10.1016/s0304-3940(01)01952-8. [DOI] [PubMed] [Google Scholar]
- 6.Cho ZH, Oleson TD, Alimi D, Niemtzow RC. Acupuncture: the search for biologic evidence with functional magnetic resonance imaging and positron emission tomography techniques. J Altern Complement Med. 2002;8(4):399–401. doi: 10.1089/107555302760253577. [DOI] [PubMed] [Google Scholar]
- 7.Villarreal Santiago M, Tumilty S, Mącznik A, Mani R. Does acupuncture alter pain-related functional connectivity of the central nervous system? A systematic review. J Acupunct Meridian Stud. 2016;9(4):167–177. doi: 10.1016/j.jams.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Hui KK, Nixon EE, Vangel MG, et al. Characterization of the “deqi” response in acupuncture. BMC Complement Altern Med. 2007;7(1):33. doi: 10.1186/1472-6882-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui KK, Sporko TN, Vangel MG, Li M, Fang J, Lao L. Perception of Deqi by Chinese and American acupuncturists: a pilot survey. Chin Med. 2011;6(1):2. doi: 10.1186/1749-8546-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Chae Y, Chang DS, Lee SH, et al. Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J Pain. 2013;14(3):215–222. doi: 10.1016/j.jpain.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Bai L, Tian J, Zhong C, et al. Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Mol Pain. 2010;6:73. doi: 10.1186/1744-8069-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla S, Torossian A, Duann JR, Leung A. The analgesic effect of electroacupuncture on acute thermal pain Perception-a central neural correlate study with fMRI. Mol Pain. 2011;7:45. doi: 10.1186/1744-8069-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X. Beijing. China: People’s Military Medical Press; 1997. Wrist-Ankle acupuncture. [Google Scholar]
- 15.Fan G, Qian L, Zhao Y, et al. Acupuncture analgesia: diversity and analysis. WJAM. 2013;23:28–35. [Google Scholar]
- 16.Zhu LB, Chan WC, Lo KC, Yum TP, Li L. Wrist-ankle Acupuncture for the treatment of pain symptoms: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2014;2014(1):1–9. doi: 10.1155/2014/261709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G, Jack CR, Jr, Yang ES. An fMRI study of somatosensory-implicated acupuncture points in stable somatosensory stroke patients. J Magn Reson Imaging. 2006;24(5):1018–1024. doi: 10.1002/jmri.20702. [DOI] [PubMed] [Google Scholar]
- 18.Hui KK, Liu J, Marina O, et al. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at St 36 as evidenced by fMRI. Neuroimage. 2005;27(3):479–496. doi: 10.1016/j.neuroimage.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Jung WM, Lee IS, Wallraven C, et al. Cortical activation patterns of bodily attention triggered by acupuncture stimulation. Sci Rep. 2015;5(1):12455. doi: 10.1038/srep12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 21.Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehteshami Rad A, Luetmer MT, Murad MH, Kallmes DF. The association between the duration of preoperative pain and pain improvement in vertebral augmentation: a meta-analysis. AJNR Am J Neuroradiol. 2012;33(2):376–381. doi: 10.3174/ajnr.A2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vas J, Perea-Milla E, Méndez C, et al. Efficacy and safety of acupuncture for chronic uncomplicated neck pain: a randomised controlled study. Pain. 2006;126(1–3):245–255. doi: 10.1016/j.pain.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Michael H, Hans-Helge M, Carmen SB, et al. German acupuncture trials (GERAC) for chronic low back pain: randomized, multi-center, blinded, parallel-group trial with 3 groups. Arch Int Med. 2007;167(17):1892–1898. doi: 10.1001/archinte.167.17.1892. [DOI] [PubMed] [Google Scholar]
- 26.Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Zeng F, Yin T, et al. Acupuncture modulates the abnormal brain-stem activity in migraine without aura patients. Neuroimage Clin. 2017;15:367–375. doi: 10.1016/j.nicl.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154 Suppl 1(1):S29–S43. doi: 10.1016/j.pain.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Raecke R, Ihle K, Ritter C, Muhtz C, Otte C, May A. Neuronal differences between chronic low back pain and depression regarding long-term habituation to pain. Eur J Pain. 2014;18(5):701–711. doi: 10.1002/j.1532-2149.2013.00407.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F, Gu L, Hong S, et al. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J Pain Res. 2018;11:165–176. doi: 10.2147/JPR.S151562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485(1):26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Spaeth RB, Freeman SG, et al. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain. 2015;11:67. doi: 10.1186/s12990-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng Y, Liang XC, Dai JP, et al. Electroacupuncture modulates cortical activities evoked by noxious somatosensory stimulations in human. Brain Res. 2006;1097(1):90–100. doi: 10.1016/j.brainres.2006.03.123. [DOI] [PubMed] [Google Scholar]
- 36.Makary MM, Lee J, Lee E, et al. Phantom acupuncture induces placebo credibility and vicarious sensations: a parallel fMRI study of low back pain patients. Sci Rep. 2018;8(1):930. doi: 10.1038/s41598-017-18870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabben T, Øye I. Interindividual differences in the analgesic response to ketamine in chronic orofacial pain. Eur J Pain. 2001;5(3):233–240. doi: 10.1053/eujp.2001.0232. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Kan H, Li C, et al. Effect of Acupuncture on Functional Connectivity of Anterior Cingulate Cortex for Bell’s Palsy Patients with Different Clinical Duration. Evid-Based Compl Alt Med. 2015;2015:7. doi: 10.1155/2015/646872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younger JW, Chu LF, D’Arcy NT, et al. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152(8):1803–1810. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sridharan D, Levitin DJ, Menon V. A critical role for the right frontoinsular cortex in switching between central-executive and Default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the Default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander O, Harald G, Afra W, et al. Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry. 2013;13(1):84. doi: 10.1186/1471-244X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Zhang JH, Yi T, et al. Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupunct Med. 2014;32(2):102–108. doi: 10.1136/acupmed-2013-010423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual and group ALFF values within significant clusters on each functional image scan.
Abbreviation: ALFF, amplitude of low-frequency fluctuation.
Individual and group fALFF values within the significant cluster on each functional image scan.
Abbreviation: fALFF, fractional amplitude of low-frequency fluctuation.