Abstract
Background
Guillain-Barré syndrome (GBS) is a progressive acute form of paralysis most probably secondary to an immune-mediated process. GBS among Saudis has been seldom investigated, which leaves both clinicians and researchers with scarcity in knowledge. Therefore, this study aims to assess the prevalence and clinical prognosis of GBS among pediatrics admitted with acute paralysis at a large healthcare facility in Riyadh, Saudi Arabia.
Methods
This retrospective study reviewed patients’ medical records between 2005 and 2015. Eligible cases were children (<14 years old) admitted to the hospital complaining of acute paralysis and later diagnosed with one form or variant of GBS. Pearson’s chi-square, Fisher’s exact test, and binary logistic regression were employed to analyze the collected data.
Results
The prevalence of GBS was 49%. The male-to-female ratio was 1.45:1. The mean ± standard deviation age was 7±3.7 years. There were 34 (69.4%) cases with progression to maximum paralysis in ≤2 weeks, while 15 (30.6%) cases occurred beyond 2 weeks. Males (n=24, 82.8%) were more likely to endure progression to maximum paralysis in ≤2 weeks after the disease onset, compared to females (n=10, 50%), P=0.014. All cases complaining of respiratory problems exhibited a progression to maximum paralysis in ≤2 weeks, compared to those with no respiratory problems, P=0.027. Residual paralysis at 60 days post disease onset was highly associated with GBS patients of age 8–14 years (n=15, 65.2%), compared to younger patients (n=8, 30.8%), P=0.016. Patients admitted in colder seasons (n=14, 63.6%) were more likely to suffer residual paralysis too, compared to those in warmer seasons (n=9, 33.3%), P=0.035. GBS cases who complained of facial weakness (n=9, 75%) and ocular abnormalities (n=10, 71.4%) were also more likely to endure residual paralysis at 60 days post disease onset, P=0.025 and P=0.03, respectively.
Conclusion
Male gender could be a determinant of rapid progression to maximum paralysis, while the older age group in pediatrics is expected to endure residual paralysis at 60 days post disease onset. GBS can be accounted as a rare disease, especially in pediatrics, so confirmed cases should be investigated comprehensively for research purposes.
Keywords: GBS, factors, prognosis, residual, paralysis, neuro
Background
Guillain-Barré syndrome (GBS) is defined as a group of clinical syndromes with acute onset of peripheral neuropathy – axonal or demyelinating – secondary to an immune-mediated process. It usually presents itself with a progressive paralysis that can involve the autonomic, bulbar, and respiratory systems.1 GBS has four subtypes, which are cute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy, acute motor and sensory axonal neuropathy, and Miller Fisher syndrome. The most common subtype of these four is acute inflammatory demyelinating polyradiculoneuropathy (AIDP) that constitutes 85%–90% of GBS cases, followed by the axonal subtypes (30%–47%) and Miller Fisher syndrome (5%).2–4
The annual incidence of GBS as reported in one 10-year study was 0.42 cases per 100,000 persons.5 Higher incidence of GBS has been reported in younger adults and the elderly aged 50 years and older.6,7 A 6-year regional study revealed that the peak age of patients diagnosed with GBS was between 41 and 60 years with a median age of 47 years.8 GBS is in fact the most common form of acute flaccid paralysis among children (25.9%–51%),9 and its annual incidence in a pediatric population ranged from 0.34 to 1.34/100,000.1,10 Reports from the Arab countries showed an incidence of 1.33–1.7 per 100,000 children.11,12
Almost 75% of GBS patients are males,4 and higher rates of GBS are reported during the winter and early summer seasons.3,8,13 GBS has been reported to be either preceded or triggered by previous infections or an immune-mediated process, especially with axonal and peripheral nerve demyelinations.8 For instance, the most common types of preceding infections were upper respiratory tract infections of viral etiologies, followed by gastrointestinal infections.4,8 Campylobacter jejuni was the most common pathogen associated with axonal degeneration and slower recovery in GBS patients. Other pathogens identified included Epstein–Barr virus, cytomegalovirus, Mycoplasma pneumoniae, Hemophilus influenzae,8 and Zika virus.14,15 It was also reported that GBS was observed after vaccination for rabies and swine influenza.8
The clinical presentation of GBS is usually a progressive flaccid weakness that can proceed to complete paralysis, often witnessed in 74% of the cases. In addition, bulbar involvement, absence of reflexes, respiratory muscle involvement, back and lower limb pain, bilateral facial or ocular weakness, and paresthesia are manifested too.8,16 Moreover, autonomic disturbances which cause arrhythmias and blood pressure fluctuations can also be observed in 4%–17% of cases which eventually increases the rates of morbidity and mortality among GBS patients.4,13 A recent study indicated that the risk of mortality associated with GBS is mostly prevalent among the elderly and severely affected patients, during which the risk increases in the recovery phase.17
GBS progresses rapidly and reaches the peak within 2 weeks before the patients undergo a plateau phase that persists from days to several months, after which they finally recover.13 In general, the prognosis of patients depends on the varying signs and symptoms of GBS which made clinicians and researchers classify GBS by the distinct subtypes. Axonal forms of GBS exhibit worse prognosis and slower recovery compared to demyelination forms of GBS.16 It is worth mentioning that the prognosis of GBS is better among pediatrics,18 as good outcomes have been observed in 61%–71% of cases.19 In one prospective study, 96% of children were either asymptomatic or had minor residual symptoms during the recovery phase.10
GBS in Saudi Arabia (the largest populated country in the Arab Gulf region) has been seldom investigated, especially among the pediatric population, which leaves both clinicians and researchers with scarcity in knowledge. A systematic review of GBS in 2014 described the findings of three studies conducted in Saudi Arabia, two of which were outdated (published in 1991 and 1994) while the third was a 6-year, single-center study published in 2009 with a very small sample size (n=12).3 The Saudi population resides in harsh desert climates with extreme temperatures, so epidemics of infections are pretty rare in Saudi Arabia, although a serious outbreak of Middle Eastern respiratory syndrome coronavirus has been recorded recently. GBS can be triggered by certain viral or bacterial infections, which might be more prevalent in certain countries than others. GBS is also a rare disease, especially among the pediatric population. Signs and symptoms of GBS usually aid clinicians in classifying the type of GBS, yet these clinical manifestations might overlap between one type and another. This has led some clinicians to report newly classified types or variants of GBS.20 Therefore, reporting the prevalence and outcomes of even a few cases of GBS will provide better insights into the care and management of GBS.
Purpose of study
The purpose of this study was to investigate the 10-year prevalence of GBS among the pediatric population who complained of acute paralysis and were admitted to the emergency department at the largest healthcare facility in Riyadh, capital of Saudi Arabia. In addition, this study determined the factors associated with GBS and described its clinical outcomes, mainly progression to maximum paralysis and the extent of residual paralysis after 60 days.
Materials and methods
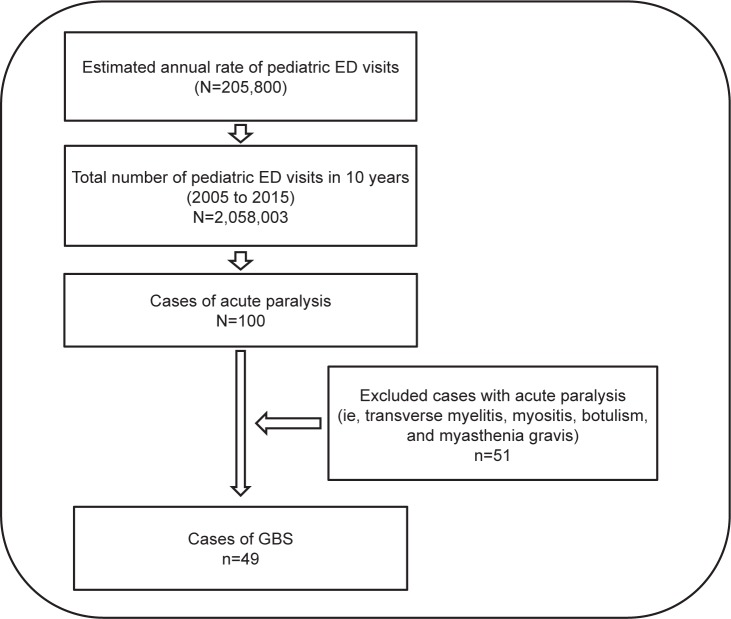
This was a retrospective cross-sectional study, based on a thorough review of medical records, between 2005 and 2015 at the largest tertiary healthcare facility in Riyadh, Saudi Arabia. Exceeding a bed capacity of 1,200 beds, King Fahad Hospital was the targeted setting, which is part of a multilevel healthcare system of the Saudi Ministry of National Guard. The estimated annual rate of pediatric visits to the emergency department is 205,800. Ethical clearance and approval to conduct this study was obtained from the Institutional Review Board at King Abdullah International Medical Research Center with reference number RC 16/130/R. Patient confidentiality was maintained as no patients’ identifiers were collected in accordance with the Declaration of Helsinki. Thus, patient parental consent to review their medical records was not required.
Eligible cases were pediatric cases (<14 years old) admitted to the setting complaining of acute paralysis and later diagnosed with one form or variant of GBS.21,22 Diagnosis of GBS was confirmed by expert neurologists based on physical examination and a series of diagnostic tests, such as nerve conduction examinations, magnetic resonance imaging (MRI), and cerebrospinal fluid tests. Diagnostic reports were read and validated by two study investigators. Other cases of acute paralysis were excluded (ie, transverse myelitis, myositis, botulism, and myasthenia gravis) (Figure 1). Patient and disease characteristics included gender, age (years), classification of GBS, season of incident, and any antecedent infections prior to disease onset. Results of physical examination and diagnostic tests as well as the type of clinical management performed were collected. Clinical outcomes mainly included the duration of progression to maximum paralysis (cutoff set at 2 weeks), residual paralysis set at 60 days, and prevalence of recurrent vs relapsed GBS.
Figure 1.
Illustration of the total number of pediatric ED visits and the number of diagnosed GBS cases.
Abbreviations: ED, emergency department; GBS, Guillain-Barré syndrome.
The SPSS version 25 (IBM Corporation, Armonk, NY, USA) was used for data analysis. Frequency and percentages were used to describe categorical variables, whereas the mean ± standard deviation was used to present continuous variables. Age was categorized into two groups at the cutoff of 7 years (50th percentile of sample). Testing the two clinical outcomes (progression to maximum paralysis within 2 weeks and residual paralysis at 60 days) across various patient disease characteristics was conducted using Pearson’s chi-square and Fisher’s exact test as applicable. Two binary logistic regression models were constructed to adjust for any confounding effect between gender, age, and antecedent infections to identify the variables significantly associated with these two clinical outcomes. Accordingly, the adjusted OR and the 95% CI were presented with statistical significance set at P<0.05.
Results
Sample characteristics and disease prevalence
Between 2005 and 2015, a total of 100 pediatric cases were admitted to the emergency department complaining of acute paralysis. The 10-year prevalence of GBS among cases with acute paralysis (N=100) was 49%. Males constituted 29 (59.2%) of the sample, whereas 20 (40.8%) were females. The mean ± standard deviation age was 7±3.7 years, that is 26 (53.1%) between 1 and 7 years old and 23 (46.9%) between 8 and 14 years old. Although 14 (28.6%) of the cases were unclassified categories of GBS, 18 (36.7%) were classified as AIDP, 14 (28.6%) as acute motor axonal neuropathy/acute motor and sensory axonal neuropathy, and 3 (6.1%) as Miller Fisher syndrome. A concurrent infection with GBS or vaccine occurred in 34 (69.4%) cases. Minimal seasonal variation in GBS admissions was observed between the summer and winter seasons, 44.9% in colder seasons vs 55.1% in warmer seasons (Table 1).
Table 1.
Patient and disease characteristics
| n (%) | |
|---|---|
|
| |
| Acute paralyses (N=100) | |
| GBS | 49 (49.0) |
| Axonal (AMAN and AMSAN) | 14 (28.6) |
| AIDP | 18 (36.7) |
| Miller Fisher syndrome | 3 (6.1) |
| Unclassified Guillain-Barre’ syndrome | 14 (28.6) |
| Others | 51 (51.0) |
|
| |
| GBS | 49 (100.0) |
|
| |
| Gender | |
| Male | 29 (59.2) |
| Female | 20 (40.8) |
|
| |
| Age category (years) | |
| 1–7 | 26 (53.1) |
| 8–14 | 23 (46.9) |
| Mean ± SD | 7±3.7 |
|
| |
| Antecedent infection | |
| Yes | 34 (69.4) |
| No | 15 (30.6) |
|
| |
| Season of incident | |
| Fall/winter | 22 (44.9) |
| Spring/summer | 27 (55.1) |
Note: n, frequency.
Abbreviations: AMAN, acute motor axonal neuropathy; AMSAN, acute motor and sensory axonal neuropathy; GBS, Guillain-Barré syndrome; SD, standard deviation.
Clinical management and outcomes
Almost half of the sample (n=27, 55.1%) complained of back or limb pain and 7 (14.3%) complained of limb paresthesia/numbness. Patients who reported facial weakness numbered 12 (24.5%), ocular abnormalities were reported by 14 (28.6%) patients, and bulbar symptoms were reported by 21 (42.9%). Only 9 (18.4%) cases reported respiratory problems and 10 (20.4%) autonomic involvement, while almost all (n=48, 98%) had impaired reflexes. A series of diagnostic tests revealed abnormal brain MRI (ie, loss of peritrigonal white matter and abnormal signal noted within the right basal ganglia) in 2 (11.8%) patients and abnormal spine MRI (ie, intense enhancement of the terminal nerve roots, significant thickening of the anterior spinal nerve root in the conus medullaris and cauda equine, abnormal enhancement noted at the peripheral sacral anterior root at the level of S1 and S2, and diffuse enhancements seen along the peripheral nerve roots of the spinal cord) in 4 (26.7%). Cerebrospinal fluid analysis showed high protein levels in 15 (62.5%) patients in the sample, with a high white blood cell level in 4 (10.2%) cases. Positive laboratory culture was observed in 7 (14.3%) of the cases (Table 2). The majority of the patients (n=40, 81.6%) received a course of intravenous immunoglobulin and 5 (10%) received a course of intravenous immunoglobulin/steroids, while 4 (8.2%) underwent plasmapheresis. Patients who exhibited a progression to maximum paralysis in ≤2 weeks numbered 34 (69.4%), while 15 (30.6%) had a progression to maximum paralysis in >2 weeks. At 60 days post emergency department visit, 23 (46.9%) patients still had residual paralysis. Three patients suffered from recurrent GBS and one case from a relapse. No deaths were reported.
Table 2.
Frequency of physical examination and diagnostic test results
| n (%) | |
|---|---|
| Physical examination* | |
| Back or limb pain | 27 (55.1) |
| Limb parathesia/numbness | 7 (14.3) |
| Facial weakness | 12 (24.5) |
| Ocular abnormalities | 14 (28.6) |
| Bulbar symptoms | 21 (42.9) |
| Respiratory problems (all intubated) | 9 (18.4) |
| Autonomic involvement | 10 (20.4) |
| Reflexes impaired | 48 (98.0) |
| Diagnostic tests* | |
| Abnormal brain MRI | 2 (11.8) |
| Abnormal spinal MRI | 4 (26.7) |
| High CSF protein | 15 (62.5) |
| High CSF WBC | 4 (10.0) |
| Positive culture (Heliobacter pylori, varicella zoster virus, Brucella, Enterobacter pneumonia, enterovirus, campylobacter) | 7 (14.3) |
| Clinical management* | |
| Plasmapheresis | 4 (8.2) |
| Intravenous immunoglobulin | 40 (81.6) |
| Intravenous immunoglobulin and steroids | 5 (10.2) |
| Intensive care unit admission | 26 (53.1) |
| Clinical outcomes | |
| Progression to maximum paralysis | |
| ≤2 weeks | 34 (69.4) |
| >2 weeks | 15 (30.6) |
| Residual paralysis (at 60 days) | 23 (46.9) |
| Recurrent Guillain-Barré syndrome | 3 (6.1) |
| Relapse | 1 (1.0) |
Note:
Mutually exclusive.
Abbreviations: CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; n, frequency; WBC, white blood cells.
Factors associated with disease outcomes
Analysis showed that male GBS patients were more likely to endure progression to maximum paralysis in ≤2 weeks after the onset of symptoms (n=24, 82.8%), compared to female GBS patients (n=10, 50%), P=0.014. All cases complaining of respiratory problems exhibited a progression to maximum paralyses in ≤2 weeks, compared to those with no respiratory problems, P=0.027. On the other hand, residual paralysis at 60 days post onset of disease was highly associated with GBS patients aged 8–14 years (n=15, 65.2%), compared to younger patients (n=8, 30.8%), P=0.016. Patients admitted in colder seasons (n=14, 63.6%) were more likely to suffer residual paralysis too, compared to warmer seasons (n=9, 33.3%), P=0.035. GBS patients who complained of facial weakness (n=9, 75%) and ocular abnormalities (n=10, 71.4%) were also more likely to endure residual paralysis at 60 days post disease onset, P=0.025 and P=0.03 respectively (Table 3).
Table 3.
Progression to maximum paralysis and residual paralysis with patient characteristics and physical examination
| Progression to maximum paralysis
|
Residual paralysis (at 60 days)
|
|||
|---|---|---|---|---|
| ≤2 weeks, 34 (69.4%) | >2 weeks, 15 (30.6%) | Yes, 23 (46.9%) | No, 26 (53.1%) | |
|
| ||||
| Gender | ||||
| Male | 24 (82.8%) | 5 (17.2%) | 15 (51.7%) | 14 (48.3%) |
| Female | 10 (50.0%) | 10 (50.0%) | 8 (40.0%) | 12 (60.0%) |
|
| ||||
| χ2=5.98, P=0.014* | χ2=0.653, P=0.419 | |||
|
| ||||
| Age category (years) | ||||
| 1–7 | 18 (69.2%) | 8 (30.8%) | 8 (30.8%) | 18 (69.2%) |
| 8–14 | 16 (69.6%) | 7 (30.4%) | 15 (65.2%) | 8 (34.8%) |
|
| ||||
| χ2=0.001, P=0.980 | χ2=5.815, P=0.016* | |||
|
| ||||
| Antecedent infection | ||||
| Yes | 23 (67.6%) | 11 (32.4%) | 18 (52.9%) | 16 (47.1%) |
| No | 11 (73.3%) | 4 (26.7%) | 5 (33.3%) | 10 (66.7%) |
|
| ||||
| χ2=0.158, P=0.691 | χ2=1.607, P=0.205 | |||
|
| ||||
| Season of incident | ||||
| Fall/winter | 17 (77.3%) | 5 (22.7%) | 14 (63.6%) | 8 (36.4%) |
| Spring/summer | 17 (63.0%) | 10 (37.0%) | 9 (33.3%) | 18 (66.7%) |
|
| ||||
| χ2=1.169, P=0.280 | χ2=4.469, P=0.035* | |||
|
| ||||
| Back or limb pain | ||||
| Yes | 18 (66.7%) | 9 (33.3%) | 14 (51.9%) | 13 (48.1%) |
| No | 16 (72.7%) | 6 (27.3%) | 9 (40.9%) | 13 (59.1%) |
|
| ||||
| χ2=0.210, P=0.647 | χ2=0.853, P=0.445 | |||
|
| ||||
| Limb parathesia/numbness | ||||
| Yes | 3 (42.9%) | 4 (57.1%) | 3 (42.9%) | 4 (57.1%) |
| No | 31 (73.8%) | 11 (26.2%) | 20 (47.6%) | 22 (52.4%) |
|
| ||||
| F-exact, P=0.117 | χ2=0.210, P=0.647 | |||
|
| ||||
| Cranial symptoms | ||||
| Yes | 20 (74.1%) | 7 (25.9%) | 16 (59.3%) | 11 (40.7%) |
| No | 14 (63.6%) | 8 (36.4%) | 7 (31.8%) | 15 (68.2%) |
|
| ||||
| χ2=0.622, P=0.430 | χ2=3.665, P=0.056 | |||
|
| ||||
| Respiratory problems | ||||
| Yes | 9 (100.0%) | 0 (0.0%) | 6 (66.7%) | 3 (33.3%) |
| No | 25 (62.5%) | 15 (37.5%) | 17 (42.5%) | 23 (57.5%) |
|
| ||||
| χ2=4.864, P=0.027* | χ2=1.723, P=0.189 | |||
|
| ||||
| Autonomic involvement | ||||
| Yes | 8 (80.0%) | 2 (20.0%) | 6 (60.0%) | 4 (40.0%) |
| No | 26 (66.7%) | 13 (33.3%) | 17 (43.6%) | 22 (56.4%) |
|
| ||||
| F-exact, P=0.344 | F-exact, P=0.283 | |||
Note:
Significant at P<0.05.
Abbreviations: F-exact, Fisher’s exact test; χ2 = Pearson’s Chi-square; P = P-value.
Two binary logistic regression models showed that males were 5.69 times more likely to exhibit progression to maximum paralysis in ≤2 weeks compared to females, adjusted P=0.017. Older GBS patients with age 8–14 years were 3.9 times more likely to sustain residual paralysis at 60 days compared to younger patients aged 1–7 years, adjusted P=0.034 (Table 4).
Table 4.
Factors associated with level of progression to maximum paralysis and residual paralysis
| Progression to maximum paralysis ≤2 weeks | Residual paralysis at 60 days | |||||
|---|---|---|---|---|---|---|
| Adj. OR | 95% CI | Adj. P-value | Adj. OR | 95% CI | Adj. P-value | |
| Gender: males vs females | 5.69 | 1.37–23.7 | 0.017* | 1.22 | 0.33–4.45 | 0.765 |
| Age: old (8–14 years) vs young (1–7 years) | 0.59 | 0.14–2.45 | 0.467 | 3.90 | 1.11–13.74 | 0.034* |
| Previous infection: yes vs none | 0.95 | 0.22–4.08 | 0.944 | 2.20 | 0.57–8.53 | 0.256 |
Note:
Significance at P<0.05.
Abbreviations: Adj, adjusted, OR, odds ratio, CI, confidence interval.
Discussion
GBS constitutes the majority of acute flaccid paralysis cases among children, yet it is less frequent compared to the prevalence among adults. The most affected age group among pediatrics was among those 1–4 years old.23 The European population-based annual incidence rate was 1.70/100,000 persons.24 One study targeting children of age <16 years showed an annual incidence of 0.4/100,000.1 Epidemiological studies of GBS in Asia are few and it is speculated that there are geographical differences with regards to the various types of GBS.25 For instance, the prevalence of AIDP in two Far Eastern settings was 20/30 (66.7%)26 and 324/661 (49%),27 much higher than that reported in this setting as 18/49 (17.9%). The average age of children who complained of GBS in this setting was 8.59±4.99 years, which was slightly higher than that reported in the literature.28 A 10-year study showed that 2.1/100,000 acute paralysis cases were GBS, of which the male-to-female ratio was 1.5:1.24 Furthermore, the incidence of GBS among males increased by 28% and among women increased by 14%, for every 10-year increment of age.24 Findings in this setting were similar to previous studies that showed a higher incidence among males.4,30
Studies have shown that GBS was preceded by infections in 40%–70% of the reported cases.31 In one Asian setting, preceding infections were prevalent in 34.9% of the GBS cases,27 a much lower figure than that reported in this setting (69.4%). Recipients of vaccines such as the varicella zoster virus vaccine were at higher risk for the demyelinating subtypes of GBS.32 Furthermore, in 50%–70% of GBS cases, respiratory or gastrointestinal infections have been reported 1–2 weeks prior to the incident.33 On the other hand, studies conducted in Western and Arab countries showed higher occurrence of GBS during the winter and early summer seasons.3,4,8,13 The seasonal incidence of GBS in one study was 22% in autumn, 25% in winter, 27% in spring, and 26% in summer.29 These figures were comparable to findings in this study where 44.9% of GBS were admitted in autumn and winter.
Rehabilitation in GBS patients is an expensive and lengthy procedure that should rapidly follow the cessation of progressive paralysis. One setting stated that 66% of their 407 GBS patients had a progression to maximum paralysis within 8 days.34 Another study stated that the majority of cases with axonal variants of GBS were more likely to complain of rapid progression to maximum paralysis in comparison to AIDP patients.35 Autonomic dysfunction and cranial nerve involvement were significantly associated with shorter time duration to maximum paralysis or nadir,28 which was similar to figures observed in this setting. Although autonomic nervous dysfunction was a significant predictor of adverse clinical outcomes in the literature,36 it had no significant effect on either the progression to maximum paralysis or to residual paralysis in this setting. Plasmapheresis conducted at early stages of the disease (within 7 days) has been proven to be a leading factor in shortening the duration to maximum paralysis.37 In this setting, only four cases underwent plasmapheresis, all of whom were observed to endure maximum paralysis in <2 weeks.
Patients complaining of GBS often exhibit a full recovery, as most patients regain ambulation, but residual paralysis or disability remains in up to 10% of the cases.38 In one regional study, residual paralysis was still observed in 64.7% and 29.4% of GBS patients after 3 to 6 months of the incident, respectively.39 In this setting, after 2 months of nadir, the clinical outcomes were better, as 46.9% complained of residual paralysis. It was reported that by the third month, 47.1% of GBS patients are expected to have a complete recovery, while 24.3% will have poor recovery (wheelchair-bound).40 One longitudinal study stated that the rates of GBS patients with complete recovery or minor limitations was 41% in the first month, 71% in the third, 86% in the sixth, and 92% in the twelfth.41 Residual paralysis at the second month post onset of disease was highly associated with patients of age 8–14 years (65.2%) in this setting, which is compatible with findings reported by a study conducted in Iraq.12 In addition, GBS cases with cranial and autonomic involvement had a poor functional outcome, which was a similar finding to this study, but with no statistically significant differences.42 Additionally, in studies where GBS and acute myelitis were concurrent, all cases suffered residual paralysis.35 Furthermore, reoccurrence or relapse of GBS is not uncommon. Findings in the literature suggest that 1%–6% of patients who have had GBS will experience a recurrent attack,43 which makes the prevalence of recurrent cases in this setting at the upper limits.
Driven by speculation that respiratory failure is rare in children with GBS, one study noted that 4/40 (10%) children required mechanical ventilation.44 The rate of GBS patients who were in need of respiratory assistance in this setting was 18.4%, which was slightly higher than a rate reported by one setting.26 Cranial nerve involvement is common in GBS patients, especially in the AIDP variant, as it ranged from 50% to 75%.5,20 Cranial nerve involvement is associated with respiratory paralysis and needs ventilator support, but overall it does not affect the clinical prognosis.45 Among the various clinical patterns of GBS, autonomic disturbances, manifested by arrhythmia and blood pressure fluctuations, were found in 4%–17% of the cases which increases the morbidity and mortality among them.4,13,19 In this setting, GBS patients with autonomic involvement were slightly higher (20.4%), yet no deaths were reported. Moreover, an elevated cerebrospinal fluid protein level has been reported in 66.7% of GBS cases, which was comparable to findings in this setting.46
Limitations
A number of limitations have been observed in this study. The retrospective analysis of previously collected data has been built on preexisting disease information recorded on medical charts. These data were originally collected for clinical rather than research purposes, which might have overlooked some patients and disease characteristics that might be of interest to readers. One of the main limitations is the fact that rehabilitation was not investigated in this study as a potential confounder for residual paralysis. Loss of follow-up, which is beyond 60 days, is another limitation, which could have reported the residual paralysis up to 12 months after the diagnosis. Due to that fact that this study reports findings from a relatively small sample size, it might not be generalizable to the populations residing in the whole Gulf region. This study has not reported the population incidence of GBS during the 10-year period, but rather limited its interest to the prevalence of GBS among acute paralytic cases. Last but not least, Zika virus has been reported to be a potential preceding trigger of GBS,14,15 yet no positive cases for Zika virus have so far been reported in Saudi Arabia.
Conclusion
This study reported the prevalence of GBS among pediatrics complaining of acute paralysis in Saudi Arabia over a 10-year period. Male gender and respiratory problems appear to be determinants of a rapid progression to maximum paralysis. Pediatrics admitted in colder seasons and, in particular, the older age group are expected to endure residual paralysis at 60 days post disease onset. GBS can be accounted as a rare disease, especially in pediatrics, so confirmed cases should be investigated comprehensively for research purposes. Multicenter pooling of GBS-related data is crucial to boost the sample representativeness of the Saudi Arabian population and to better understand both the etiology and prognosis of this disease. Having a national GBS database will also aid in better understanding the various GBS variants with their distinctive clinical characteristics. The availability of the Saudi national biobank facility in Riyadh city could be seized to store GBS health-pertinent information and biological samples for future research analytical purposes.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. Neuroepidemiology. 2009;32(2):150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 2.Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366(9497):1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 3.Benamer HT, Bredan A. Guillain-Barré syndrome in Arab countries: a systematic review. J Neurol Sci. 2014;343(1–2):221–223. doi: 10.1016/j.jns.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Mukerji S, Aloka F, Farooq MU, Kassab MY, Abela GS. Cardiovascular complications of the Guillain-Barré syndrome. Am J Cardiol. 2009;104(10):1452–1455. doi: 10.1016/j.amjcard.2009.06.069. [DOI] [PubMed] [Google Scholar]
- 5.Matsui N, Nodera H, Kuzume D, et al. Guillain-Barré syndrome in a local area in Japan, 2006–2015: an epidemiological and clinical study of 108 patients. Eur J Neurol. 2018;25(5):718–724. doi: 10.1111/ene.13569. [DOI] [PubMed] [Google Scholar]
- 6.Hauck LJ, White C, Feasby TE, Zochodne DW, Svenson LW, Hill MD. Incidence of Guillain-Barre syndrome in Alberta, Canada: an administrative data study. J Neurol Neurosurg Psychiatry. 2008;79(3):318–320. doi: 10.1136/jnnp.2007.118810. [DOI] [PubMed] [Google Scholar]
- 7.Govoni V, Granieri E, Manconi M, Capone J, Casetta I. Is there a decrease in Guillain-Barré syndrome incidence after bovine ganglioside withdrawal in Italy? A population-based study in the local health district of Ferrara, Italy. J Neurol Sci. 2003;216(1):99–103. doi: 10.1016/s0022-510x(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 8.Dahbour SS. Clinical experience with Gullain Barre syndrome over a 6-year period in one hospital in the Middle East. Jordan Med J. 2010;43(4):280–285. [Google Scholar]
- 9.National Committee for the Certification of Wild Poliovirus Eradication in Hong Kong Fifteen years of acute flaccid paralysis surveillance in Hong Kong: findings from 1997 to 2011. J Paediatr Child Health. 2014;50(7):545–552. doi: 10.1111/jpc.12492. [DOI] [PubMed] [Google Scholar]
- 10.Korinthenberg R, Schessl J, Kirschner J. Clinical presentation and course of childhood Guillain-Barré syndrome: a prospective multicentre study. Neuropediatrics. 2007;38(1):10–17. doi: 10.1055/s-2007-981686. [DOI] [PubMed] [Google Scholar]
- 11.Nagarajan V, Al-Shubaili A. Clinical and neurophysiological pattern of Guillain-Barré syndrome in Kuwait. Med Princ Pract. 2006;15(2):120–125. doi: 10.1159/000090916. [DOI] [PubMed] [Google Scholar]
- 12.Jasem J, Marof K, Nawar A, et al. Guillain-Barré syndrome as a cause of acute flaccid paralysis in Iraqi children: a result of 15 years of nationwide study. BMC Neurol. 2013;13(1):195. doi: 10.1186/1471-2377-13-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Doorn PA, Kuitwaard K, Walgaard C, van Koningsveld R, Ruts L, Jacobs BC. IVIG treatment and prognosis in Guillain-Barré syndrome. J Clin Immunol. 2010;30(S1):74–78. doi: 10.1007/s10875-010-9407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauer F, Riesen M, Reveiz L, et al. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: systematic review. PLoS Med. 2017;14(1):e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucchese G, Kanduc D. Zika virus and autoimmunity: from microcephaly to Guillain-Barré syndrome, and beyond. Autoimmun Rev. 2016;15(8):801–808. doi: 10.1016/j.autrev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Dhar R, Stitt L, Hahn AF. The morbidity and outcome of patients with Guillain-Barré syndrome admitted to the intensive care unit. J Neurol Sci. 2008;264(1–2):121–128. doi: 10.1016/j.jns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg B, Bunschoten C, van Doorn PA, et al. Mortality in Guillain-Barre syndrome. Neurology. 2013;80(18):1650–1654. doi: 10.1212/WNL.0b013e3182904fcc. [DOI] [PubMed] [Google Scholar]
- 18.Walgaard C, Lingsma HF, Ruts L, van Doorn PA, Steyerberg EW, Jacobs BC. Early recognition of poor prognosis in Guillain-Barre syndrome. Neurology. 2011;76(11):968–975. doi: 10.1212/WNL.0b013e3182104407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govoni V, Granieri E. Epidemiology of the Guillain-Barré syndrome. Curr Opin Neurol. 2001;14(5):605–613. doi: 10.1097/00019052-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Dimachkie MM, Barohn RJ. Guillain-Barré syndrome and variants. Neurol Clin. 2013;31(2):491–510. doi: 10.1016/j.ncl.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain. 2014;137(Pt 1):33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 22.Bvd B, Walgaard C, Drenthen J, et al. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 23.Landaverde JM, Danovaro-Holliday MC, Pierson Trumbo S, Cl P-T, Ruiz-Matus C. Guillain-Barré syndrome in children aged. J Infect Dis. 2010;201(5):746–750. doi: 10.1086/650530. [DOI] [PubMed] [Google Scholar]
- 24.Sipilä JOT, Soilu-Hänninen M, Ruuskanen JO, Rautava P, Kytö V. Epidemiology of Guillain-Barré syndrome in Finland 2004–2014. J Peripher Nerv Syst. 2017;22(4):440–445. doi: 10.1111/jns.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanada K, Matsui N, Nodera H, et al. Guillain-Barré syndrome in a local area in Japan, 2006–2015: an epidemiological and clinical study of 108 patients. J Neurolog Sci. 2017;381:371. doi: 10.1111/ene.13569. [DOI] [PubMed] [Google Scholar]
- 26.Kulkantrakorn K, Sukphulloprat P. Outcome of Guillain-Barré syndrome in tertiary care centers in Thailand. J Clin Neuromuscul Dis. 2017;19(2):51–56. doi: 10.1097/CND.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Xiao Z, Lou M, et al. Guillain-Barré syndrome in southern China: retrospective analysis of hospitalised patients from 14 provinces in the area south of the Huaihe river. J Neurol Neurosurg Psychiatry. 2018;89(6):618–626. doi: 10.1136/jnnp-2017-316930. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Sung IY, Rew IS. Clinical presentation and prognosis of childhood Guillain-Barré syndrome. J Paediatr Child Health. 2008;44(7–8):449–454. doi: 10.1111/j.1440-1754.2008.01325.x. [DOI] [PubMed] [Google Scholar]
- 29.Rivera-Lillo G, Torres-Castro R, Burgos PI, et al. Incidence of Guillain-Barré syndrome in Chile: a population-based study. J Peripher Nerv Syst. 2016;21(4):339–344. doi: 10.1111/jns.12182. [DOI] [PubMed] [Google Scholar]
- 30.Alzaidi MA, Nouri KA. Guillain-Barre syndrome. Pattern of muscle weakness. Neurosciences (Riyadh) 2002;7(3):176–178. [PubMed] [Google Scholar]
- 31.Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10(9):643–651. doi: 10.1016/S1473-3099(10)70140-7. [DOI] [PubMed] [Google Scholar]
- 32.Islam B, Islam Z, Geurtsvankessel CH, et al. Guillain-Barré syndrome following varicella-zoster virus infection. Eur J Clin Microbiol Infect Dis. 2018;37(3):511–518. doi: 10.1007/s10096-018-3199-5. [DOI] [PubMed] [Google Scholar]
- 33.Esposito S, Longo MR. Guillain-Barré syndrome. Autoimmun Rev. 2017;16(1):96–101. doi: 10.1016/j.autrev.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Ishaque T, Islam MB, Ara G, et al. High mortality from Guillain-Barré syndrome in Bangladesh. J Peripher Nerv Syst. 2017;22(2):121–126. doi: 10.1111/jns.12215. [DOI] [PubMed] [Google Scholar]
- 35.Dourado ME, Félix RH, da Silva WK, et al. Clinical characteristics of Guillain-Barré syndrome in a tropical country: a Brazilian experience. Acta Neurol Scand. 2012;125(1):47–53. doi: 10.1111/j.1600-0404.2011.01503.x. [DOI] [PubMed] [Google Scholar]
- 36.Sri-Udomkajorn S, Suwannachote S, Demographics SS. Demographics, clinical features, outcome and prognostic factors of Guillain-Barre syndrome in Thai children. J Med Assoc Thai. 2014;97(Suppl 6):S101–S107. [PubMed] [Google Scholar]
- 37.Amin B, Meghnathi H, Gajjar MD, et al. Impact of electrophysiological and clinical variants, and timing of plasmapheresis on outcome of Guillain-Barré syndrome. J Assoc Physicians India. 2017;65(11):14–15. [PubMed] [Google Scholar]
- 38.Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92(3):467–479. doi: 10.1016/j.mayocp.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Salehiomran MR, Nikkhah A, Mahdavi M. Prognosis of Guillain-Barré syndrome in children. Iran J Child Neurol. 2016;10(2):38. [PMC free article] [PubMed] [Google Scholar]
- 40.Kalita J, Kumar M, Misra UK. Prospective comparison of acute motor axonal neuropathy and acute inflammatory demyelinating polyradiculoneuropathy in 140 children with Guillain-Barré syndrome in India. Muscle Nerve. 2018;57(5):761–765. doi: 10.1002/mus.25992. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Lang W, Zhang Y, Ma X, Zhou C, Zhang HL. Long-term prognosis of Guillain-Barré syndrome not determined by treatment options? Oncotarget. 2017;8(45):79991. doi: 10.18632/oncotarget.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barzegar M, Toopchizadeh V, Maher MHK, Sadeghi P, Jahanjoo F, Pishgahi A. Predictive factors for achieving independent walking in children with Guillain-Barre syndrome. Pediatr Res. 2017;82(2):333–339. doi: 10.1038/pr.2017.67. [DOI] [PubMed] [Google Scholar]
- 43.Jo YS, Choi JY, Chung H, Kim Y, Na SJ. Recurrent Guillain-Barre syndrome following urinary tract infection by Escherichia coli. J Korean Med Sci. 2017;33(4):e29. doi: 10.3346/jkms.2018.33.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu MH, Chen CM, Lin KL, et al. Risk factors of respiratory failure in children with Guillain-Barré syndrome. Pediatr Neonatol. 2012;53(5):295–299. doi: 10.1016/j.pedneo.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Bhargava A, Banakar BF, Pujar GS, Khichar S. A study of Guillain-Barré syndrome with reference to cranial neuropathy and its prognostic implication. J Neurosci Rural Pract. 2014;5(Suppl 1):S43–S47. doi: 10.4103/0976-3147.145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan J, Zhang J, Zhang B, Hu W. The clinical features of patients concurrent with Guillain-Barre syndrome and myasthenia gravis. Neurosciences (Riyadh) 2018;23(1):66–70. doi: 10.17712/nsj.2018.1.20170209. [DOI] [PMC free article] [PubMed] [Google Scholar]