Abstract
Aims/Introduction
Research has proved a correlation between glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and gastrointestinal adverse events. Predominantly, nausea and vomiting are frequent gastrointestinal adverse events that lead to the discontinuation of GLP‐1 RAs treatment. The present study aims to investigate clinical factors related to nausea and vomiting, considering diabetic complications and agents affecting the gastrointestinal tract, such as proton pump inhibitors (PPIs) and histamine‐2 receptor antagonists (H2RAs), in patients with type 2 diabetes treated with GLP‐1 RAs.
Materials and Methods
This retrospective study included Japanese patients with type 2 diabetes who started receiving GLP‐1 RAs therapy. We assessed nausea and vomiting up to 48 weeks after treatment with GLP‐1 RAs and used Fine–Gray's proportional hazards model to investigate clinical factors related to nausea and vomiting.
Results
A total of 130 patients were included in this study. Patients with PPIs or H2RAs showed a higher incidence of nausea and vomiting at 48 weeks than those without PPIs or H2RAs. The multivariate analysis revealed that female sex, retinopathy and treatment with PPIs or H2RAs were statistically significant risk factors for nausea and vomiting. Analysis of patients without PPIs or H2RAs showed that female sex and retinopathy were also statistically significant risk factors.
Conclusions
The present study showed a significant correlation of PPIs or H2RAs, female sex, and diabetic retinopathy with nausea and vomiting in patients with type 2 diabetes treated with GLP‐1 RAs. Hence, the occurrence of nausea and vomiting in patients with these factors warrants attention.
Keywords: Glucagon‐like peptide‐1 receptor agonists, Nausea, Vomiting
Introduction
Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) improve glycemic control because of their ability to promote insulin secretion in a glucose concentration‐dependent manner and inhibit glucagon secretion without the risk of hypoglycemia1, 2. In addition, they show diverse physiological effects, including weight loss by a decrease in appetite and suppression of cardiovascular events2, 3, 4, 5, 6, 7. Research has established the safety and efficacy of GLP‐1 RAs, and they are widely used for the treatment of type 2 diabetes in the clinical practice8.
The major adverse events (AEs) of GLP‐1 RAs are gastrointestinal (GI) disorders, including nausea, vomiting and diarrhea, which are perhaps caused by the delay of gastric emptying because of GI motility suppression or centrally mediated effects1, 9, 10. GI AEs caused by GLP‐1 RAs occur in a dose‐dependent manner; however, they are alleviated by treatment with lower doses of GLP‐1 RAs and gradually increasing the doses11, 12, 13. Furthermore, other studies have reported that GI AEs depend on short‐ or long‐acting characteristics of GLP‐1 RAs13, 14, 15, implying that GI AEs are associated with delayed gastric emptying in the short‐acting GLP‐1 RAs1. However, some patients experience GI AEs despite a gradual increase in the dose and being administered the long‐acting GLP‐1 RAs. As factors focused on patients’ clinical background, GI AEs are known to be positively associated with age, renal function and background of glucose‐lowering medication13, 16, 17, 18, 19. Although the studies discussed above have shown the importance of identifying the risk factors for GI AEs, agents affecting GI tracts, including proton pump inhibitors (PPIs) and histamine‐2 receptor antagonists (H2RAs), have not been considered. In addition, whether diabetic complications, which are characteristic of diabetes, could predict the risk factor for GI AEs remains unclear. Among GI AEs, nausea and vomiting (nausea/vomiting) have been recognized as frequent AEs that lead to the discontinuation of GLP‐1 RAs treatment20, 21. Hence, exploring factors that can predict the occurrence of nausea/vomiting is imperative. The present study aims to determine the risk factors for nausea/vomiting, considering diabetic complications and agents affecting the GI tract in patients with type 2 diabetes treated with GLP‐1 RAs.
Methods
Study design and participants
The present retrospective study included Japanese patients with type 2 diabetes who started receiving GLP‐1 RAs therapy (e.g., liraglutide or lixisenatide) at Kitasato University Medical Center (Kitamoto, Japan) between November 2010 and July 2017. First, liraglutide was administered at a dose of 0.3 mg once daily for 1 week or longer, followed by an increment in the dose to 0.6 mg once daily for 1 week or longer and, finally, to 0.9 mg once daily depending on patients’ conditions, which is the maximum dose approved in Japan. Then, lixisenatide was administered at a dose of 10 μg once daily for 1 week or longer, followed by the dose increment to 15 μg once daily for 1 week or longer and, finally, 20 μg once daily depending on patients’ conditions. Oral hypoglycemic agents, except dipeptidyl peptidase‐4 inhibitors, and insulin therapy were continued after administering GLP‐1 RAs. Patients who discontinued GLP‐1 RAs therapy for reasons other than nausea/vomiting without increasing the dose, as it is proved to be GI AEs of GLP‐1 RAs that occurs in a dose‐independent manner11, 12, 13, were excluded from the study. Other exclusion criteria of the study were as follows: (i) administration of other GLP‐1 RAs before starting liraglutide or lixisenatide; (ii) use of anti‐emetics; (iii) showing poor drug compliance based on regular prescribing from their medical records; and (iv) the presence of malignancy. The investigation lasted up to 48 weeks after GLP‐1 RAs treatment. This study was approved by the ethics committee of Kitasato University Medical Center, and was carried out in accordance with the principles of the Declaration of Helsinki.
Parameters evaluated
We collected the patients’ baseline characteristics using medical records, including sex, age, duration of diabetes, body mass index, glycated hemoglobin, systolic and diastolic blood pressure, aspartate aminotransferase, alanine aminotransferase, estimated glomerular filtration rate (eGFR), creatinine clearance, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, diabetes complications (such as retinopathy and nephropathy), and the use of oral hypoglycemic agents, insulin and agents affecting the GI tract. The development of nausea/vomiting was extracted from the medical records, which were confirmed on examination. In addition, the glycated hemoglobin values were recorded as National Glycohemoglobin Standardization Program values; if recorded as the Japan Diabetes Society values, they were converted into National Glycohemoglobin Standardization Program values22. While eGFR was calculated using the Japan Nephrology Society equation23, creatinine clearance was estimated using the Cockcroft–Gault equation24. We defined diabetic retinopathy as simple retinopathy or more, and the presence and severity of diabetic retinopathy were determined by a qualified ophthalmologist. Diabetic nephropathy was defined as the urine albumin : creatinine ratio ≥30 mg/g creatinine and/or eGFR <30 mL/min/1.73 m2 25.
Statistical analysis
In the present study, data are expressed as the mean ± standard deviation, median and interquartile range (IQR) or numbers and percentages. We compared the difference between the two groups using the Student's t‐test or the Mann–Whitney U‐test for continuous variables. Categorical variables were compared using the Fisher's exact test. The cumulative incidence of nausea/vomiting was estimated using the Fine–Gray method26, and compared using the Gray's test. In addition, any discontinuation of GLP‐1 RAs was considered a competing risk in the analysis. Univariate analysis was carried out using the Fine–Gray's proportional hazards model to determine the predictor of nausea/vomiting. Using univariate analysis, we determined factors with P < 0.10 to be potential risk factors for nausea/vomiting and further investigated these factors using multivariate analysis. If the factors determined by univariate analysis were continuous variables, multivariate analysis was carried out after obtaining the cut‐off values using the receiver operating characteristic curve analysis to evaluate the performance of the prognostic parameters predicting nausea/vomiting. Pearson's correlation coefficient was used to measure collinearity. Statistical analyses were carried out using the R software (version 3.4.1; The R Foundation for Statistical Computing, Vienna, Austria)27. We considered P < 0.05 to be statistically significant.
Results
During the study period, liraglutide and lixisenatide therapy was given to 181 patients. We excluded nine patients who discontinued GLP‐1 RAs for reasons other than nausea/vomiting without increasing its dose, 13 patients who were administered GLP‐1 RAs other than liraglutide or lixisenatide in the beginning, one patient who used an anti‐emetic, six patients who showed poor drug compliance and one patient with malignancy. In addition, 21 patients were excluded because of incomplete data. In total, 130 patients, who were administered liraglutide and lixisenatide, were included in the present study. The median follow‐up period was 48 weeks (IQR 20–48 weeks). Table 1 presents the demographic and clinical characteristics of 130 patients at the baseline. The mean age of the study population was 56.8 ± 13.3 years, and the mean duration of diabetes was 12.2 ± 9.6 years. Diabetic retinopathy and nephropathy were 37.7 and 41.5%, respectively. During the previous antidiabetic treatment, metformin was the most frequently used drug (41.5%), and its median dose was 875 mg (IQR 750–1,500 mg). In the present study, 14.6% of all the patients were treated with PPIs or H2RAs as agents affecting the GI tract. The therapeutic targets with PPIs or H2RAs in this study were gastroesophageal reflux disease (GERD), non‐steroidal anti‐inflammatory drugs (NSAIDs)‐induced gastropathy and gastric ulcer (GU). Before receiving GLP‐1 RAs treatment, symptoms of nausea/vomiting were controlled by PPIs or H2RAs. The median doses of liraglutide and lixisenatide at the occurrence of nausea/vomiting were 0.6 mg (IQR 0.3–0.6 mg) and 10 μg (IQR 10–15 μg), respectively. At the last follow up, the median doses of liraglutide and lixisenatide were 0.9 mg (IQR 0.75–0.9 mg) and 15 μg (IQR 15–20 μg), respectively. Table 1 also shows the demographic and clinical characteristics of patients in their respective groups (with and without nausea/vomiting). Furthermore, 34.6% of all patients experienced nausea/vomiting during the follow‐up period. Patients with nausea/vomiting comprised a significantly high number of women (P = 0.026) and had a higher occurrence of diabetic retinopathy (P = 0.013) than those without nausea/vomiting.
Table 1.
Baseline demographic and clinical characteristics of the patients
| Total | Nausea and vomiting (−) | Nausea and vomiting (+) | P‐value | |
|---|---|---|---|---|
| n (Liraglutide/lixisenatide) | 130 (83 / 47) | 85 (59 / 26) | 45 (24 / 21) | |
| Sex (% male) | 45.4 | 52.9 | 31.1 | 0.026 |
| Age (years) | 56.8 ± 13.3 | 56.3 ± 13.7 | 57.8 ± 12.6 | 0.529 |
| Duration of diabetes (years) | 12.2 ± 9.6 | 11.5 ± 9.3 | 13.4 ± 10.1 | 0.309 |
| BMI (kg/m2) | 27.5 (24.6–32.2) | 27.8 (25.4–32.3) | 25.9 (24.1–31.1) | 0.292 |
| HbA1c (%) | 9.0 ± 1.7 | 9.0 ± 1.7 | 8.9 ± 1.8 | 0.673 |
| SBP (mmHg) | 133.9 ± 18.8 | 133.8 ± 17.4 | 134.2 ± 21.2 | 0.899 |
| DBP (mmHg) | 75.4 ± 13.5 | 75.2 ± 13.5 | 75.8 ± 13.6 | 0.796 |
| AST (U/L) | 22 (17–31) | 21 (17–29) | 23 (17–32) | 0.448 |
| ALT (U/L) | 24 (17–39) | 24 (17–39) | 24 (20–37) | 0.609 |
| eGFR (mL/min/1.73 m2) | 75.1 ± 20.5 | 77.0 ± 19.5 | 71.5 ± 22 | 0.146 |
| CrCl (mL/min) | 110.7 ± 54.0 | 116.2 ± 55.6 | 100.3 ± 49.8 | 0.140 |
| HDL cholesterol (mmol/L) | 50.8 ± 11.1 | 50.1 ± 10.8 | 52.1 ± 11.7 | 0.350 |
| LDL cholesterol (mmol/L) | 115.2 ± 31.6 | 118.4 ± 31.5 | 109.1 ± 31.1 | 0.111 |
| TG (mmol/L) | 145 (99–227) | 146 (99–228) | 144 (102–214) | 0.619 |
| Diabetes complications (%) | ||||
| Retinopathy | 37.7 | 29.4 | 53.3 | 0.013 |
| Nephropathy | 41.5 | 37.6 | 48.9 | 0.160 |
| Previous antidiabetic treatment (%) | ||||
| Diet only | 14.6 | 17.6 | 8.9 | 0.204 |
| Sulfonylureas | 39.2 | 40.0 | 37.8 | 0.852 |
| Metformin | 41.5 | 41.2 | 42.2 | 1 |
| Glinides | 6.2 | 5.9 | 6.7 | 1 |
| α‐Glycosidase inhibitors | 15.4 | 14.1 | 17.8 | 0.615 |
| Pioglitazone | 8.5 | 10.6 | 4.4 | 0.328 |
| DPP‐4 inhibitors | 27.7 | 24.7 | 33.3 | 0.310 |
| Insulin | 29.2 | 30.6 | 26.7 | 0.690 |
| Treatment with PPIs or H2RAs (%) | 14.6 | 10.6 | 22.2 | 0.115 |
Data are expressed as the mean ± standard deviation, median and interquartile range or numbers and percentages. The statistical significance was estimated using Student's t‐test or the Mann–Whitney U‐test for continuous variables, and Fisher's exact test for categorical variables. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CrCl, creatinine clearance; DBP, diastolic blood pressure; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; H2RAs, histamine‐2 receptor antagonists; LDL, low‐density lipoprotein; PPIs, proton pump inhibitors; SBP, systolic blood pressure; TG, triglyceride.
Figure 1 shows the results of the cumulative incidence of nausea/vomiting in the present study using the Fine–Gray method26, accounting for the competing risk of any discontinuation of GLP‐1 RAs. During the 48‐week follow‐up period, the total cumulative incidence was 35.8%. More than 90% of patients developed nausea/vomiting by 8 weeks after the initiation of GLP‐1 RAs (Figure 1a). Analysis of with or without treatment with PPIs or H2RAs showed a higher incidence rate of nausea/vomiting among patients with PPIs or H2RAs than in those without PPIs or H2RAs, the cumulative incidence rate of nausea/vomiting of 52.6% at 48 weeks in patients with PPIs or H2RAs, and 32.9% in patients without PPIs or H2RAs (Gray's test, P = 0.06; Figure 1b). Table 2 shows the results of univariate analysis using the Fine–Gray models. Nausea/vomiting was associated with sex (women hazard ratio [HR] 1.95, 95% confidence interval [CI] 1.10–3.45; P = 0.023), retinopathy (HR 1.99, 95% CI: 1.18–3.35; P = 0.009) and treatment with PPIs or H2RAs (HR 1.78, 95% CI: 0.99–3.21; P = 0.053). In multivariate analysis, the risks of nausea/vomiting included sex (women HR, 2.08, 95% CI: 1.18–3.65; P = 0.011), retinopathy (HR 1.89, 95% CI: 1.13–3.15; P = 0.016) and treatment with PPIs or H2RAs (HR 2.05, 95% CI: 1.12–3.74; P = 0.020). We observed no significant differences in the incidence of nausea/vomiting in the previous antidiabetic treatment. In addition, no significant differences were noted in antidiabetic treatment 8 weeks after the initiation of GLP‐1 RAs. We carried out the subgroup analysis to assess the incidence of nausea/vomiting using the Fine–Gray models by assigning patients to either of the two groups: patients treated with liraglutide and lixisenatide. In patients treated with liraglutide, sex, diabetic nephropathy and retinopathy were the determining factors (P < 0.10; Table S1). The treatment with PPIs or H2RAs was more likely to increase the risk of nausea/vomiting (without nausea/vomiting 11.9%, with nausea/vomiting 20.8%; HR 1.56, 95% CI: 0.68–3.57), although it was not a potential risk factor (P < 0.10). In patients treated with lixisenatide, the treatment with PPIs or H2RAs was the determining factor (P < 0.10; Table S2). Sex and diabetic retinopathy were more likely to increase the risk of nausea/vomiting (women without nausea/vomiting 50%; women with nausea/vomiting 61.9%, HR 1.31, 95% CI: 0.60–2.88; retinopathy without nausea/vomiting 42.3%, retinopathy with nausea/vomiting 61.9%, HR 1.71, 95% CI: 0.77–3.80), although these were not potential risk factors (P < 0.10). Of note, we did not carry out the multivariate analysis because of the limited number of participants in the present study28.
Figure 1.
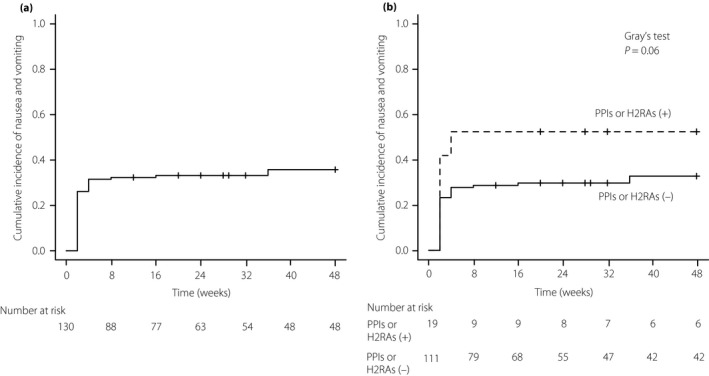
Cumulative incidence of nausea/vomiting caused by glucagon‐like peptide‐1 receptor agonists in type 2 diabetes patients. (a) Total (b) with or without treatment with proton pump inhibitors (PPIs) or histamine‐2 receptor antagonists (H2RAs), PPIs or H2RAs (+), PPIs or H2RAs (−). Cumulative incidence of nausea/vomiting was determined using the Fine–Gray method. The P‐value was determined using Gray's test.
Table 2.
Risk factors for nausea and vomiting as assessed by Fine–Gray's proportional hazards model
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Sex (female) | 1.95 | 1.10–3.45 | 0.023 | 2.08 | 1.18–3.65 | 0.011 |
| Diabetic retinopathy | 1.99 | 1.18–3.35 | 0.009 | 1.89 | 1.13–3.15 | 0.016 |
| Treatment with PPIs or H2RAs | 1.78 | 0.99–3.21 | 0.053 | 2.05 | 1.12–3.74 | 0.020 |
CI, confidence interval; HR, hazard ratio; H2RAs, histamine‐2 receptor antagonists; PPIs, proton pump inhibitors.
Table 3 shows the results of univariate analysis using the Fine–Gray models for patients without treatment with PPIs or H2RAs. Nausea/vomiting was associated with sex (women HR 2.63, 95% CI: 1.26–5.51; P = 0.01), eGFR (HR 0.98, 95% CI: 0.97–0.99; P = 0.005) and retinopathy (HR 2.13, 95% CI: 1.17–3.89; P = 0.014). In multivariate analysis, the risks of nausea/vomiting included sex (women HR 2.49, 95% CI: 1.20–5.18; P = 0.015) and retinopathy (HR 2.00, 95% CI: 1.11–3.63; P = 0.022; Table 3). In the subgroup analysis, systolic blood pressure, high‐density lipoprotein cholesterol, sex and diabetic retinopathy were the determining factors (P < 0.10) in patients treated with liraglutide (Table S3). In patients treated with lixisenatide, eGFR was a determining factor (P < 0.10; Table S4). Both sex and diabetic retinopathy were more likely to increase the risk for nausea/vomiting (women without nausea/vomiting 48.0%, women with nausea/vomiting 73.3%, HR 2.12, 95% CI: 0.72–6.23; retinopathy without nausea/vomiting 40.0%, retinopathy with nausea/vomiting 66.7%, HR 2.31; 95% CI: 0.83–6.39), although these were not potential risk factors (P < 0.10). The multivariate analysis was not carried out because of the limited number of participants in the present study28. Figure 2 shows the cumulative incidence of nausea/vomiting in sex and retinopathy (women and retinopathy 61.0%, women and no retinopathy 32.9%, men and retinopathy 25.0%) using the Fine–Gray method26, accounting for the competing risk of any discontinuation of GLP‐1 RAs. We observed a significantly higher incidence of nausea/vomiting among patients with female sex and retinopathy than those with other factors (Gray's test, P = 0.004).
Table 3.
Receiver operating characteristic curve analysis and risk factors for nausea and vomiting in patients without treatment with proton pump inhibitors or histamine‐2 receptor antagonists as assessed by Fine–Gray's proportional hazards model
| Univariate | ROC | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | Cut‐off value | AUC | 95% CI | HR | 95% CI | P‐value | |
| Sex (female) | 2.63 | 1.26–5.51 | 0.010 | 2.49 | 1.20–5.18 | 0.015 | |||
| eGFR (mL/min/1.73 m2) | 0.98 | 0.97–0.99 | 0.005 | 75.3 | 0.614 | 0.50–0.72 | 0.77 | 0.42–1.44 | 0.420 |
| Diabetic retinopathy | 2.13 | 1.17–3.89 | 0.014 | 2.00 | 1.11–3.63 | 0.022 | |||
AUC, area under the curve; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; H2RAs, histamine‐2 receptor antagonists; PPIs, proton pump inhibitors; ROC, receiver operating characteristic.
Figure 2.
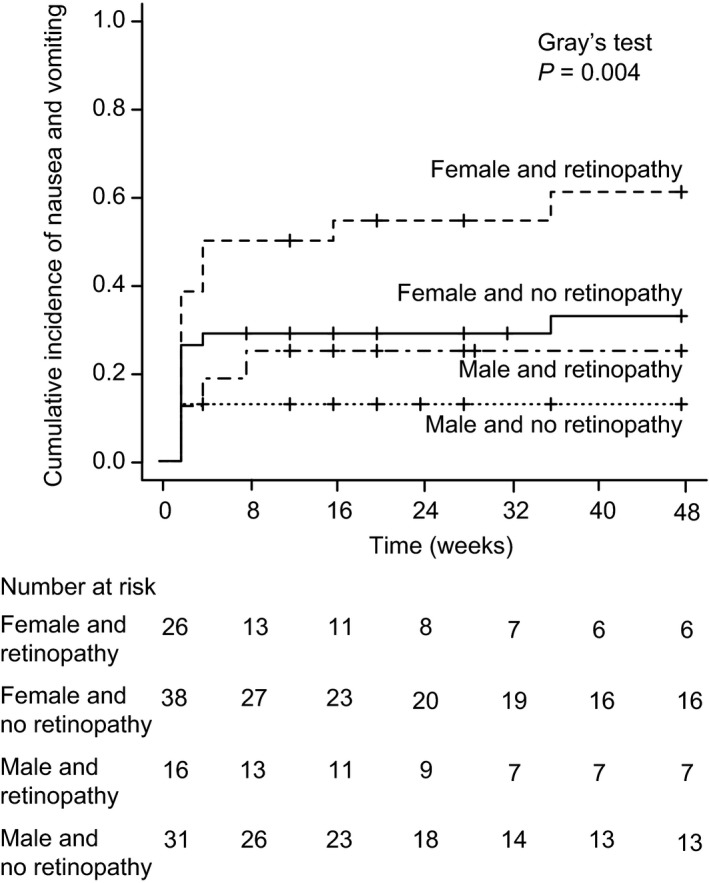
Cumulative incidence of nausea/vomiting caused by glucagon‐like peptide‐1 receptor agonists by sex and retinopathy in type 2 diabetes patients without proton pump inhibitors (PPIs) or histamine‐2 receptor antagonists (H2RAs) treatment. The cumulative incidence of nausea/vomiting was determined using the Fine–Gray method. The P‐value was determined using Gray's test.
Discussion
The present study suggests that treatment with PPIs or H2RAs, female sex and diabetic retinopathy are risk factors for nausea/vomiting in patients with type 2 diabetes treated with GLP‐1 RAs. In addition, female sex and diabetic retinopathy are recognized as risk factors in patients not treated with PPIs or H2RAs. To the best of our knowledge, this is the first study to investigate the risk factors for nausea/vomiting considering diabetic complications and agents affecting the GI tract in patients with type 2 diabetes treated with GLP‐1 RAs.
Nausea/vomiting are major symptoms of GERD, NSAIDs‐induced gastropathy and GU, which were the therapeutic targets of PPIs or H2RAs in the present study. GERD is attributed to various conditions, including lower esophageal pressure caused by lower esophageal sphincter relaxation, increased intragastric pressure and increased gastric acid production29, and is also one of the AEs of GLP‐1 RAs. As GLP‐1 RAs primarily inhibit gastric peristalsis through the vagal reflex30, it is hypothesized that the gastric peristalsis‐suppressing effect caused elevated intragastric pressure, potentiating GERD symptoms. Because NSAIDs and GU cause delayed gastric emptying action31, 32, 33, 34, the gastric emptying rate of patients treated with NSAIDs and those with GU might be delayed by GLP‐1 RAs. Regarding the correlation between PPIs and GLP‐1, some studies have reported that the elevation of serum gastrin levels by PPIs stimulates the GLP‐1 secretion35, 36. However, whether indirect GLP‐1 secretion by PPIs affects the development of nausea/vomiting remains unclear. Therefore, symptoms and actions of GERD, NSAIDs and GU, which are therapeutic targets of PPIs or H2RAs, could have been exacerbated by the treatment of GLP‐1 RAs. In the present study, although few patients were endoscopically examined before initiating GLP‐1 RAs, we could not confirm the relationship between GI AEs after initiating GLP‐1 RAs and GERD or GU severity. Further studies are thus required for clarifying these relationships.
Metformin causes GI AEs. Nausea, vomiting and diarrhea caused by GLP‐1 RAs have been observed in background treatment with metformin13. However, no other study has reported any association between metformin and GI AEs37. Thong et al.16 have highlighted that non‐metformin use is associated with more significant GI AEs, leading to the discontinuation of liraglutide treatment. In the present study, metformin used before and after the initiation of GLP‐1 RAs was not a risk factor for nausea/vomiting caused by GLP‐1 RAs. Metformin has been reported to result in GI AEs in a dose‐dependent manner38, but previous studies did not provide details about the dose. However, as patients in the present study used a small dose of metformin, its association with nausea/vomiting was not apparent. In our study, although two patients added metformin after the initiation of GLP‐1 RAs, we did not consider the duration of all metformin use. Furthermore, as it was used only for patients who could tolerate metformin, it might not have been associated with the development of nausea/vomiting.
A previous study has suggested that GI AEs are positively associated with age16. That study reported that eGFR was highly collinear with age, and that it did not achieve significance in multivariate models. However, other studies have reported that GI AEs are positively associated with renal function18, 19, 39. Idorn et al.18 showed that nausea/vomiting occurred in the liraglutide‐treated group with end‐stage renal disease. Hanefeld et al.19 reported that in patients receiving lixisenatide, those with mild renal impairment (eGFR = 60–89 mL/min) were at an increased risk of experiencing any GI AEs compared with patients with normal renal function (eGFR ≥90 mL/min). In addition, Davidson et al.39 observed an increasing trend of nausea among patients with moderate or severe renal impairment (creatinine clearance <60 mL/min) receiving liraglutide. In the present study, we did not confirm collinearity in eGFR and age, and approximately 80% patients showed normal renal function or mild renal impairment. Hence, age and renal function were not related to nausea/vomiting. However, eGFR was significantly associated with nausea/vomiting in univariate analysis. As GLP‐1 RAs show different eliminations depending on their type, the influence of renal impairment also varies. Liraglutide is catabolized in a manner similar to that of large proteins, without a specific organ as the primary route of elimination. Conversely, lixisenatide is eliminated through glomerular filtration with subsequent proteolytic degradation, resulting in smaller peptides and amino acids reintroduced into protein metabolism. In patients receiving liraglutide, it has been reported that renal impairment has not been found to increase the exposure of liraglutide40. However, another study has reported that the plasma liraglutide concentration increased in patients with type 2 diabetes and end‐stage renal disease18. In patients receiving lixisenatide, a significant increase has been reported in the area under the plasma concentration–time curve of lixisenatide in type 2 diabetes patients with severe renal impairment17. Although a causal relationship between GI AEs and GLP‐1 RAs has not been shown, advanced renal dysfunction might be associated with the occurrence of GI AEs because of the elevated blood concentration, necessitating further investigation by considering the type of GLP‐1 RAs.
The present study shows that female sex was a risk factor for nausea/vomiting in patients with type 2 diabetes treated with GLP‐1 RAs. A post‐hoc analysis of GLP‐1 RAs showed that upper GI AEs were more frequent in women than in men37. Regarding GI disorders, a study has reported a higher prevalence of GI symptoms among women than men, regardless of diabetes41. In addition, female patients with non‐insulin‐dependent diabetes have been reported to show a higher prevalence of GI symptoms, especially nausea, than men42. Furthermore, studies have recognized female sex as a risk factor for postoperative and chemotherapy‐induced nausea/vomiting in fields other than diabetes43, 44, 45, 46, 47. Although the underlying reason for female sex being a risk factor for nausea/vomiting is unclear, female sex might be a risk factor for nausea/vomiting caused by GLP‐1 RAs, and it is considered to be necessary when starting therapy with GLP‐1 RAs.
Furthermore, the present study highlighted diabetic retinopathy as a risk factor. Reportedly, nausea and vomiting, diabetic GI motility disorders, are caused by autonomic neuropathy48, and autonomic neuropathy has been associated with delayed gastric emptying in diabetic patients49, 50, 51, 52, 53. Diabetic neuropathy is associated with diabetic retinopathy and nephropathy54, 55, 56. In retinopathy, it is reported that the prevalence of neuropathy increases with the severity of retinopathy57, and that patients with neuropathy with impaired glucose tolerance and impaired fasting glycemia are twofold more likely to have albuminuria, and fourfold more likely to have retinopathy58. A recent study has shown that diabetic retinopathy is a risk factor for constipation, which is a diabetic GI disorder in patients with diabetes59. Hence, diabetes patients with retinopathy also have neuropathy; both neuropathy and GLP‐1RAs could enhance delayed gastric emptying and might have been related to the development of nausea/vomiting. However, the relationship between diabetic retinopathy and nausea/vomiting remains unclear. In the present study, diabetes patients with retinopathy were at significantly higher risk for nausea/vomiting caused by GLP‐1 RAs. Based on these findings and previous studies, diabetes patients with retinopathy might be at a higher risk of nausea/vomiting caused by GLP‐1 RAs through diabetic neuropathy.
The incidence of nausea/vomiting in the present study was consistent with some previous studies of Japanese patients treated with lixisenatide60, 61, 62. However, in patients treated with liraglutide, the present study showed a high incidence of nausea/vomiting compared with previous studies in which the occurrence of GI AEs was reported to be 44–60%, of which nausea occurred in 5–14%63, 64, 65, 66. In addition, symptoms of nausea/vomiting were confirmed by an individual physician's method. Therefore, patients might have complained about upper gastrointestinal AEs, including heartburn, dyspepsia and stomach discomfort as nausea.
Regrettably, our subgroup analysis could not determine risk factors in patients treated with liraglutide and lixisenatide; however, it showed a tendency for female sex and diabetic retinopathy to be risk factors in patients treated with liraglutide, and treatment with PPIs or H2RAs is a risk factor, as well as GLP‐1RAs, in patients treated with lixisenatide. Perhaps, by increasing the sample size, the risk factors for liraglutide and lixisenatide could be determined.
The present study had several limitations. First, we did not evaluate diabetic polyneuropathy, especially autonomic neuropathy by the heart rate variability. There are no symptoms or tests specific to diabetic polyneuropathy, and no diagnostic criteria have been established for obtaining international consensus. Definitions of minimal criteria for typical diabetic polyneuropathy proposed by the Toronto Diabetic Neuropathy Expert Group have high relevance and can be used in daily clinical practice67; however, in the present study, only the presence of symptoms or signs of diabetic polyneuropathy, only ankle reflexes, only nerve conduction or their combination existed. Hence, we could not confirm diabetic polyneuropathy. However, considering the result of the present study, the application of the assessment according to certain criteria of diabetic polyneuropathy would be beneficial to ensure risk factors for nausea/vomiting in patients with type 2 diabetes treated with GLP‐1 RAs. Second, the present study was retrospective. Accordingly, nausea and vomiting were confirmed by individual physicians’ methods at the time of examination without specific criteria for appropriate assessment. Perhaps this limitation might have introduced some bias, and thus a prospective study with predefined criteria of nausea/vomiting is warranted. Finally, the present study comprised a limited sample size. Accordingly, an extensive prospective study with a larger sample size is required to confirm the present results.
In conclusion, the present study highlights that agents affecting the GI tract, such as PPIs or H2RAs, are risk factors for nausea/vomiting in patients with type 2 diabetes treated with GLP‐1 RAs. Furthermore, we determined that female sex and diabetic retinopathy are risk factors for nausea/vomiting in patients with type 2 diabetes treated with GLP‐1 RAs regardless of treatment with PPIs or H2RAs. Nevertheless, further extensive research is required; it is necessary to pay attention to the occurrence of nausea/vomiting when receiving GLP‐1 RAs for patients with these factors. The results of the present study are expected to be helpful to clinicians administering GLP‐1 RAs.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Risk factors for nausea and vomiting in patients treated with liraglutide as assessed by the Fine–Gray's proportional hazards model.
Table S2 ¦ Risk factors for nausea and vomiting in patients treated with lixisenatide as assessed by the Fine–Gray's proportional hazards model.
Table S3 ¦ Risk factors for nausea and vomiting in patients treated with liraglutide and without treatment with proton pump inhibitors or histamine‐2 receptor antagonists as assessed by the Fine–Gray's proportional hazards model.
Table S4 ¦ Risk factors for nausea and vomiting in patients treated with lixisenatide and without treatment with proton pump inhibitors or histamine‐2 receptor antagonists as assessed by the Fine–Gray's proportional hazards model.
J Diabetes Investig 2019; 10: 408–417
References
- 1. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- 2. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 3. van Can J, Sloth B, Jensen CB, et al Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond) 2014; 38: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Bloemendaal L, Ten Kulve JS, la Fleur SE, et al Effects of glucagon‐like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol 2014; 221: T1–T16. [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer MA, Claggett B, Diaz R, et al Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373: 2247–2257. [DOI] [PubMed] [Google Scholar]
- 6. Marso SP, Bain SC, Consoli A, et al Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 7. Marso SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran KL, Park YI, Pandya S, et al Overview of glucagon‐like peptide‐1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits 2017; 10: 178–188. [PMC free article] [PubMed] [Google Scholar]
- 9. Dalvi PS, Nazarians‐Armavil A, Purser MJ, et al Glucagon‐like peptide‐1 receptor agonist, exendin‐4, regulates feeding‐associated neuropeptides in hypothalamic neurons in vivo and in vitro . Endocrinology 2012; 153: 2208–2222. [DOI] [PubMed] [Google Scholar]
- 10. Jones KL, Russo A, Stevens JE, et al Predictors of delayed gastric emptying in diabetes. Diabetes Care 2001; 24: 1264–1269. [DOI] [PubMed] [Google Scholar]
- 11. Nauck M, Frid A, Hermansen K, et al Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fineman MS, Shen LZ, Taylor K, et al Effectiveness of progressive dose‐escalation of exenatide (exendin‐4) in reducing dose‐limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev 2004; 20: 411–417. [DOI] [PubMed] [Google Scholar]
- 13. Bettge K, Kahle M, Abd El Aziz MS, et al Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes Metab 2017; 19: 336–347. [DOI] [PubMed] [Google Scholar]
- 14. Buse JB, Rosenstock J, Sesti G, et al Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 15. Sun F, Chai S, Yu K, et al Gastrointestinal adverse events of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Technol Ther 2015; 17: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thong KY, Gupta PS, Blann AD, et al The influence of age and metformin treatment status on reported gastrointestinal side effects with liraglutide treatment in type 2 diabetes. Diabetes Res Clin Pract 2015; 109: 124–129. [DOI] [PubMed] [Google Scholar]
- 17. Scheen AJ. Pharmacokinetics and clinical use of incretin‐based therapies in patients with chronic kidney disease and type 2 diabetes. Clin Pharmacokinet 2015; 54: 1–21. [DOI] [PubMed] [Google Scholar]
- 18. Idorn T, Knop FK, Jorgensen MB, et al Safety and efficacy of liraglutide in patients with type 2 diabetes and end‐stage renal disease: an investigator‐initiated, placebo‐controlled, double‐blind, parallel‐group, randomized trial. Diabetes Care 2016; 39: 206–213. [DOI] [PubMed] [Google Scholar]
- 19. Hanefeld M, Arteaga JM, Leiter LA, et al Efficacy and safety of lixisenatide in patients with type 2 diabetes and renal impairment. Diabetes Obes Metab 2017; 19: 1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sikirica MV, Martin AA, Wood R, et al Reasons for discontinuation of GLP1 receptor agonists: data from a real‐world cross‐sectional survey of physicians and their patients with type 2 diabetes. Diabetes Metab Syndr Obes 2017; 10: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu S, Chai S, Yang J, et al Gastrointestinal adverse events of dipeptidyl peptidase 4 inhibitors in type 2 diabetes: a systematic review and network meta‐analysis. Clin Ther 2017; 39: 1780–1789. [DOI] [PubMed] [Google Scholar]
- 22. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 24. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 25. Haneda M, Utsunomiya K, Koya D, et al A new classification of diabetic nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy. J Diabetes Investig 2015; 6: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 27. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peduzzi P, Concato J, Kemper E, et al A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49: 1373–1379. [DOI] [PubMed] [Google Scholar]
- 29. Usai Satta P, Oppia F, Cabras F. Overview of pathophysiological features of GERD. Minerva Gastroenterol Dietol 2017; 63: 184–197. [DOI] [PubMed] [Google Scholar]
- 30. Imeryuz N, Yegen BC, Bozkurt A, et al Glucagon‐like peptide‐1 inhibits gastric emptying via vagal afferent‐mediated central mechanisms. Am J Physiol 1997; 273: G920–G927. [DOI] [PubMed] [Google Scholar]
- 31. Kechagias S, Jonsson KA, Norlander B, et al Low‐dose aspirin decreases blood alcohol concentrations by delaying gastric emptying. Eur J Clin Pharmacol 1997; 53: 241–246. [DOI] [PubMed] [Google Scholar]
- 32. Kulkarni SG, Parikh SS, Shankhpal PD, et al Gastric emptying of solids in long‐term NSAID users: correlation with endoscopic findings and Helicobacter pylori status. Am J Gastroenterol 1999; 94: 382–386. [DOI] [PubMed] [Google Scholar]
- 33. Koch TR, Petro A, Darrabie M, et al Effects of esomeprazole magnesium on nonsteroidal anti‐inflammatory drug gastropathy. Dig Dis Sci 2005; 50: 86–93. [DOI] [PubMed] [Google Scholar]
- 34. Harasawa S, Tani N, Suzuki S, et al Gastric emptying in normal subjects and patients with peptic ulcer: a study using the acetaminophen method. Gastroenterol Jpn 1979; 14: 1–10. [DOI] [PubMed] [Google Scholar]
- 35. Cao Y, Cao X, Liu XM. Expression of cholecystokinin2‐receptor in rat and human L cells and the stimulation of glucagon‐like peptide‐1 secretion by gastrin treatment. Acta Histochem 2015; 117: 205–210. [DOI] [PubMed] [Google Scholar]
- 36. Takebayashi K, Inukai T. Effect of proton pump inhibitors on glycemic control in patients with diabetes. World J Diabetes 2015; 6: 1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horowitz M, Aroda VR, Han J, et al Upper and/or lower gastrointestinal adverse events with glucagon‐like peptide‐1 receptor agonists: Incidence and consequences. Diabetes Obes Metab 2017; 19: 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonnet F, Scheen A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes Metab 2017; 19: 473–481. [DOI] [PubMed] [Google Scholar]
- 39. Davidson JA, Brett J, Falahati A, et al Mild renal impairment and the efficacy and safety of liraglutide. Endocr Pract 2011; 17: 345–355. [DOI] [PubMed] [Google Scholar]
- 40. Jacobsen LV, Hindsberger C, Robson R, et al Effect of renal impairment on the pharmacokinetics of the GLP‐1 analogue liraglutide. Br J Clin Pharmacol 2009; 68: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bytzer P, Talley NJ, Leemon M, et al Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population‐based survey of 15,000 adults. Arch Intern Med 2001; 161: 1989–1996. [DOI] [PubMed] [Google Scholar]
- 42. Oh JH, Choi MG, Kang MI, et al The prevalence of gastrointestinal symptoms in patients with non‐insulin dependent diabetes mellitus. Korean J Intern Med 2009; 24: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gan TJ, Diemunsch P, Habib AS, et al Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2014; 118: 85–113. [DOI] [PubMed] [Google Scholar]
- 44. Pollera CF, Giannarelli D. Prognostic factors influencing cisplatin‐induced emesis. Definition and validation of a predictive logistic model. Cancer 1989; 64: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 45. du Bois A, Meerpohl HG, Vach W, et al Course, patterns, and risk‐factors for chemotherapy‐induced emesis in cisplatin‐pretreated patients: a study with ondansetron. Eur J Cancer 1992; 28: 450–457. [DOI] [PubMed] [Google Scholar]
- 46. Osoba D, Zee B, Pater J, et al Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1997; 15: 116–123. [DOI] [PubMed] [Google Scholar]
- 47. Roscoe JA, Bushunow P, Morrow GR, et al Patient expectation is a strong predictor of severe nausea after chemotherapy: a University of Rochester Community Clinical Oncology Program study of patients with breast carcinoma. Cancer 2004; 101: 2701–2708. [DOI] [PubMed] [Google Scholar]
- 48. Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil 2014; 26: 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bharucha AE, Camilleri M, Low PA, et al Autonomic dysfunction in gastrointestinal motility disorders. Gut 1993; 34: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merio R, Festa A, Bergmann H, et al Slow gastric emptying in type I diabetes: relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care 1997; 20: 419–423. [DOI] [PubMed] [Google Scholar]
- 51. Stacher G, Lenglinger J, Bergmann H, et al Impaired gastric emptying and altered intragastric meal distribution in diabetes mellitus related to autonomic neuropathy? Dig Dis Sci 2003; 48: 1027–1034. [DOI] [PubMed] [Google Scholar]
- 52. Kotani K, Kawabe J, Kawamura E, et al Clinical assessment of delayed gastric emptying and diabetic complications using gastric emptying scintigraphy: involvement of vascular disorder. Clin Physiol Funct Imaging 2014; 34: 151–158. [DOI] [PubMed] [Google Scholar]
- 53. Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia 2016; 59: 404–408. [DOI] [PubMed] [Google Scholar]
- 54. Hotta N, Kawamori R, Fukuda M, et al Long‐term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med 2012; 29: 1529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Charles M, Soedamah‐Muthu SS, Tesfaye S, et al Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2010; 33: 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dyck PJ, Davies JL, Wilson DM, et al Risk factors for severity of diabetic polyneuropathy: intensive longitudinal assessment of the Rochester Diabetic Neuropathy Study cohort. Diabetes Care 1999; 22: 1479–1486. [DOI] [PubMed] [Google Scholar]
- 57. Karvestedt L, Martensson E, Grill V, et al Peripheral sensory neuropathy associates with micro‐ or macroangiopathy: results from a population‐based study of type 2 diabetic patients in Sweden. Diabetes Care 2009; 32: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barr EL, Wong TY, Tapp RJ, et al Is peripheral neuropathy associated with retinopathy and albuminuria in individuals with impaired glucose metabolism? The 1999‐2000 AusDiab. Diabetes Care 2006; 29: 1114–1116. [DOI] [PubMed] [Google Scholar]
- 59. Yamada E, Namiki Y, Takano Y, et al Clinical factors associated with the symptoms of constipation in patients with diabetes mellitus: a multicenter study. J Gastroenterol Hepatol 2018; 33: 863–868. [DOI] [PubMed] [Google Scholar]
- 60. Seino Y, Yabe D, Takami A, et al Long‐term safety of once‐daily lixisenatide in Japanese patients with type 2 diabetes mellitus: GetGoal‐Mono‐Japan. J Diabetes Complications 2015; 29: 1304–1309. [DOI] [PubMed] [Google Scholar]
- 61. Seino Y, Terauchi Y, Wang X, et al Safety, tolerability and efficacy of lixisenatide as monotherapy in Japanese patients with type 2 diabetes mellitus: An open‐label, multicenter study. J Diabetes Investig 2018; 9: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seino Y, Stjepanovic A, Takami A, et al Safety, tolerability and efficacy of lixisenatide in combination with oral antidiabetic treatment in Japanese patients with type 2 diabetes: An open‐label, multicenter study. J Diabetes Investig 2018; 9: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaku K, Rasmussen MF, Nishida T, et al Fifty‐two‐week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon‐like peptide‐1 analog liraglutide vs glibenclamide in patients with type 2 diabetes. J Diabetes Investig 2011; 2: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kaku K, Kiyosue A, Ono Y, et al Liraglutide is effective and well tolerated in combination with an oral antidiabetic drug in Japanese patients with type 2 diabetes: A randomized, 52‐week, open‐label, parallel‐group trial. J Diabetes Investig 2016; 7: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seino Y, Kaneko S, Fukuda S, et al Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: A 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Investig 2016; 7: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kaneko S, Nishijima K, Bosch‐Traberg H, et al Efficacy and safety of adding liraglutide to existing insulin regimens in Japanese patients with type 2 diabetes mellitus: A post‐hoc analysis of a phase 3 randomized clinical trial. J Diabetes Investig 2018; 9: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tesfaye S, Boulton AJ, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Risk factors for nausea and vomiting in patients treated with liraglutide as assessed by the Fine–Gray's proportional hazards model.
Table S2 ¦ Risk factors for nausea and vomiting in patients treated with lixisenatide as assessed by the Fine–Gray's proportional hazards model.
Table S3 ¦ Risk factors for nausea and vomiting in patients treated with liraglutide and without treatment with proton pump inhibitors or histamine‐2 receptor antagonists as assessed by the Fine–Gray's proportional hazards model.
Table S4 ¦ Risk factors for nausea and vomiting in patients treated with lixisenatide and without treatment with proton pump inhibitors or histamine‐2 receptor antagonists as assessed by the Fine–Gray's proportional hazards model.


