Abstract
Background:
In renal cell carcinoma (RCC), angiopoietin (Ang) 2 is elevated at the time of progression on anti-vascular endothelial growth factor (VEGF) therapy and may contribute to resistance.
Objective:
We tested trebananib, an Ang 1 and 2 neutralizing peptibody in patients with RCC progressing on anti-VEGF treatment.
Methods:
Patients with measurable RCC progressing despite an anti-VEGF agent within 12 weeks, any number of prior treatments, and good PS were randomized to trebananib 15 mg/kg IV weekly without (Arm A) or with (Arm B) continuation of the prior anti-VEGF agent. The primary endpoint for each arm was tumor response (RECIST 1.1). Secondary endpoints included progression free survival and adverse events.
Results:
Of 41 enrolled patients, 35 were eligible and started treatment (17 Arm A, 18 Arm B) with median age 60 (46–76) and 3 prior treatments (1–8). Four died prior to documented progression and 27 progressed as their first event. Both arms were stopped after interim analysis, 2 responses (11%; 95% C.I. 1–35%) were observed in Arm B. Median PFS of 2.7 (95% C.I. 2.3–4.7) months in Arm A and 5.2 (95% C.I. 2.7–10.8) months in Arm B did not support continued study. Common adverse events including fatigue, nausea, and increased creatinine were generally grade 1–2 and numerically higher in Arm B. The most common grade 3 or higher adverse events were hypertension and dyspnea.
Conclusions:
While tolerable, trebananib either without or with continued anti-VEGF therapy did not show promising activity in RCC patients who recently progressed on anti-VEGF therapy alone.
Keywords: Renal cell carcinoma, trebananib, angiopoietin, vascular endothelial growth factor
INTRODUCTION
Angiogenesis through the vascular endothelial growth factor (VEGF) pathway is a hallmark of clear cell renal cell carcinoma (RCC). There are multiple agents targeting the VEGF receptor that improve outcomes in patients with metastatic RCC, including the tyrosine kinase inhibitors axitinib, lenvatinib, pazopanib, sorafenib, and sunitinib as well as the monoclonal antibody bevacizumab. Nonetheless, the development of clinical resistance to these agents is universal. Resistance to anti-VEGF therapy may develop from selection for or up-regulation of alternative pro-angiogenic pathways, recruitment of bone marrow derived vascular progenitors and pro-angiogenic monocytes, increased involvement of pericytes in blood vessel stability, and an increased capacity for vascular invasion [1–3].
The angiopoietin-Tie signaling system is involved in normal vascular development and maintenance. Angiopoietin 1 (Ang1) and Angiopoietin 2 (Ang2) are ligands of Tie2, a receptor tyrosine kinase expressed on endothelial cells [4]. In RCC, the angiopoietin-Tie signaling system has been implicated as a potential mechanism of resistance to anti-VEGF agents. The expression of Ang2 is higher in RCC as compared to normal kidney and in RCC compared to other tumor types [5, 6]. In patients with advanced RCC, Ang2 is elevated at the time of progression on anti-VEGF therapy [5]. Thus, angiogenesis through the angiopoietin-Tie signaling system may contribute to acquired resistance in patients treated with VEGF pathway targeted agents. Moreover, Ang2 inhibition combined with VEGF receptor inhibition slows tumor progression in mouse models of RCC, [7–9] suggesting a possible benefit for the combination of VEGF and angiopoietin pathway inhibition in RCC.
Trebananib (AMG 386) is an investigational peptide-Fc fusion protein that sequesters both Ang1 and Ang2 and prevents their interaction with the Tie2 receptor. While no in vitro activity was seen in cell line studies with trebananib as a single agent, significant inhibition of tumor xenograft growth was observed in preclinical models [10]. As monotherapy, trebananib was well tolerated up to doses of 30 mg/kg weekly and evidence of an antiangiogenic effect was observed by dynamic contrast-enhanced magnetic resonance imaging [11]. Prior studies of trebananib in RCC confirmed the feasibility and safety of combining trebananib with sorafenib and sunitinib at clinically relevant doses, [12, 13] and demonstrated promising activity for the combination of trebananib and sunitinib in the first line treatment of patients with metastatic RCC [14]. As observed in ovarian cancer, [15] there is evidence that a dose of trebananib above 10 mg/kg may be more effective than lower doses when used in combination therapy in metastatic RCC [14]. In this study, we tested trebananib at a 15 mg/kg dose in patients with RCC that had progressed on anti-VEGF agents to test the hypothesis that potent inhibition of angiopoitin-Tie2 angiogenesis would be active in this setting. Additionally, we explored whether continued anti-VEGF inhibition with trebananib might result in a more effective regimen for future clinical development.
PATIENTS and Methods
This phase II study was sponsored by the National Cancer Institute and conducted by the California Cancer Consortium. Trebananib was provided through a Cooperative Research and Development Agreement between NCI Cancer Therapy Evaluation Program (CTEP) and Amgen. The institutional review board at each participating site approved the study protocol and written informed consent was obtained from each enrolled patient. The study was registered at www.clinicaltrials.gov with the identifier NCT01664182.
Patients
Eligible adult (age≥18) patients with ECOG performance status 0–1 had histologically or cytologically confirmed renal cell carcinoma except medullary or collecting duct subtypes, RECIST 1.1 measurable disease, and documented radiologic or clinical progressive disease following at least one prior anti-VEGF regimen administered either as a single agent or in combination with other agents for at least 8 weeks. A prior anti-VEGF treatment regimen must have included bevacizumab, pazopanib, sorafenib or sunitinib administered not more than 12 weeks before study entry (intercurrent therapy with an mTOR inhibitor was allowed if progression on that treatment was observed within 12 weeks of the prior anti-VEGF therapy). There was no limit to number of prior therapies. Acceptable hematologic function was required as was a total bilirubin < institutional upper limits of normal, transaminases≤2.5 X institutional upper limit of normal, PTT or apt≤upper limits of normal and INR≤1.5, creatinine within normal institutional limits or creatinine clearance >40 mL/min per 24 h urine collection or calculated according to the Cockcroft-Gault formula, and urinary protein≤100 mg/dL in urinalysis or≤1+ on dipstick, unless quantitative protein is <1000 mg in a 24 h urine sample. Generally well-controlled blood pressure with systolic blood pressure≤140 mmHg and diastolic blood pressure≤90 mmHg was required prior to enrollment. The use of anti-hypertensive medications to control hypertension was permitted. For pre-treatment research biopsies, patients must have had a tumor site amenable to biopsy as determined by the treating investigator and willingness to consent to tumor biopsy for research purposes.
Patients were excluded if they were intolerant of prior treatment with bevacizumab, pazopanib, sorafenib, or sunitinib, had central nervous system metastases unless: (1) metastases had been treated and have remained controlled for at least two weeks following treatment, and (2) patient had no residual neurological dysfunction off corticosteroids for at least one week, had a history of venous or arterial thromboembolism within 12 months prior to enrollment/randomization, or history of clinically significant bleeding within 6 months of enrollment/randomization. Additional exclusion criteria included clinically significant cardiovascular disease within 12 months prior to enrollment/randomization, major surgery within 28 days prior to enrollment or still recovering from prior surgery, minor surgical procedures except placement of tunneled central venous access device within 3 days prior to enrollment, non-healing wound, ulcer (including gastrointestinal), or fracture. Patients receiving any medications or substances that are strong inhibitors or inducers of CYP450 3A4 were ineligible due to the potential for interaction with pazopoanib, sorafenib, or sunitinib.
Study procedures and treatment plan
Fresh tissue from biopsy was required of all patients. Patients were then randomized to treatment with trebananib monotherapy (Arm A) or trebananib plus bevacizumab, pazopanib, sorafenib, or sunitinib (Arm B). In both arms, trebananib was administered at a dose of 15 mg/kg intravenously once per week on an outpatient basis (Table 1). Patients on Arm B continued their prior anti-VEGF agent in combination with trebananib. The bevacizumab dose was 10 mg/kg intravenously every 2 weeks. The standard doses of acceptable prior oral anti-VEGF kinase inhibitors were pazopanib 800 mg orally daily, sorafenib 400 mg orally twice per day, or sunitinib 50 mg daily for day 1–28 of each 42-day cycle. Up to two dose level dose reductions due to toxicity according to the prescribing information of these anti-VEGF kinase inhibitors were allowed prior to study entry. For the oral agents, patients were requested to maintain a medication diary during the study of each dose of medication. Cycle length in each arm was defined as 6 weeks.
Table 1.
Summary of Treatment Regimensa
| Agent | Dose | Route | Schedule |
| Trebananib Monotherapy (Arm A) | |||
| Trebananib | 15 mg/kg | IV | Day 1, 8, 15, 22, 29, and 36 |
| Trebananib + continued anti-VEGF therapy (Arm B) | |||
| Trebananib | 15 mg/kg | IV | Day 1, 8, 15, 22, 29, and 36 |
| plus ONE of the following anti-VEGF agents: | |||
| Bevacizumab | 10 mg/kg | IV | Day 1, 15, and 29 |
| Pazopanib | 800 mg once dailyb | Oral | Days 1–42 |
| Sorafenib | 400 mg twice dailyb | Oral | Days 1–42 |
| Sunitinib | 50 mg once dailyb | Oral | Days 1–28 |
aCycle length is 42 days on both Arms.
bThe standard starting dose is given. Patients may have started at a lower dose based on toxicity during prior administration.
Physical examination and laboratory tests were repeated within 72 hours of treatment on week 1, week 2, week 4 and then every 3 weeks (twice per cycle). Tumor measurements were repeated every 12 weeks (+/–7 days). At study entry, patients were required to have measureable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1) [16]. All patients underwent radiographic evaluation and tumor measurements at baseline and then after every 2 cycles (every 12 weeks). Confirmatory scans were required at≥4 weeks following initial documentation of an objective response. Overall response was graded according to RECIST v1.1. Overall complete response (CR) and partial response (PR) were considered objective responses. Toxicity was graded using the Common Terminology Criteria for Adverse Events (CTCAE) criteria, version 4.0. Treatment could continue until disease progression, intercurrent illness that prevented further administration of treatment, treatment delay of greater than 4 weeks for any reason, unacceptable adverse event(s), patient’s decision to withdraw from the study, or general or specific changes in the patient’s condition that rendered the patient unacceptable for further treatment in the judgment of the investigator.
Correlative studies
Research tumor biopsies were performed on all registered patients prior to treatment. Analysis of these specimens will be the subject of a separate report. Blood for correlatives was collected at baseline, prior to cycle 2, and prior to cycle 3. For pharmacodynamic correlative studies based on analysis of plasma, biomarker levels were multiplexed using bead suspension arrays and analyzed in duplicate using the Luminex xMAP® system (Luminex Corporation, Austin, Tx). Markers were grouped into three categories: markers of the angiopoietin-Tie2 pathway (Ang2, soluble Tie2), markers of the VEGF pathway (VEGF-A, placental growth factor [PlGF], VEGFR-3 and VEGF-C) and markers of alternative pro-angiogenic pathways (interleukin-8 [IL-8], intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1], fibroblast growth factor-2 [FGF2], platelet derived growth factor [PDGF-AA]).
Statistical analysis
This randomized phase II study was designed to assess efficacy of each arm. The primary endpoint in each treatment arm was overall tumor response rate (ORR) defined as the total number of efficacy-evaluable patients who achieve a complete or partial response by RECIST 1.1 criteria. For the purposes of this study, any eligible patient who began therapy (and received any amount of the first dose of trebananib) was considered efficacy-evaluable. The secondary objectives were to evaluate progression free survival in each arm, and to evaluate the tolerance and toxicity of trebananib alone and in combination with continuation of the prior VEGF targeted agent.
For assessment of clinical outcome, we considered a 15% response rate as interesting enough to encourage future study. Both arms were evaluated separately using a Simon Optimum design with a maximum of 39 patients, an interim evaluation after 17 patients, and with alpha = 0.10 (when the response rate is≤3%) and beta = 0.10 (when the response rate is 15%). With this design, if 0/17 or <2/39 patients experienced an objective response within each arm, this was taken as evidence that the response rate in that arm is less than 15%. Conversely, if 3 or more patients out of 39 experienced an objective response, that would be taken as evidence that the true response rate is greater than 3%. Secondary endpoints included progression free survival in each arm and the tolerance and toxicity of trebananib alone and in combination with continuation of the prior VEGF targeted regimen. Kaplan-Meier curves were constructed to summarize PFS in each arm.
For pharmacodynamic correlative studies of the plasma-based biomarkers, median, 25th percentile (lower quartile: Q1) and 75th percentile (upper quartile: Q3) were calculated for each biomarker in each arm at baseline, prior to cycle 2, and prior to cycle 3. The Wilcoxon Rank Sum test was used to compare the 2 arms in terms of the change in the biomarker levels prior to cycle 2 or prior to cycle 3: the ratio of the post-treatment levels divided by the baseline was tested. In these exploratory analyses, all p-values were two-sided and no attempt was made to control for multiple testing; the resulting p-values were regarded as additional descriptive statistics. Statistical analysis was performed using SAS version 9.4 software (SAS Institute, Inc., Cary, North Carolina).
Results
Patient characteristics
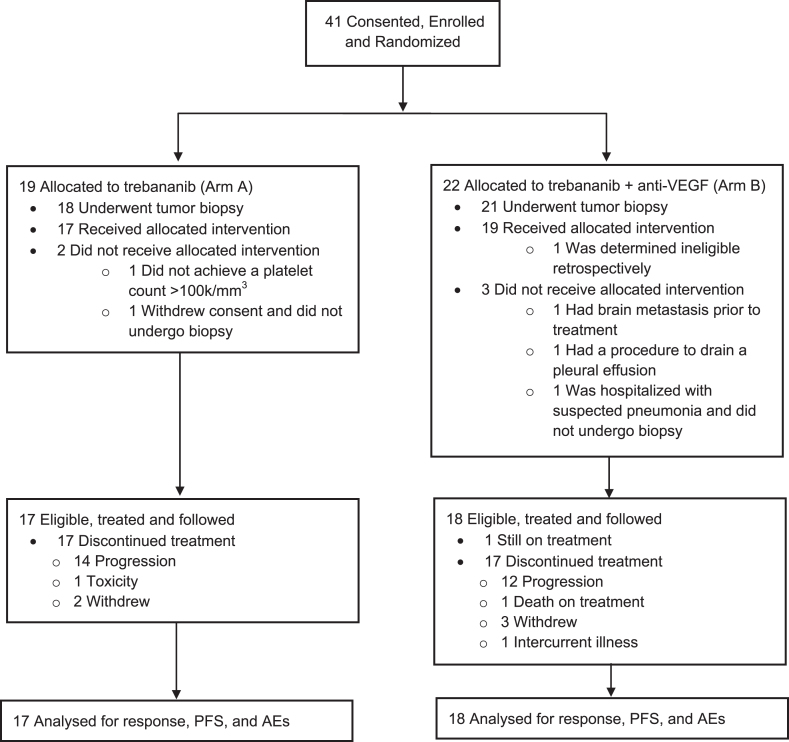
Forty-one patients were enrolled and randomized from March 2013 through November 2015, 35 were eligible and started treatment (17 Arm A, 18 Arm B). One patient was retrospectively determined to be ineligible after enrollment; 5 randomized patients (1 on Arm A and 4 on Arm B) underwent biopsy but did not start treatment and are not included in the analysis of response and toxicity (Fig. 1). The overall treated cohort was mostly male and white with excellent performance status and had a median age of 60 (Table 2). The most common prior anti-VEGF agent was bevacizumab and the median time from prior anti-VEGF therapy was 28 days.
Fig. 1.
Enrollment, Randomization and Follow Up. AEs indicates adverse events; PFS, progression-free survival.
Table 2.
Demographic, Clinical, and Treatment Characteristics for Patients Who were Eligible and Started Treatment
| Characteristic | Overall (n = 35) n (%) | Arm A (n = 17) n (%) | Arm B (n = 18) n (%) |
| Age, years | |||
| Median (range) | 60 (46–76) | 64 (49–76) | 59 (46–74) |
| ECOG Performance Status | |||
| 0 | 23 (66) | 12 (71) | 11 (61) |
| 1 | 12 (34) | 5 (29) | 7 (39) |
| Prior Anti-VEGF Agent | |||
| Bevacizumab | 15 (43) | 5 (29) | 10 (56) |
| Pazopanib | 11 (31) | 6 (35) | 5 (28) |
| Sorafenib | 4 (11) | 2 (12) | 2 (11) |
| Sunitinib | 5 (14) | 4 (24) | 1 (6) |
| Time from Last Anti-VEGF Treatment, days | |||
| Median (range) | 28 (10–76) | 26 (10–44) | 29 (13–76) |
| # greater than 30 days | 14 (40) | 6 (35) | 8 (44) |
| Number of Prior Regimens | |||
| Median (range) | 3 (1–8) | 3 (1–6) | 3 (1–8) |
| Gender | |||
| Female | 8 (23) | 4 (24) | 4 (22) |
| Male | 27 (77) | 13 (76) | 14 (78) |
| Race/Ethnicity | |||
| American Indian/AlaskaNative | 2 (6) | 2 (12) | 0 |
| Asian/Pacific Islander | 1 (3) | 0 | 1 (6) |
| Black | 3 (9) | 2 (12) | 1 (6) |
| Hispanic | 8 (23) | 4 (24) | 4 (22) |
| White | 21 (60) | 9 (53) | 12 (67) |
Antitumor activity
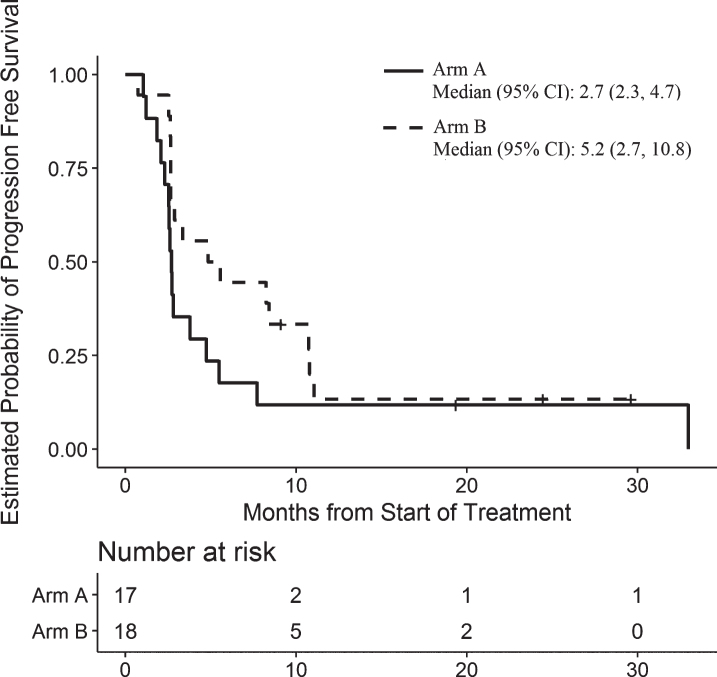
In the overall treated cohort, four patients died prior to documented progression and 27 progressed as their first event. In Arm A, 17 patients were enrolled. At the time of interim analysis, there were no responders and this arm was closed to further accrual. The best response of stable disease was observed in 5 patients (29%) and the median progression free survival was 2.7 (95% C.I. 2.3–4.7) months (Table 3 and Fig. 2). In the subgroup of 10 patients on Arm A who had received either prior sunitinib or prior pazopanib, there were no responses to trebananib monotherapy and the median PFS was 2.6 (95% C.I. 2.3–8.3) months.
Table 3.
Disease Response and Duration of Treatment
| Characteristic | Overall (n = 35) n (%) | Arm A (n = 17) n (%) | Arm B (n = 18) n (%) |
| Tumor Response | |||
| Evaluable | |||
| Partial Response | 2 (6) | 0 | 2 (11) |
| Stable Disease | 13 (37) | 5 (29) | 8 (44) |
| Progressive Disease | 17 (49) | 11 (65) | 6 (33) |
| Not Evaluable – Off too early | 3 (9) | 1 (6) | 2 (11) |
| Observed Response Rate | |||
| % (95% CI) | 6% (1–19%) | 0% (0–20%) | 11% (1–35%) |
| Cycles Received | |||
| Median | 2 | 2 | 3 |
| Rangea | 1–25 | 1–25 | 1–8 |
| Number≥4 Cycles | 14 (40) | 5 (29) | 9 (50) |
| Reason Off Treatment | |||
| Progression | 26 (74) | 14 (82) | 12 (67) |
| Early Death | 1 (3) | 0 | 1 (6) |
| Toxicity | 1 (3) | 1 (6) | 0 |
| Patient Decision | 5 (14) | 2 (12) | 3 (17) |
| Intercurrent Illness | 1 (3)b | 0 | 1 (6) |
| Still Ona | 1 (3) | 0 | 1 (6) |
| Progression-Free Survival (months) | |||
| Median (95% confidence interval) | 2.9 (2.6–8.4) | 2.7 (2.3–4.7) | 5.2 (2.7–10.8) |
a1 patient on Arm B (on for 21 cycles) is still on treatment and is not included in these ranges. bPatient’s renal function declined unrelated to treatment, required dialysis and became ineligible for further treatment.
Fig. 2.
Progression-Free Survival for Patients in Arm A and Arm B.
In Arm B, there was a single response amongst 17 enrolled patients at the time of interim analysis. Given this low level of observed activity, we examined the PFS. The estimated median PFS in Arm B of 3.4 (95% C.I. 2.7–8.3) months did not support continued expansion of this study arm and the decision was made to close it to further accrual. One of these 17 patients was found to be ineligible retrospectively and thus removed from final analysis. During the time confirming and analyzing the preliminary estimate of PFS, two additional patients were enrolled onto Arm B. The final best overall response in Arm B was partial response in 2 patients (both treated with pazopanib + trebananib), with a median PFS of 5.2 (95% C.I. 2.7–10.8) months (Table 3 and Fig. 2). In the subgroup of 6 patients on Arm B who had received trebananib in combination with either sunitinib or pazopanib, there were 2 responses and the median PFS was 8.3 (95% C.I. 2.6-not reached) months.
Thirty-four patients are off treatment; 1 patient remains on treatment after 20 cycles (Arm B). Fourteen patients (82%) on Arm A and 12 (67%) on Arm B stopped treatment due to disease progression. Of those off treatment, a median of 2 cycles were administered (range: 1–25) on Arm A and a median of 3 cycles were administered on Arm B (range: 1–8).
Adverse events
The most common treatment related toxicities in both arms were generally mild and included fatigue, nausea, hyponatremia, and increased creatinine (Table 4). Overall adverse events were numerically higher in Arm B. Edema is a known adverse event related to trebananib therapy, it was generally of low grade and more common in the combination arm. In Arm A, 6 of 17 treated patients (35%) experienced a grade 3 or higher adverse event attributed to trebananib. In Arm B, 10 of 18 patients (56%) experienced a grade 3 or higher adverse event attributed to combination treatment. The most common severe events were hypertension and dyspnea. A possibly related unwitnessed fatal cardiac arrest occurred on Arm B.
Table 4.
Major Treatment Related Toxicities
| Arm A (n = 17) | Arm B (n = 18) | |||
| Grade 1-2a | Grade 3 + b | Grade 1-2a | Grade 3 + b | |
| Adverse Event (CTCAE term) | n (%) | n (%) | n (%) | n (%) |
| Overall | 10 (59) | 6 (35) | 8 (44) | 10 (56) |
| All non-hematologic | 10 (59) | 6 (35) | 8 (44) | 10 (56) |
| Blood and Lymphatic System Disorders | ||||
| Anemia | 4 (24) | 0 | 3 (17) | 0 |
| Cardiac Disorders | ||||
| Cardiac arrest | 0 | 0 | 0 | 1 (6) |
| Sinus bradycardia | 2 (12) | 0 | 5 (28) | 0 |
| Eye Disorders | ||||
| Blurred Vision | 4 (24) | 0 | 0 | 0 |
| Gastrointestinal Disorders | ||||
| Diarrhea | 3 (18) | 0 | 5 (28) | 0 |
| Nausea | 7 (41) | 0 | 7 (39) | 0 |
| General Disorders | ||||
| Edema limbs | 3 (18) | 0 | 7 (39) | 0 |
| Fatigue | 7 (41) | 1 (6) | 8 (44) | 1 (6) |
| Pain | 2 (12) | 0 | 2 (11) | 0 |
| Investigations | ||||
| Creatinine increased | 5 (29) | 0 | 10 (56) | 0 |
| Lymphocyte count decreased | 2 (12) | 0 | 2 (11) | 1 (6) |
| Platelet count decreased | 3 (18) | 0 | 3 (17) | 0 |
| Weight gain | 2 (12) | 0 | 4 (22) | 1 (6) |
| White blood cells decreased | 1 (6) | 0 | 4 (22) | 0 |
| Metabolism and Nutrition Disorders | ||||
| Anorexia | 2 (12) | 0 | 2 (11) | 0 |
| Hyperglycemia | 0 | 1 (6) | 0 | 0 |
| Hyperkalemia | 2 (12) | 0 | 4 (22) | 0 |
| Hypoalbuminemia | 1 (6) | 0 | 6 (33) | 0 |
| Hypocalcemia | 2 (12) | 0 | 3 (17) | 0 |
| Hypokalemia | 0 | 1 (6) | 0 | 0 |
| Hyponatremia | 3 (18) | 0 | 4 (22) | 1 (6) |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 3 (18) | 0 | 2 (11) | 0 |
| Back pain | 2 (12) | 1 (6) | 3 (17) | 1 (6) |
| Neck pain | 0 | 0 | 0 | 1 (6) |
| Pain in extremity | 3 (18) | 0 | 4 (22) | 0 |
| Nervous System Disorders | ||||
| Dizziness | 3 (18) | 0 | 2 (11) | 0 |
| Headache | 4 (24) | 0 | 1 (6) | 0 |
| Psychiatric disorders | ||||
| Insomnia | 2 (12) | 0 | 2 (11) | 0 |
| Renal and Urinary Disorders | ||||
| Proteinuria | 4 (24) | 0 | 7 (39) | 0 |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Dyspnea | 5 (29) | 1 (6) | 1 (6) | 1 (6) |
| Pleural effusion | 0 | 1 (6) | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Rash maculo-papular | 1 (6) | 0 | 3 (17) | 0 |
| Vascular Disorders | ||||
| Hypertension | 2 (12) | 3 (18) | 7 (39) | 4 (22) |
aToxicities of any grade occurring in 4 or more individuals in either arm.
bGrade 3 or higher toxicities occurring in 1 or more individuals in either arm.
Molecular correlates
Peripheral blood samples were available at baseline in 30 patients, prior to cycle 2 in 29 patients, and prior to cycle 3 in 20 patients. In both arms, the circulating level of Ang2 prior to both cycle 2 and cycle 3 had increased significantly from baseline (Supplementary Table 1 and Supplementary Figure 1): p-value < 0.01 in both arms and at both timepoints (based on the Wilcoxon signed-rank test). Compared to pretreatment values, the VEGF ligands VEGF-A and PlGF tended to decrease in Arm A and stay the same or increase in Arm B. These differences between the two arms were significant for VEGF-A prior to cycle 3 (p = 0.046) and PlGF prior to cycle 2 (p = 0.029). Changes in markers of alternative pro-angiogenic pathways were similar between arms except for VCAM-1. The magnitude of increase was significantly higher in Arm B than in Arm A for VCAM-1 prior to cycle 3 (p = 0.046).
Discussion
In this multicenter randomized phase II study, trebananib at 15 mg/kg was well tolerated but there was no evidence that trebananib could overcome resistance to anti-VEGF therapy either as monotherapy or in combination with continued anti-VEGF treatment given at a previously tolerated dose. Nonetheless, the results provide several insights into tumors that have progressed on prior anti-VEGF therapy. We speculated that targeting an alternative angiogenic pathway would result in subsequent response in tumors driven by that alternative pathway, but that the depth and duration of response might be enhanced by continuing to target VEGF signaling. Our negative results could potentially be explained by insufficient inhibition of the angiopoietin-Tie signaling by trebananib; however, trebananib at doses below those used in this study impacts tumor blood flow and permeability using dynamic contrast enhanced MRI [11]. We demonstrated pharmacodynamics effects of trebananib on serum Ang2 that are consistent with inhibition of angiopoietin-Tie signaling system. Other potential explanations for these results include the absence of angiopoietin-Tie signaling as a clinically important resistance mechanism to anti-VEGF therapy in RCC or a flawed clinical definition of anti-VEGF therapy resistance.
That the angiopoietin-Tie signaling may be a mode of resistance to anti-VEGF therapy is inferred from studies demonstrating an elevation of Ang2 in non-responders and at the time of clinical progression on anti-VEGF agents [5, 17, 18]. Preclinical studies suggest that the combination of angiopoietin-Tie and VEGF signaling inhibition has the potential to improve outcomes over anti-VEGF therapy alone in RCC and other tumor types [5, 7, 19]. Prior clinical studies of trebananib in RCC have evaluated the agent in combination with sorafenib and sunitinib in patients with previously untreated advanced RCC. In a randomized phase II study of trebananib 3 mg/kg and 10 mg/kg plus sorafenib in previously untreated patients with RCC, the response rates were numerically higher in the combination arms but there was no difference in PFS [13]. A phase II study tested sequential cohorts of 10 mg/kg and 15 mg/kg in combination with sunitinib in patients with untreated RCC. Confirmed objective response rate in both cohorts were promisingly high (58% and 63% respectively) with median PFS of 13.9 and 16.3 months. A phase Ib study of trebananib combined with sorafenib or sunitinib explored trebananib doses of 3 mg/kg and 10 mg/kg in a mixed cohort of untreated and previously treated patients with advanced RCC [12]. The efficacy in this mixed cohort early phase study was considered promising. To our knowledge, the study reported here is the first phase II study to systemically evaluate trebananib plus anti-VEGF therapy in previously treated RCC. We did not confirm that angiopoietin-Tie signaling is a clinically relevant resistance mechanism to anti-VEGF therapy in RCC. Future analysis of the pretreatment tumor biopsies performed as part of this study may provide further insights into angiogenic pathway signaling after anti-VEGF therapy.
In this study, anti-VEGF therapy resistance was defined by the enrollment criteria as progression of disease within the prior 12 weeks while receiving at least 8 weeks of treatment with an anti-VEGF agent. The overall response rates observed as monotherapy or in combination were disappointing; however, two of five patients treated with the combination of pazopanib and trebananib responded to therapy for 8 months and over 24 months respectively. These numbers are too small to make any conclusions about this combination and the study was not designed or powered as a direct comparison of the two arms. Nonetheless, response to re-targeting VEGF has frequently been observed in patients with advanced RCC, both with the use of alternative anti-VEGF agents [20, 21] and after retreatment with a previously given anti-VEGF agent [22]. Moreover, in advanced colorectal cancer there is a benefit of continuing anti-VEGF therapy while switching the backbone cytotoxic chemotherapy regimen upon progression on an anti-VEGF containing regimen [23]. Taken together, these results suggest that the phenotype of anti-VEGF therapy resistance may be transient and is strongly context dependent.
This study is limited by its small size and heterogeneity of prior anti-VEGF targeted therapies included. It remains possible that the angiopoietin-Tie system is a relevant resistance mechanism in a small fraction of RCC tumors treated with anti-VEGF agents, which this study did not have the power to detect. It is likely that anti-VEGF resistance mechanisms are dependent on the spectrum of other targets inhibited by the anti-VEGF agent. Given the variety of anti-VEGF regimens used prior to entry in this study and small numbers, we cannot conclude that a particular anti-VEGF agent is more or less likely to induce angiogenesis through the angiopoietin-Tie system.
In conclusion, trebananib was not effective as monotherapy in patients with pre-treated RCC than had progressed on anti-VEGF therapy. Combination of trebananib with the prior anti-VEGF therapy was also insufficiently active in these patients to support further development. Further research is needed to understand the mechanisms of progression on anti-VEGF therapy in order to define effective combinations that overcome acquired resistance and improve treatment options for these patients.
CONFLICT OF INTEREST
Dr. Semrad has nothing to disclose. Dr. Groshen reports grants from NCI UM1 CA 186717 and NCI P30 CA 014089 during the conduct of the study. Ms. Luo has nothing to disclose. Dr. Pal reports consulting fees from Pfizer, Genetech, Novartis, and Exelexis, outside the submitted work, during the conduct of the study. Dr. Vaishampayan has nothing to disclose. Dr. Joshi has nothing to disclose. Dr. Quinn reports grants from NCI CTEP and consulting fees from Genentech, Amgen, Pfizer, Exelixis, BMS, Merck Sharp and Dohme, EMD Serono, Novartis, outside the submitted work during the conduct of the study. Dr. Mack reports grants from Boehringer Ingelheim, personal fees from Guardant Health, AstraZeneca, Pfizer, Celgene, and Apton Biosystems, outside the submitted work during the conduct of the study. Dr. Gandara has nothing to disclose. Dr. Lara has nothing to disclose.
Supplementary Material
ACKNOWLEDGMENTS
The research reported was supported by the National Cancer Institute of the National Institutes of Health under Award Number UM1CA186717 and NO1-CM-2011-00,038. Additional support was provided under National Institutes of Health awards P30CA33572, P30CA093373, P30CA014089, and P30CA014089 from the National Cancer Institute. During the study, Dr. Semrad was also supported by the NCI under Award Number K12CA138464. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/KCA-180041.
REFERENCES
- [1]. Abdollahi A, Folkman J. Evading tumor evasion: Current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist Updat. 2010;13(1-2):16–28. [DOI] [PubMed] [Google Scholar]
- [2]. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14(20):6371–5. [DOI] [PubMed] [Google Scholar]
- [4]. Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–77. [DOI] [PubMed] [Google Scholar]
- [5]. Wang X, Bullock AJ, Zhang L, Wei L, Yu D, Mahagaokar K, Alsop DC, Mier JW, Atkins MB, Coxon A, Oliner J, Bhatt RS. The role of angiopoietins as potential therapeutic targets in renal cell carcinoma. Transl Oncol. 2014;7(2):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Currie MJ, Gunningham SP, Turner K, Han C, Scott PA, Robinson BA, Chong W, Harris AL, Fox SB. Expression of the angiopoietins and their receptor Tie2 in human renal clear cell carcinomas; regulation by the von Hippel-Lindau gene and hypoxia. J Pathol. 2002;198(4):502–10. [DOI] [PubMed] [Google Scholar]
- [7]. Hashizume H, Falcon BL, Kuroda T, Baluk P, Coxon A, Yu D, Bready JV, Oliner JD, McDonald DM. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Research. 2010;70(6):2213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Huang H, Lai JY, Do J, Liu D, Li L, Del Rosario J, Doppalapudi VR, Pirie-Shepherd S, Levin N, Bradshaw C, Woodnutt G, Lappe R, Bhat A. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res. 2011;17(5):1001–11. [DOI] [PubMed] [Google Scholar]
- [9]. Huang J, Bae JO, Tsai JP, Kadenhe-Chiweshe A, Papa J, Lee A, Zeng S, Kornfeld ZN, Ullner P, Zaghloul N, Ioffe E, Nandor S, Burova E, Holash J, Thurston G, Rudge J, Yancopoulos GD, Yamashiro DJ, Kandel JJ. Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. International Journal of Oncology. 2009;34(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- [10]. Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E, Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T, Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun JR, Zhang N, Lu J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S, Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A, Hayward I, Radinsky R, Boone T, Kendall R. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6(5):507–16. [DOI] [PubMed] [Google Scholar]
- [11]. Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Sil-verman JM, Rasmussen E, Sun YN, Zhong D, Hwang YC, Evelhoch JL, Oliner JD, Le N, Rosen LS. Safety, pharma-cokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27(21):3557–65. [DOI] [PubMed] [Google Scholar]
- [12]. Hong DS, Gordon MS, Samlowski WE, Kurzrock R, Tannir N, Friedland D, Mendelson DS, Vogelzang NJ, Rasmussen E, Wu BM, Bass MB, Zhong ZD, Friberg G, Appleman LJ. A phase I, open-label study of trebananib combined with sorafenib or sunitinib in patients with advanced renal cell carcinoma. Clinical Genitourinary Cancer. 2014;12(3):167–77 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Rini B, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, Dirix LY, Fishman M, Ramlau R, Ravaud A, Rogowski W, Kracht K, Sun YN, Bass MB, Puhlmann M, Escudier B. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: A randomized, double-blind, placebo-controlled, phase 2 study. Cancer. 2012;118(24):6152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Atkins MB, Gravis G, Drosik K, Demkow T, Tomczak P, Wong SS, Michaelson MD, Choueiri TK, Wu B, Navale L, Warner D, Ravaud A. Trebananib (AMG 386) in Combination With Sunitinib in Patients With Metastatic Renal Cell Cancer: An Open-Label, Multicenter, Phase II Study. J Clin Oncol. 2015;33(30):3431–8. [DOI] [PubMed] [Google Scholar]
- [15]. Lu JF, Rasmussen E, Karlan BY, Vergote IB, Navale L, Kuchimanchi M, Melara R, Stepan DE, Weinreich DM, Sun YN. Exposure-response relationship of AMG 386 in combination with weekly paclitaxel in recurrent ovarian cancer and its implication for dose selection. Cancer Chemother Pharmacol. 2012;69(5):1135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- [17]. Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, Louis DN, Cohen KS, Chea H, Exarhopou-los A, Loeffler JS, Moses MA, Ivy P, Sorensen AG, Wen PY, Jain RK. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky TC, Weihrauch MR, Cremer B, Kashkar H, Odenthal M, Augustin HG, Schmiegel W, Hallek M, Hacker UT. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. British Journal of Cancer. 2010;103(9):1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Chae SS, Kamoun WS, Farrar CT, Kirkpatrick ND, Niemeyer E, de Graaf AM, Sorensen AG, Munn LL, Jain RK, Fukumura D. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin Cancer Res. 2010;16(14):3618–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J, Hariharan S, Chen C, Rosbrook B, Kim S, Rini BI. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. The Lancet Oncology. 2013;14(6):552–62. [DOI] [PubMed] [Google Scholar]
- [21]. Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomase kJ, Kremer A, Kim HJ, Wood K, Dutcus C, Larkin J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. The Lancet Oncology. 2015;16(15):1473–82. [DOI] [PubMed] [Google Scholar]
- [22]. Porta C, Paglino C, Grunwald V. Sunitinib re-challenge in advanced renal-cell carcinoma. British Journal of Cancer. 2014;111(6):1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso-Orduna V, Schlichting C, Reyes-Rivera I, Bendahmane B, Andre T, Kubicka S, Investigators MLS. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. The Lancet Oncology. 2013;14(1):29–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.