Abstract
Background
Polymeric delivery systems have been elucidated over the last few years as an approach of achieving high therapeutic effect to the local site of malignant disease patients who have cancer. Polypyrrole (Ppy) is a potential organic conducting polymer which has long been recognized as a versatile material due to its excellent stability, conductive properties, and great absorbance in the range of near-infrared (NIR). It is tremendously versatile for use in various biomedical fields such as cancer therapy. NIR irradiation-activated treatment platform technologies are now being considered to be novel and exciting options in potential nanomedicine. However, the realistic photothermal use of Ppy-applied nanomaterials is yet in its early phase, and there are a few disadvantages of Ppy, such as its water insolubility. In the clinic, the common approach for treatment of lung cancer is the delivery of therapeutic active substances through intratumoral administration. Nevertheless, the tumor uptake, regional retention, mechanism of treatment, and tissue organ penetration regarding the developed strategy of this nanomaterial with photothermal hyperthermia are important issues for exerting effective cancer therapy.
Materials and methods
In this study, we developed a cationic Ppy–polyethylenimine nanocomplex (NC) with photothermal hyperthermia to study its physicochemical characteristics, including size distribution, zeta potential, and transmission electron microscopy, scanning electron microscopy, and Fourier transform infrared morphology. We also examined the cellular uptake effect on lung cancer cells, the photothermal properties, intracellularly generated reactive oxygen species (ROS), and cytotoxicity.
Results
The results suggested that this nanocarrier system was able to effectively attach onto lung cancer cells for subsequent endocytosis. The NCs taken up were able to absorb NIR and then converted the NIR light into local hyperthermia with its intracellular photothermal performance to provide local hyperthermic treatment. This regionally generated hyperthermia also induced ROS formation and improved the killing of lung cancer cells as a promising local photothermal therapy.
Conclusion
This development of a nanocarrier would bring a novel therapeutic strategy for lung cancer in the future.
Keywords: polypyrrole, photothermal, lung cancer therapy, nanomaterial, near-infrared
Introduction
Chemotherapy has been mainly used for cancer in these middle and later stage and is able to be administered before or after medical resection, in place of surgery when the tumorous region is unresectable. However, therapeutic approaches would cause a drug sensitized response, low bioavailability, and other harmful side effects.1,2 Since systemic chemotherapy intravenously administrated is not exclusively distributed to the tumorous site, it is actually hard to attain beneficial dosage levels of active medicine inside or around the tumorous region. Moreover, a substantial amount of active medicine regularly accumulates within normal tissues, causing toxic reactions and undesired side effects.3
Thermo-brachytherapy for instance, providing a means of local hyperthermia, involves the grafting of combining radiated seeds of point source intratumorally, near the tumorous region, or at the operating margins of resection, and they have been confirmed as a useful local therapeutic choice to treat the inoperable tumor and to prevent the recurrence of localized tumors. This locally therapeutic approach has been regularly administered to patients who have slow growing tumors, to be a substitute for the surgical option and has revealed efficacy in numerous dangerous cancers such as cervical, breast, and lung tumors.4–6
Polymerically delivered systems providing a therapeutic effect have been considered over several years as an approach for improving on the absence of targeting of lung tumor and serious morbidities related to systemically chemotherapeutic therapies. Some biomaterial depots offer biocompatibility and are formulated to preserve therapeutic effects at sites of tumor for a sustained period of time. Passive targeting also involves the usage of characteristic features of polymeric nanomaterials such as the charged effect on targeting the cancer cell. Cancer cells bear a rather high negative surface charged property compared with normal healthy cells, thus allowing favored attaching by cationic nanoparticle systems.7 Targeting of a positively charged nanomaterials system is achievable by electrostatic cellular binding onto negatively charged phospholipid head-groups favorably expressed on cancer cells with retained properties. Cationically polymeric materials with net positive surface charge feature emerge as a promising option because of their excellent cellular uptake and extremely strong cancer cellular interaction properties. Among the positively charged polymers, polyethylenimine (PEI) has been proved to have the highest delivery efficiency of active substances toward cells owing to its large positively charged feature that facilitates a high degree of drug incorporation and an efficient ability to escape the cell endosome upon an internalized process.8
In hyperthermia treatment, biocompatible conductive polymers have photothermal convertible efficiencies and photostability that would be higher than commercially available inorganic-based photothermal ablation agents.9 Poly-pyrrole (Ppy) functions as one potential organic conductive polymeric material and is extensively applied for organic electronic use due to its excellent stability, conductivity, and great absorbance in the near-infrared (NIR) range.10 Ppy is tremendously useful for various biological applications.11,12 NIR irradiation-activated therapy platform technologies are now being considered as novel and exciting options for potential nanomedicine uses.13 As a valuable photothermal reagent, Ppy has been studied for hyperthermia therapy because of its excellent NIR absorption band gap of photothermal behavior with promising biocompatibility.14,15 However, the practical photothermal use of Ppy-applied nanomaterials is yet in its infant period, and there are a few disadvantages of Ppy, such as its water insolubility.16,17
Ppy-based multifunctional nanomaterials for clinical cancer treatment are an emerging medical trend and an aim for cancer therapy. In the study, synthesis of a PEI nanoencapsulating Ppy (Ppy–PEI NC) and outcomes of its photothermal properties after NIR-triggered alteration are discussed. In the clinic, hyperthermic treatment can be deliberately induced using medicinal or biomedical strategies and has been elucidated and routinely used in hospitals as a therapy for dangerous cancers.18 Local hyperthermic treatment provides a good therapeutic choice for patients who may have issues regarding recurrent or serious cancer.6 In the clinic, the common way for bronchoscopic treating toward lung cancer is delivering of therapeutic active anticancer substances through intratumoral administration.19 However, the tumor uptake, regional retention, and tissue organ penetration regarding the developed strategy of this cationic nanomaterial with photothermal hyperthermia are other important issues for exerting effective cancer therapy.20 For instance, cationic nanoparticles enable increased active substance concentration in the tumor site and improved therapeutic effects.21
In addition, the mechanism of local hyperthermia is related to the localized generating of reactive oxygen species (ROS) for example free radicals and the practical damage to mitochondria in numerous types of cancer cell lines. ROS and mitochondrial dysfunction might be important functions in the cell apoptotic procedure.22 ROS are generally well defined as containing oxygen and active chemical substances.22,23 Usually, ROS are classified as two kinds.23 ROS which are commonly observed in biological systems include hydroxyl radicals, superoxide, and nitric oxide, ozone, hydroxide, peroxynitrite, and hydrogen peroxide.24
In this study, we developed a Ppy–PEI NC and elucidated its physicochemical properties. Its internalization behavior in lung cancer cells, photothermal properties, intracellularly generated ROS, and cytotoxicity mechanism were also elucidated. We hypothesized that this designed carrier system attaches onto the surface of lung cancer cells for subsequent endocytosis. Next, the endocytosed NCs would be able to receive NIR and provide local hyperthermia. This locally generated hyperthermia could induce intracellularly generated ROS and facilitate ablation of lung cancer as a promising photothermal treatment in cancer therapy.
Materials and methods
Preparation of the test samples
Tested chemical products and experimental kits were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) except where announced otherwise and were in analytical grade. Chemicals applied in cell researched works were obtained from Life Technologies (Thermo Fisher Scientific, Waltham, MA, USA).
Ppy–PEI NCs were manufactured with a nano-formulated process that was slightly modified from that previously described.25 In brief, the cationic polymeric PEI (200 mg, 600 Da) was dissolved in a deionized (DI) water (10–20 mL) and subsequently mixed with a prepared amount of monomer of pyrrole (12.5 µL). The above mixed solution was then stirred for 30 min in an acid environment (at pH 0.8). Ferric chloride hexahydrate (12.5 mg/mL, 1 mL) was then added to this aqueous. Next, in a volume polymerized chemical reaction lasting 0.5 h, the acquired black blended fluid was purified with a dialysis bag against DI water to eliminate free PEI and ferric ions. To calculate the NC amount and to obtain NC, the procedure of centrifugation (20 min) and oven dry were performed.
Physicochemical characterization of the test Ppy–PEI NCs
Ppy–PEI NCs were first suspended in different pH concentrations, and then the zeta potential and size distribution data were recorded by dynamic light scattering (DLS) (Malvern Instruments, Malvern, UK), and morphological consequences were visualized by the transmission electron microscopy (TEM) or the scanning electron microscopy (SEM). The Fourier transform infrared (FTIR) spectroscopy was carefully used to resolve molecular structural changes of the test sample. To understand the photothermal behavior, the test sample was immersed in a DI water at different concentrations or the formulations were at different pH environments then exposed to an NIR laser (808 nm, power 2.0 W cm−2, 5 min). The infrared thermographic photographic images were gotten with a thermal camera. The Thermocouple was used to study the quantitative temperature profile. To test the ability of the test Ppy–PEI NCs to attach onto the gelatin surface, a cationic gelatin (A) or anionic gelatin (B) was placed on a confocal dish at room temperature. Before this study, the test gelatin and Ppy–PEI NCs were respectively labeled with cyanine 5 (Cy5) and fluorescein N-hydroxysuccinimide (NHS) esters through covalent conjugation. Next, the fluorescent fluorescein-Ppy–PEI NCs (2.4 mg/mL) were placed on a gelation state of fluorescent Cy5-gelatin (20%, 25°C) on a confocal dish. After 60 min, the unbound fluorescein-Ppy–PEI NCs were flushed with phosphate-buffered saline (PBS) for subsequent confocal laser scanning microscopy (CLSM) examination. A dual image of fluorescent fluorescein-Ppy–PEI NCs and Cy5-gelatin was then spatially imaged by CLSM.
Cellular interactions with the test formulation
Lung cancer cells H460, human lung large cell carcinoma (from ATCC® HTB-177™; American Type Culture Collection, Manassas, VA, USA) were kept in cellgrowth culture medium (5% CO2, 37°C, 10% fetal bovine serum). Test cells were seeded into the confocal dishes, and then these dishes were kept in a cell incubator at 37°C and 5% CO2 overnight. Before study, the attached cells were kept in Hank’s balanced salt solution (HBSS) for 1 h. Afterward, cells on the dish were incubated with different tested formulations for 1 h. The test sample was then flushed 3 times with PBS and stained by 4′,6-diamidino-2-phenylindole (DAPI), a solution of 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, ROS dye), or Amplex Red (hydrogen peroxide dye) to elucidate biocellular interactions with the developed Cy5-NC system for visualizing the NC and cell biological interactions.26 To create a hyperthermia environment, the test sample with cells were placed in a water bath incubator with an additional heat source, or the cells were treated with NC/NIR. Fluorescent results were visualized through CLSM. The fluorescence signal intensity was studied using ImageJ software.
Cell viability
Test H460 cells were cultured in 96-well plates with cell growth medium at 104 cells/well and 37°C with 5% CO2 overnight. The tested cancer cells were flushed twice with HBSS and then supplemented with 200 µL test Ppy–PEI NCs dissolved in HBSS. After 1 h, the above solution was withdrawn by flushing twice with PBS. Next, with or without remote NIR irradiation (10 min under 2 W/cm2), 200 µL freshly prepared medium together with 20 µL of MTT chemical reagent with a stock mixture (5 mg/mL dissolved in PBS) was used, followed by subsequent cell culture in 4 h at 37°C. Once withdrawing the medium, the formazan formed chemicals were extracted by dimethyl sulfoxide (DMSO) and incubated for 20 min. The recorded absorbance information (formazan) was attained using a microplate reader. For qualitatively imaging cellular viability following incubation with different formulated treatments lasting 1 h, as mentioned (MTT) above, with subsequent NIR irradiation (10 min), flushed cells were then stained by live/dead chemical reagent in a commercial kit, as described in the standard protocol. Briefly, the live/dead viability/cytotoxicity assay kit (Molecular Probes, Eugene, OR, USA) contained dead cell dye (ethidium homodimer) with live cell dye (acetoxymethyl ester of calcein [calcein AM]) stock solutions diluted to their formulated mixture (4 and 2 µM, respectively) in PBS. Next, the cells were placed in this aqueous reagent at room temperature for 30-min reaction. Fluorescent data were measured with microscopy.
Statistical analysis
The experimental data are expressed as average±SD. The Student t-test was conducted to calculate differences between pairs of groups. P<0.05 was deemed to show statistical significance.
Results and discussion
Characteristics of Ppy–PEI NCs
Recently, researchers have observed the extraordinary development of nanotechnologies.27 There has been growing hope that nanoscience will achieve practical noteworthy progress in medication and clinical use in analyses, therapeutic strategies, and avoidance of diseases. Increasing attention in forthcoming biomedical requests for nanoscale treatment is guiding the appearance of the novel arena called nanomedicine.28,29
The versatility of conductive polymers, for instance Ppy, has been widely studied for their exclusive chemical and physical features, and they have various functions in numerous fields such as biology and chemistry sensors, organic electronic devices, and electromagnetic sheltering applications. Conducting polymeric Ppy was also described as having poor dispersion in aqueous phases which restricts its applicability.30
One of the main difficulties in generating dispersed nano-Ppy is the poor homogeneity of Ppy molecules in aqueous systems. Dispersion is worse for coated polymers without polar groups, as the polarity of the coating polymer has an impact on the dispersion of Ppy. In order to overcome this dispersion problem, the Ppy interface is usually coated with a dispersion polymeric agent in an nano-formulation process, such as (eg, heparin,31 polyvinyl acetate,32 and chi-tosan33) previously exposed to polymerized pyrrole under mechanical stirring. These dispersed polymeric molecules are appropriately functionalized organic molecules which allow stabilization of Ppy polymeric molecules in aqueous solutions.
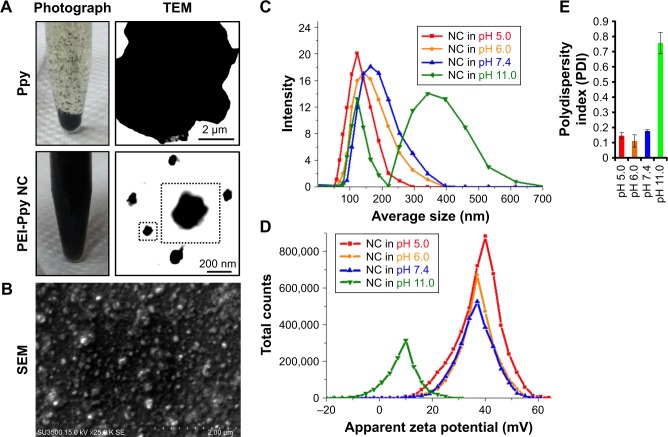
As shown by TEM and photographic data in Figure 1A, Ppy lacks stability in the aqueous phase owing to its hydro-phobicity, so that precipitation and aggregation behaviors occurred after the Ppy material was introduced into water. Once Ppy was stabilized with the polymeric PEI, nanoscale Ppy–PEI NCs were obtained as shown in Figure 1A as a uniformly dark suspended fluid. SEM results suggested that the nano-dimension of Ppy–PEI NCs with a well-dispersed arrangement was possibly due to the smaller size together with stronger repulsive cationic electronic fields of each nanoformulated particle (Figure 1A and B). Details of the size properties were measured by DLS. The DLS results indicated that particle size tends to increase with increasing pH value, while the zeta potential was reduced (Figure 1C–E). The PEI-containing nanoparticle should be deprotonated at high pH,34 leading to the lack of interaction from each particle so that PEI would lack a strongly charged surface to generate repulsive force, resulting in particle aggregation.
Figure 1.
(A) Results indicating photographic images of polypyrrole (Ppy) and Ppy–PEI NCs and their microscopic TEM. (B) Results of SEM showing prepared Ppy–PEI NCs with nano-size. DLS study shows the size distribution (C), zeta potential (D), and polydispersity index (E) of the different pH environments.
Abbreviations: NC, nanocomplex; PEI, polyethylenimine; SEM, scanning electron microscopy; TEM, transmission electron microscopy; DLS, dynamic light scattering.
Photothermal properties of Ppy–PEI NCs
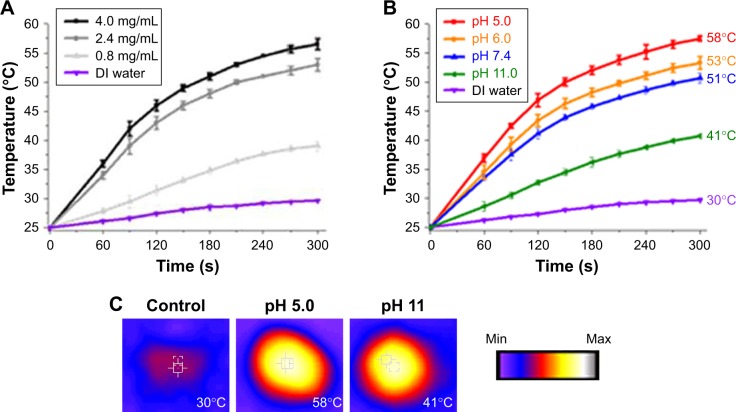
The results for the photothermal properties of prepared Ppy–PEI NCs at different concentrations and pH environments after NIR treatment studied by Thermocouple and thermal camera are shown in Figure 2. The temperature was slightly increased after NIR irradiation in the DI water group. After applying NIR, the recorded temperature of Ppy–PEI NC solutions was increased in a concentration-dependent manner (Figure 2A). Owing to the presence of an aromatic ring of Ppy, the prepared Ppy–PEI NCs have the advantage of noninvasively remotely generating local hyperthermia once exposed to NIR.25 The temperature profile of Ppy–PEI NC solutions irradiated with NIR would be reduced on increasing the pH value (Figure 2B and C). It was described that the smaller size of photoablation agent particles would relate to higher conductivity as mentioned previously.35,36 Larger nanoparticles or aggregated particle resultant inhomogeneous phase might associate with lower photothermal conversion efficiency.37 Hyperthermic temperature (HT) is a cancer treatment used together with surgical processes, gene immunotherapy, radiotherapy, and chemotherapy.38 In oncology, clinical doctor uses an exterior heat source to locally raise the temperature of tissues and eradicate cancerous cells or obstruct their additional growth. A locally high temperature, as many studies discovered, sensitizes cells to handling modalities and triggers direct damage to cancer cells, thus increasing the irradiation and chemotherapeutic effects with minor or no damage to healthy tissues and organs. The healing abilities, therapeutic outlay, technical issues, and confirmation of efficiency diverge according to the HT method. Management of cancer with hyperthermia has been used previously, but use of this skill was discontinued due to its limitations, including failure to elevate the local temperature toward the object without harming nearby cells, difficulty reaching a uniform heat distribution in the cancer, and intrinsic issues with imperceptible micrometastases.
Figure 2.
Photothermal property results. Temperature profile (A) at different concentrations of prepared NC and (B) at different pH environments. (C) Photothermal images (temperature value recorded by Thermocouple). The temperature elevated to 25 degree celsius is considered time 0 second.
Abbreviations: DI, deionized; NC, nanocomplex.
Surface properties of Ppy–PEI NCs
Around solid cancerous biology, neutrophils usually act as a dominant cell species in the tumorous tissue region surrounding infiltration.39 The neutrophils with tumor surrounding tissue are the cells with cationic charged peptides/substances covering the surface.40 Besides, the anionic charges were found to be generated from the huge amount of lactate secretions, a recognized feature of an entirely metabolically active cancerous cell line.40 Thus, this different surface charged feature indicated that targeting negative surface charges of cancer cells by cationic nanoparticles with photothermal properties would provide selective targeting toward negatively charged lung cancer with cytotoxicity. To test this hypothesis of binding affinity, the cationic gelatin represents inflammation cationic tissue and anionic gelatin represents cancer cell surface.41 In pathological conditions, cancer cells also have rather high negatively charged cellular membranes compared to surrounding cells.40,42,43
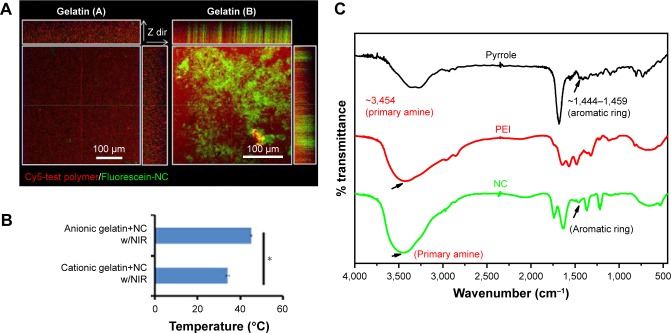
For elucidation of the binding affinity toward different electronically charged surfaces, the prepared fluorescent Ppy–PEI NC was incubated with a gelatin (A) or gelatin (B) hydrogel, and then the unbound Ppy–PEI NC was flushed with PBS for subsequent CLSM examination. As shown in Figure 3A, the cationic Ppy–PEI NCs that accumulated on the negatively charged gelatin (B) hydrogel were greater than those on the gelatin (A) cationic hydrogel. The repulsive forces generated between the polyelectrolytes of the same charge that prevent interaction; in contrast, different electronic charges will generate an attractive force for molecularly enhanced binding affinity. Additionally, the generated internal heat in the tested hydrogels (anionic hydrogel) with residual cationic PEI-Ppy NP by irradiated NIR around 45°C. The temperature of cationic gel treated group was around 34°C (Figure 3B). Theoretically, these effects from temperature and binding affinity also should be an advantage for local administration of PEI-Ppy NPs with photothermal treatment (PTT) against lung cancer and could cause less damage to surrounding cationic inflammatory tissue.
Figure 3.
(A) Fluorescence results show binding affinity of prepared NC in differently charged hydrogels and (B) their thermal properties. (C) Chemical structural change studied by Fourier transform infrared spectroscopy.
Note: *Statistically significant, P < 0.05.
Abbreviations: Cy, cyanine; NC, nanocomplex; NIR, near-infrared; PEI, polyethylenimine.
Chemical structure
To further analyse the molecule construction, FTIR was used to study the chemical structure of the synthesized compounds. The pyrrole–PEI NC product was indicated by characteristic peaks contributed by its respective chemicals, such as PEI and Ppy for comparison. The IR spectrum around 1,444~1,459 cm−1 44 possibly originated from stretching vibrations of aromatic ring. According to the chemical characteristic peak of PEI, around 3,454 (probable primary amine) with corresponding pyrrole–PEI NC compound (Figure 3C).45
Recent developments in nanoparticle-assisted hyperthermia treatment support the capability to overcome most of targeted tissues, but some concern regarding the use of nanomaterials remains. It is known that surrounding healthy tissues and organs might display improved thermotolerance toward cancer cells, also the mediating mechanisms are mostly unclear. According to the location of the cancer (eg, deep-seated or superficial), various modalities of treatment should be implemented. Cellular apoptosis is associated with a trigger HT to cause death of cells with producing intracellular oxidative stress. Several studies confirmed that HT should be an applicable cancer treatment. As for terminology, hyperthermia (also called thermotherapy or thermal therapy) is a sort of cancer treatment where body tissues are exposed to high temperatures. An investigation revealed that high temperatures can destroy and eradicate cancer cells, typically with negligible damage to healthy tissues and organs. In PTT, NIR light (at around 650–900 nm) is chosen for its convenient use, its slight absorbance by tissues and skin to permit noninvasive penetration of deeper tissues and organs, and its aptitude to be locally intensive in a particular region.46 The important constituent of this method is a transducer with photothermal properties that can efficiently absorb and convert remote NIR light into heat via a mechanism of nonradiation.
As a useful substitute or enhancement to traditional cancer treatment methods, PTT has garnered substantial attention due to its benefits, including slight invasiveness, few difficulties, and quick recovery. PTT, also called optical hyperthermia or photothermal ablation, requires photo absorbers and a source of NIR light energy, and offers an exact and negligibly invasive substitute for cancer management.46 It is a technique based on local heating due to absorption of light to selectively destroy cancer cells. The photostability of Ppy-based nanoparticles would be contributed from the aromatic structure with dark color in aqueous.47
Over the past decade, numerous distinct categories of photothermal therapeutic agents (PTAs) have been discussed, comprising organic materials and chemicals (eg, indo-cyanine green, polyaniline, and inorganic nanomaterials).48 Once exposed to NIR light, all of these materials are able to create adequate heat to increase the local temperature to achieve hyperthermia and consequently kill cancer cells. It was noted that bioorganic PTT is multifunctional and has good biocompatibility, and thus should be able to be applied for nanobiotechnology.
Cancer cell interactions
A positively charged nanomaterial can interact with negatively charged (anionic) cancer cellular membranes to trigger depolarization of local cell membranes and also trigger subsequent intracellular endocytosis.40,49 Maintaining and preserving nanostructures on external cellular membranes, even with subsequent cellular interaction, and chemical and physical binding approaches to reactive groups characteristically present on their outer cellular interfaces were needed to understand. Cationic nanomaterials are rapidly attracted to cancer cells because of the positive attractive forces.40 It is thought that positively charged nanocarriers may develop optimal bioapplications for cancer treatments in sustainable regional and mucosal therapies. In addition, these positively charged nanocarriers would offer protective effects against enzyme degradation and were revealed to influence the release of medicines or active therapeutic substances in a sustained, controllable manner.50
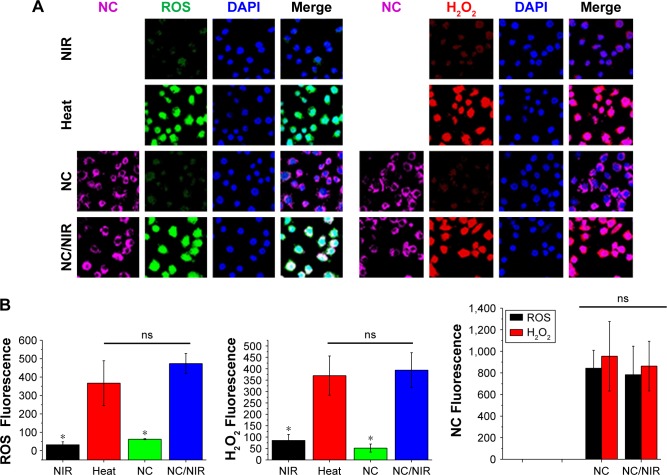
Fluorescence image showed that Ppy–PEI NCs were endocytosed by H460 cells after 1 h of incubation. The accumulation of fluorescent Ppy–PEI NCs was appeared within cellular organelles near nuclei of H460 cells (Figure 4A). As is known, PEI-complexes cell uptake mechanism might be through clathrin-dependent pathways.51 Furthermore, this developed endocytosed pathway through clathrin mediation can be a potential target for a therapeutic approach for overcoming tumor resistance.52
Figure 4.
(A) Fluorescent images show cell interaction with different formulations. (B) Fluorescence intensity was calculated by ImageJ software, and the statistical data analysis compared with NC/NIR (cy5-). NC Fluorescence group, left: NC, right: NC/NIR).
Notes: Black bar is ROS studied group; red bar is H2O2 studied group. *Statistically significant, P < 0.05.
Abbreviations: H2O2, hydrogen peroxide; NC, nanocomplex; NIR, near-infrared; ns, nonsignificant; ROS, reactive oxygen species; DAPI, 4′,6-diamidino-2-phenylindole.
Previous results suggested that positively charged nanoparticles (NPs) might be internalized via clathrin coated vesicles.53 Additionally, the size of nanomaterials may also affect the uptake kinetics and efficiency, the sub-cellular biodistribution, and the internalization mechanism. The dimension-dependent uptake of various biomaterials in diverse cellular lines has been studied with suitable cell internalization at a nanomaterial core dimension in a range of around 60–400 nm, which indicates that there is an ideal size distribution for active cellular uptake.54
ROS detection
As known, hyperthermia stimulates intracellular ROS, and mitochondria functional disorders in numerous cancerous cell lines were found.22 Mitochondria dysfunction and ROS act important roles in the cell apoptotic process. As indicated in Figure 4A and B, test cells treated with NIR alone or the group received with only Ppy–PEI NCs generated few ROS and few hydrogen peroxide was observed. However, test cells treated with an additional heat source or the group of Ppy–PEI NCs with NIR treatment generated highly ROS including hydrogen peroxide fluorescent products, as analyzed by CLSM and ImageJ software (Figure 4A and B). The greater amounts of ROS generated may be attributed to local hyperthermia triggered by the intracellular uptake of NIR-photothermal transformable fluorescent Ppy–PEI NCs once NIR treatment. Interestingly, the prepared formulation with NIR transferring to local hyperthermia-caused biological response was suggested to increase ROS, which would be consistent with previous publications.55,56
Cell viability
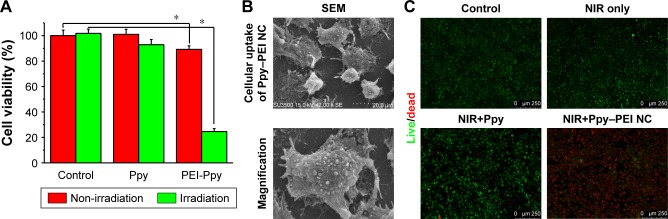
Hyperthermic treatment noticeably improved the initiation effects of a locally elevated temperature on cellular apoptosis and involvement of intracellular ROS creation. The anticancer effects of this novel treatment might be closely related to the local temperature. Results suggest that hyperthermia therapy can be applied as a potential approach for cancer treatment.57 As shown in Figure 5A, test cells treated with only NIR exhibited lower cytotoxicity as untreated control group (without NIR). After incubating with Ppy–PEI NCs, test cells exhibited relatively low cytotoxicity (above 80% metabolic activity of live cells). In the Ppy group, cells may show a poor adhesion on to hydrophobic and aggregated materials (Ppy), resulting that the low cytotoxic effect after washing with or without NIR treatment. However, the group that received Ppy–PEI NCs applied with NIR treatment displayed a cytotoxic effect compared to the control group. SEM examination was then used to elucidate the detailed morphological changes. Ppy–PEI NCs possibly were seen on the surface of H460 cell membranes, suggesting that receptors-mediated endocytosis would be occurred for subsequent intracellular trafficking (Figure 5B), and NIR provided a local hyperthermic effect. Even heterogenous shape of Ectosomes, the size of Ectosomes (100–1000 nm) would be possibly overlaped with the prepared nanocarrier (100–200 nm) so that the precisely identification way is needed in the future work.
Figure 5.
(A) Quantitative results of MTT cell viability with different treatments, comparison of control and PEI-Ppy. (B) The group of Ppy–PEI NCs attached onto cell membranes morphologically imaged by SEM. (C) The cell viability was qualitatively tested with a Live/Dead method.
Note: *Statistically significant, P < 0.05.
Abbreviations: NC, nanocomplex; NIR, near-infrared; PEI, polyethylenimine; Ppy, polypyrrole; SEM, scanning electron microscopy.
The further elucidation of live/dead cells using ethidium homodimer-1 and calcein AM showed a noteworthy difference in the indicator between dead and living cells. These fluorescent tracer dyes function as a powerful means to evaluate the cell death mechanism in high-content screening tests, as they can record numerous biological actions during the experiment. A high viability was detected with similar tendency observed in the untreated, NIR, and Ppy (with wash) groups. However, the group that received Ppy–PEI NCs and then noninvasive NIR treatment exhibited significant cytotoxicity compared to the other groups (Figure 5C). Thus, our study reveals a role for hyperthermia in vitro provided by the accumulated Ppy–PEI NCs with NIR treatment, which induced lung cancer cell death.
Conclusions
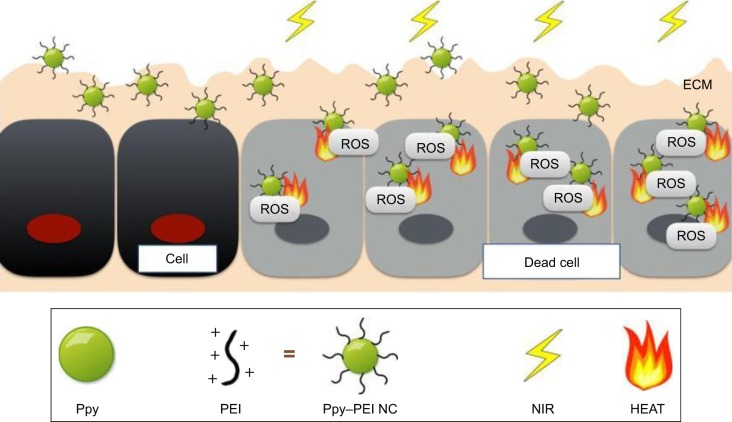
Ppy has been recognized as a versatile conductive biomaterial, which is widely utilized in biomedical applications and appears to have actual promise for progressive biotechnological bioapplications and is a widely used electroresponsive or photothermal material that might have the capability used to treat cancers. In this study, a Ppy–PEI NC was effectively made, and its fundamental features were explored using FTIR, TEM, SEM, and DLS methods. We also examined the lung cancer cell interaction with test Ppy–PEI NCs via fluorescent approaches. The photothermal features and cellular ROS with cytotoxicity were studied. These designed Ppy–PEI NCs were capable of interacting with cultured H460 lung cancer cells. The photothermal behavior, morphological cellular interactions, and cell ROS generation after treating NC/NIR were illustrated in Figure 6. In future studies, this photothermal nanotechnology might be applied intratumor for treating lung cancer. The in vivo biodistribution, toxicity, and encapsulation of cancer medicine as combination therapy will be further investigated in the future.
Figure 6.
Schematic illustration showing the developed Ppy–PEI NCs bioapplied for lung cancer treatment.
Abbreviations: ECM, extracellular matrix; NC, nanocomplex; NIR, near-infrared; PEI, polyethylenimine; Ppy, polypyrrole; ROS, reactive oxygen species.
Acknowledgments
This paper was supported by the government project from Ministry of Science and Technology (MOST) of Taiwan (106-2314-B-038-022-MY2).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 2.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 3.Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH, Distribution T. Tissue distribution, metabolism and excretion of paclitaxel in mice. Anticancer Drugs. 1996;7(1):78–86. doi: 10.1097/00001813-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Polgár C, Major T. Current status and perspectives of brachytherapy for breast cancer. Int J Clin Oncol. 2009;14(1):7–24. doi: 10.1007/s10147-008-0867-y. [DOI] [PubMed] [Google Scholar]
- 5.Odell DD, Kent MS, Fernando HC. Sublobar resection with brachytherapy mesh for stage I non-small cell lung cancer. Semin Thorac Cardiovasc Surg. 2010;22(1):32–7. doi: 10.1053/j.semtcvs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Wolinsky JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release. 2012;159(1):14–26. doi: 10.1016/j.jconrel.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomedicine. 2015;10:1001. doi: 10.2147/IJN.S56932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veiseh O, Kievit FM, Gunn JW, Ratner BD, Zhang M. A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells. Biomaterials. 2009;30(4):649–657. doi: 10.1016/j.biomaterials.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park C, Lee C, Kwon O. Conducting Polymer Based Nanobiosensors. Polymers. 2016;8(7):249. doi: 10.3390/polym8070249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Serna F, Nickels J, Schmidt CE. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules. 2006;7(6):1692–1695. doi: 10.1021/bm060220q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JW, Serna F, Schmidt CE. Carboxy-endcapped conductive polypyr-role: biomimetic conducting polymer for cell scaffolds and electrodes. Langmuir. 2006;22(24):9816–9819. doi: 10.1021/la062129d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Xu H, Liang C, et al. Iron oxide @ polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano. 2013;7(8):6782–6795. doi: 10.1021/nn4017179. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, He N, He Q, et al. A novel self-assembled sandwich nano-medicine for NIR-responsive release of NO. Nanoscale. 2015;7(47):20055–20062. doi: 10.1039/c5nr06630a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R, Sun X, Sun J, Wang L, Han J. Polypyrrole-modified CuS nanoprisms for efficient near-infrared photothermal therapy. RSC Adv. 2017;7(17):10143–10149. [Google Scholar]
- 15.Manivasagan P, Quang Bui N, Bharathiraja S, et al. Multifunctional biocompatible chitosan-polypyrrole nanocomposites as novel agents for photoacoustic imaging-guided photothermal ablation of cancer. Sci Rep. 2017;7:43593. doi: 10.1038/srep43593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Cai W. Nanomedicine for targeted photothermal cancer therapy: where are we now? Nanomedicine. 2015;10(1):1–3. doi: 10.2217/nnm.14.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dallas P, Niarchos D, Vrbanic D, et al. Interfacial polymerization of pyrrole and in situ synthesis of polypyrrole/silver nanocomposites. Polymer. 2007;48(7):2007–2013. [Google Scholar]
- 18.Song CW, Lokshina A, Rhee JG, Patten M, Levitt SH. Implication of blood flow in hyperthermic treatment of tumors. IEEE Trans Biomed Eng. 1984;31(1):9–16. doi: 10.1109/TBME.1984.325364. [DOI] [PubMed] [Google Scholar]
- 19.Harris K, Puchalski J, Sterman D. Recent advances in bronchoscopic treatment of peripheral lung cancers. Chest. 2017;151(3):674–685. doi: 10.1016/j.chest.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Huo S, Ma H, Huang K, et al. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013;73(1):319–330. doi: 10.1158/0008-5472.CAN-12-2071. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Zeng ZW, Xiao RZ, et al. Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine. 2011;6:765. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou CH, Lin FL, Hou SM, Liu JF. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int J Mol Sci. 2014;15(10):17380–17395. doi: 10.3390/ijms151017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89. [PMC free article] [PubMed] [Google Scholar]
- 25.Mi FL, Burnouf T, Lu SY, Fl M, Sy L, et al. Self-targeting, immune transparent plasma protein coated nanocomplex for noninvasive photo-thermal anticancer therapy. Adv Healthc Mater. 2017;6(14):1700181. doi: 10.1002/adhm.201700181. [DOI] [PubMed] [Google Scholar]
- 26.Lu KY, Lin PY, Chuang EY, et al. H2O2-Depleting and O2-Generating Selenium Nanoparticles for Fluorescence Imaging and Photodynamic Treatment of Proinflammatory-Activated Macrophages. ACS Appl Mater Interfaces. 2017;9(6):5158–5172. doi: 10.1021/acsami.6b15515. [DOI] [PubMed] [Google Scholar]
- 27.Dowling AP. Nanotechnologies Dof. Materials Today. 2004;7(12):30–35. [Google Scholar]
- 28.Li J, Yoon SJ, Hsieh BY, Tai W, O’Donnell M, Gao X. Stably doped conducting polymer nanoshells by surface initiated polymerization. Nano Lett. 2015;15(12):8217–8222. doi: 10.1021/acs.nanolett.5b03728. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Arnal B, Wei CW, et al. Magneto-optical nanoparticles for cyclic magnetomotive photoacoustic imaging. ACS Nano. 2015;9(2):1964–1976. doi: 10.1021/nn5069258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. Applications of conducting polymers and their issues in biomedical engineering. J R Soc Interface. 2010;7(Suppl 5):S559–S579. doi: 10.1098/rsif.2010.0120.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong GM, Yap YZ, Choong C. Single-step synthesis of heparin-doped polypyrrole nanoparticles for delivery of angiogenic factor. Nanomedicine. 2016;11(7):749–765. doi: 10.2217/nnm.16.13. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira HP, dos Santos MVB, dos Santos CG, de Melo CP. Preparation and electrical and dielectric characterization of PVA/PPY blends. Mater Charact. 2003;50(2–3):223–226. [Google Scholar]
- 33.Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J Biomed Mater Res A. 2010;93(1):164–174. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 34.Rac O, Suchorska-Woźniak P, Fiedot M, Teterycz H. Influence of stabilising agents and pH on the size of SnO2 nanoparticles. Beilstein J Nanotechnol. 2014;5:2192–2201. doi: 10.3762/bjnano.5.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghalib H, Abdullah I, Daik R. Electrical conductivity of anionic surfactant-doped polypyrrole nanoparticles prepared via emulsion polymerization. Sci Technol. 2013;21(2):459–471. [Google Scholar]
- 36.Li X-G LA, Huang M-R, Liao Y, Y-G L. Efficient and scalable synthesis of pure polypyrrole nanoparticles applicable for advanced nanocomposites and carbon nanoparticles. The Journal of Physical Chemistry C. 2010;114(45):19244–19255. [Google Scholar]
- 37.Qin Z, Wang Y, Randrianalisoa J, et al. Quantitative Comparison of Photothermal Heat Generation between Gold Nanospheres and Nanorods. Sci Rep. 2016;6:29836. doi: 10.1038/srep29836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197–1201. [PubMed] [Google Scholar]
- 39.Pabinger I, Posch F. Flamethrowers: blood cells and cancer thrombosis risk. Hematology Am Soc Hematol Educ Program. 2014;2014(1):410–417. doi: 10.1182/asheducation-2014.1.410. [DOI] [PubMed] [Google Scholar]
- 40.Chen B, Le W, Wang Y, et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics. 2016;6(11):1887–1898. doi: 10.7150/thno.16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamko DJ, Wu Y, Ajamian F, Ilarraza R, Moqbel R, Gleich GJ. The effect of cationic charge on release of eosinophil mediators. J Allergy Clin Immunol. 2008;122(2):383–390. doi: 10.1016/j.jaci.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9(Suppl 1):51. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi D. Cancer cell surface negative charges: a bio-physical manifestation of the warburg effect. Nano Life. 2017;071771001(03n04):1771001. [Google Scholar]
- 44.Achour B, Wu Q. Advances in Energy and Environment Research: Proceedings of the International Conference on Advances in Energy and Environment Research. CRC Press; [Google Scholar]
- 45.Zaikov GE. Trends in molecular and high molecular science. Nova; Publishers: 2005. [Google Scholar]
- 46.Bao Z, Liu X, Liu Y, Liu H, Zhao K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J Pharm Sci. 2016;11(3):349–364. [Google Scholar]
- 47.Emanuel NNM, Buchachenko AL. Chemical Physics of Polymer Degradation and Stabilization. Vol. 1. Utrecht: VSP; 1987. [Google Scholar]
- 48.Tian Q, Tang M, Sun Y, et al. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv Mater. 2011;23(31):3542–3547. doi: 10.1002/adma.201101295. [DOI] [PubMed] [Google Scholar]
- 49.Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release. 2014;190:485–499. doi: 10.1016/j.jconrel.2014.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan ML, Choong PF, Dass CR, Cancer DCR. Cancer, chitosan nanoparticles and catalytic nucleic acids. J Pharm Pharmacol. 2009;61(1):3–12. doi: 10.1211/jpp/61.01.0002. [DOI] [PubMed] [Google Scholar]
- 51.van der Aa MA, Huth US, Häfele SY, et al. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm Res. 2007;24(8):1590–1598. doi: 10.1007/s11095-007-9287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6(12):1861–1871. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 53.Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiu L, Chen T, Öçsoy I, Öçsoy I, et al. A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2015;15(1):457–463. doi: 10.1021/nl503777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volkov RA, Panchuk II, Mullineaux PM, Schöffl F. Heat stress-induced H(2)O (2) is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol. 2006;61(4–5):733–746. doi: 10.1007/s11103-006-0045-4. [DOI] [PubMed] [Google Scholar]
- 56.Li FJ, Kondo T, Zhao QL, et al. Enhancement of hyperthermia-induced apoptosis by a free radical initiator, 2,2′-azobis (2-amidinopropane) dihydrochloride, in human histiocytic lymphoma U937 cells. Free Radic Res. 2001;35(3):281–299. doi: 10.1080/10715760100300821. [DOI] [PubMed] [Google Scholar]
- 57.Luk KH, Hulse RM, Phillips TL. Hyperthermia in cancer therapy. West J Med. 1980;132(3):179. [PMC free article] [PubMed] [Google Scholar]