Abstract
Aims/Introduction
Dipeptidyl peptidase‐4 inhibitor has been proven to improve glycemic control and β‐cell function in latent autoimmune diabetes in adults (LADA). The potential immune modulation mechanism is still unknown. Thus, we tested T‐lymphocyte subsets and expression of relevant transcription factors in LADA patients with sitagliptin intervention for up to 1‐year.
Materials and Methods
A total of 40 LADA patients were randomly assigned to sitagliptin and/or insulin treatment (SITA group; n = 20) or insulin alone treatment (CONT group; n = 20). Peripheral blood mononuclear cells were isolated at baseline, 6 months and 12 months. The percentage of T‐lymphocyte subsets (T helper 1, T helper 2, T helper 17 and regulatory T cells) tested by flow cytometry, and the messenger ribonucleic acid expression (T box expressed in T cells [T‐BET], GATA binding protein 3 [GATA3], forkhead box protein 3 [FOXP3] and related orphan receptor C [RORC]) tested by real‐time polymerase chain reaction were determined at baseline, 6 months and 12 months.
Results
The percentage of regulatory T cells in the SITA group was significantly lower than that of the CONT group at baseline. The percentage of T helper 2 cells was higher than that of the CONT group at 6 months and 12 months. At 12 months, the percentage of T helper 17 cells was lower in the SITA group than that of the CONT group. After a 1‐year visit, the messenger ribonucleic acid expression levels of T‐BET expressed in T cells and RORC in the SITA group were significantly lower than at baseline. Whereas that of RORC in the CONT group were significantly lower than that at baseline.
Conclusions
The data confirmed that sitagliptin altered the phenotype of T cells and downregulated the expression of T‐BET and RORC in LADA patients, and ameliorated glycemic control in LADA patients.
Keywords: Dipeptidyl peptidase‐4 inhibitor, Latent autoimmune diabetes in adults, T‐lymphocyte subsets
Introduction
Latent autoimmune diabetes in adults (LADA) is a subset of autoimmune diabetes, with clinical manifestation overlapping both type 1 diabetes and type 2 diabetes1, 2. A variety of oral agents are applicable to LADA, such as sulfonylureas, insulin sensitizers (metformin and thiazolidinediones), incretins and sodium–glucose cotransporter inhibitors3, 4, 5. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors, a novel hypoglycemic medication, have been widely applied in type 2 diabetes patients because of their cardiovascular safety, excellent glycemic controlling ability and rare relevant hypoglycemic events in large‐scale multiple‐centered randomized controlled trials, as reported6, 7, 8.
Autoimmune diabetes is considered to be dominantly modulated by T cells. A potential pathogenic mechanism is the imbalance between pathogenic and regulatory T lymphocytes, which results in the actual destruction of the insulin‐producing β‐cells in the pancreatic islets. DPP‐4, known as lymphocyte cell surface protein CD26, has multiple functions in diabetes9, the cardiovascular system, solid tumor and so on because of the diversity of substrates cleaved by DPP‐4. DPP‐4 inhibitors could increase the incretin levels by blocking their degradation. It has also been proven to play a crucial role in immune regulation by several signaling pathways. In a non‐obese diabetic mouse model10, 11, 12, 13, 14, DPP‐4 inhibitors were reported to delay the onset of diabetes, preserve β‐cell mass and attenuate autoimmunity.
Several clinical trials15, 16, 17, 18, 19 of DPP‐4 inhibitors in autoimmune diabetes also confirmed that DPP‐4 inhibitors might improve glycemic control and preserve β‐cell function. However, DPP‐4‐mediated cell signaling and possible immune modulation is still not clearly identified in autoimmune diabetes.
In the present prospective randomized control trial, we investigated whether there are any differences in the profiles of the T‐cell subpopulation and its associated transcription factors in LADA patients with sitagliptin intervention for up to 1 year of follow up.
Methods
Patient selection
The criteria of LADA were: (i) diabetes diagnosed according to the report of the World Health Organization in 1999; (ii) age range 30–70 years; (iii) glutamic acid decarboxylase antibody (GADA)‐positive; (iv) insulin independent within the first 6 months after the diagnosis of diabetes; (v) fasting C‐peptide level >200 pmol/L or a 2‐h postprandial C‐peptide >400 pmol/L; and (vi) duration of diabetes of ≤3 years. The exclusion criteria were: (i) total insulin doses >0.8 U/kg/day; (ii) evidence of any other autoimmune diseases; (iii) evidence of chronic or acute infection; (iv) a history of any malignancy, congestive heart failure or secondary diabetes; (v) renal diseases or renal dysfunction with serum creatinine of ≥1.5 mg/dL for men and ≥1.4 mg/dL for women; (vi) women who were pregnant, had several miscarriages or were breast‐feeding; and (vii) patients unable to abide by the treatment protocol.
A total of 40 LADA patients were recruited from December 2014 to December 2016. All of them were given written information and consent was obtained. The protocols were approved by the ethics committee of the Second Xiangya Hospital, Central South University. This study has been registered online (www.clinicaltrials.gov/identifier NCT01159847).
Treatment protocol
After a 3‐month washing‐in period, the 40 patients enrolled in this study were randomized at a 1:1 ratio to insulin with sitagliptin 100 mg/day (SITA group; n = 20) or insulin without sitagliptin (CONT group; n = 20). Patients included in this study had similar diet and lifestyle modifications, self‐monitoring of blood glucose, and insulin dose adjustment. The regimen of insulin is administration of premixed insulin (30% insulin aspart and 70% insulin aspart protamine) twice or three times daily according to the patient's glycemic profile. Sulfonylureas, insulin sensitizers, other types of DPP‐4 inhibitors and sodium–glucose cotransporter inhibitors were not applied to the patients in the present study. The intervention lasted for 12 months, and the follow‐up visits took place at baseline, 6 months and 12 months. All 40 participants completed the treatment protocol and attended the visits at all three time‐points.
Fasting blood samples were tested for hemoglobin A1c (HbA1c), GADA, fasting blood glucose and C‐peptide; 2‐h postprandial blood samples were tested for postprandial blood glucose and C‐peptide.
GADA assay
GADA was tested by radioligand assay confirmed by the Diabetes Antibody Standardization Program (2012) and sponsored by the Immunology of Diabetes Society. The cut‐off indices of positivity for GADA was ≥18 U/mL (World Health Organization units), and the sensitivity was 78.0% and specificity was 96.7% (Diabetes Autoantibody Standardization Program 2010)20.
Assessment of HbA1c and β‐cell function
A standard 543.6‐kcal, mixed‐meal tolerance test (44.4% of calories as carbohydrate, 47.7% calories as fat and 7.9% calories as protein) was given. The HbA1c levels were measured by automated liquid chromatography (HLC‐723G8; Tosoh, Yamaguchi, Japan). Plasma glucose and C‐peptide levels were measured at 0 and 2 h after the standard meal. C‐peptide was detected by a chemiluminescence method (Advia Centaur System, Munich, Germany).
T helper 1/T helper 2/T helper 17/regulatory T Cell phenotype testing
Fresh blood samples, collected at baseline, 6 and 12 months, were gathered in sodium heparin tubes from fasting participants and processed in 2 h. Peripheral blood mononuclear cells were separated by Ficoll‐Paque density‐gradient. Cells were stimulated with 1 μg/mL ionomycin and 50 ng/mL phorbol‐12‐myristate‐13‐acetate for 6 h in the presence of Brefeldin A and GolgiStop. Cells were harvested and stained with viability dye (Invitrogen, Carlsbad, CA, USA) and flurophore conjugated anti‐interferon‐γ, anti‐interleukin‐4, anti‐interleukin‐17A and anti‐forkhead box protein 3 (FOXP3) by fluorescence‐activated cell sorting. Data were gathered in a Canto flow cytometer (BD, Franklin Lakes, NJ, USA) and analyzed with Flowjo 7.6 software (Tree Star, Inc., Ashland, OR, USA). The gating strategy is shown in Figure 1.
Figure 1.
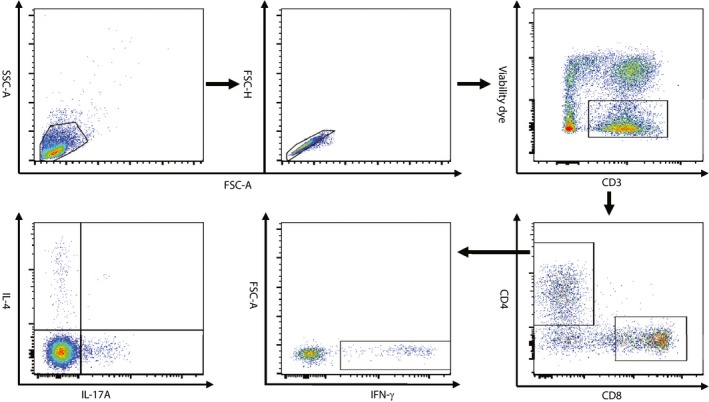
Representative plots and the gating strategy for the fluorescence‐activated cell sorting analysis of CD4 helper T cell subsets. CD4+ T cells were sequentially gated on lymphocytes, singlets, live T cells and CD4+ T cells, and the expression of interferon (IFN)‐γ, interleukin (IL)‐4 and IL‐17A was further analyzed. FSC‐A, forward scatter‐area; FSC‐H, forward scatter‐height; SSC‐A, side scatter‐area.
Quantification of T‐cell transcription factors
Total ribonucleic acid (RNA) was obtained by the TRIZOL (Invitrogen) method and frozen at −70°C according to the manufacturer's protocol. The RNA was first isolated with a RNA extraction kit (Zymo Research, Orange County, CA, USA), and was then converted to complementary deoxyribonucleic acid (cDNA) with a cDNA Reverse Transcription Kit (GoScript™; Promega, Madison, WI, USA). The cDNAs collected were amplified with specific oligonucleotides for T box expressed in T cells (T‐BET), GATA binding protein 3 (GATA3), forkhead box protein 3 (FOXP3) and related orphan receptor C (RORC) with SYBR Green I and quantified by real‐time polymerase chain reaction in an ABI PRISM Step One Sequence Detection System (PE Applied Biosystems™ machine; Carlsbad, CA, USA). Primers are listed in Table 1. The relative messenger RNA (mRNA) gene expression of T‐cell transcription factors (T helper 1 [Th1] = T‐BET, T helper 2 [Th2] = GATA3, T helper 17 [Th17] = RORC, regulatory T cells [Treg] = FOXP3) were quantified as a fold change against the actin control sample by using the 2−∆∆CT method. These associated transcription factors were tested at baseline, 6 and 12 months.
Table 1.
Forward and reverse primers for T‐cell transcription factors
| Target gene | Primer forward | Primer reverse |
|---|---|---|
| β‐Actin | 5′‐CGGGAAATCGTGCGTGAC‐3′ | 5′‐GGAAGGAAGGCTGGAAGAG‐3′ |
| T‐BET | 5′‐CAACGCTTCCAACACGCAT‐3′ | 5′‐GACTCAAAGTTCTCCCGGAA‐3′ |
| GATA3 | 5′‐TCATTAAGCCCAAGCGAAGG‐3′ | 5′‐GTCCCCATTGGCATTCCTC‐3′ |
| RORC | 5′‐GCAGCGCTCCAACATCTTCT‐3′ | 5′‐ACGTACTGAATGGCCTCGGT‐3′ |
| FOXP3 | 5′‐CACCTGGCTGGGAAAATGG‐3′ | 5′‐GGAGCCCTTGTCGGATGA‐3′ |
FOXP3, forkhead box protein 3; GATA3, GATA binding protein 3; RORC, related orphan receptor C; T‐BET, T box expressed in T cells.
Statistical analysis
Data are presented as mean ± standard error of the mean or median (25th–75th percentile). A paired t‐test was used for a comparison from the baseline within the same group. Categorical variables were compared by one‐way anova. We used SPSS version 17.0 (IBM Corporation, Chicago, IL, USA) and GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). A two‐tailed P < 0.05 was considered a significant difference.
Results
Anthropometric and metabolic data
We randomly assigned these 40 LADA patients into the SITA group (n = 20, women/men 7/13) and CONT group (n = 20, women/men 9/11). The mean age in the SITA group and CONT group was 47.8 ± 13.1 vs 51.9 ± 10.2 years, respectively. The GADA titer in the SITA group was 483.6 U/mL (30.9–1,134.7 U/mL), and 285.1 U/mL (18.5–1,190.5 U/mL) in the CONT group. No significant differences were present among duration, sex, age and GADA titer. No patient progressed to an insulin‐dependent state during the 1‐year follow up.
Table 2 summarizes the characteristics of the participants in the SITA group and CONT group at baseline, 6 and 12 months. After the 12‐month visit, postprandial blood glucose and fasting blood glucose in the CONT group were significantly higher than baseline. No significant difference could be found in body mass index, insulin dose, HbA1c, fasting blood glucose, fasting C‐peptide, postprandial C‐peptide.
Table 2.
Demographic and clinical characteristics at baseline, 6 months and 12 months
| Baseline | 6 months | 12 months | ||||
|---|---|---|---|---|---|---|
| SITA group (n = 20) | CONT group (n = 20) | SITA group (n = 20) | CONT group (n = 20) | SITA group (n = 20) | CONT group (n = 20) | |
| BMI (kg/m2) | 23.19 ± 0.64 | 24.46 ± 0.63 | 23.11 ± 0.65 | 24.41 ± 0.62 | 23.05 ± 0.67 | 24.27 ± 0.58 |
| Insulin dose (U/day) | 12.0 ± 2.6 | 14.0 ± 2.4 | 11.5 ± 2.5 | 15.2 ± 2.8 | 12.3 ± 2.6 | 18.9 ± 3.4 |
| HbA1c (%) | 6.33 ± 0.20 | 6.94 ± 0.41 | 6.35 ± 0.17 | 7.09 ± 0.43 | 6.48 ± 0.21 | 7.12 ± 0.36 |
| FBG (mmol/L) | 6.68 ± 0.40 | 7.54 ± 0.61 | 6.82 ± 0.42 | 7.62 ± 0.67 | 6.29 ± 0.29 | 7.50 ± 0.63 |
| PBG (mmol/L) | 12.72 ± 1.07 | 14.02 ± 1.36 | 12.45 ± 0.90 | 14.79 ± 1.30 | 11.68 ± 1.19 | 14.90 ± 1.28* |
| ▵BG (mmol/L) | 6.05 ± 1.12 | 5.95 ± 0.92 | 5.63 ± 1.01 | 6.08 ± 1.13 | 5.37 ± 1.26 | 7.22 ± 0.92* |
| FCP (pmol/L) | 479.5 ± 71.2 | 477.1 ± 55.5 | 420.9 ± 59.7 | 522.2 ± 59.7 | 441.3 ± 51.8 | 458.3 ± 43.4 |
| 2hCP (pmol/L) | 1,458.9 ± 200.7 | 1,487.9 ± 175.5 | 1,557.7 ± 191.1 | 1,658.4 ± 184.8 | 1,691.3 ± 220.8 | 1,516.7 ± 150.9 |
| ▵CP (pmol/L) | 979.4 ± 166.2 | 1,010.8 ± 137.2 | 1,136.9 ± 148.5 | 1,136.15 ± 157.3 | 1,250.0 ± 179.7 | 1,058.4 ± 125.8 |
Data presented as mean ± standard error of the mean. *P < 0.05, compared with the insulin alone treatment (CONT) group at baseline. ▵CP, 2‐h postprandial C‐peptide – fasting C‐peptide; 2hCP, 2‐h postprandial C‐peptide; BG, blood glucose; BMI, body mass index, FBG, fasting blood glucose; FCP, fasting C‐peptide; CP, postprandial C‐peptide; HbA1c, hemoglobin A1c; PBG, postprandial blood glucose; SITA, sitagliptin and insulin treatment.
Th1/Th2/Th17/Treg phenotype
We investigated the percentage of T‐lymphocyte subsets (Th1, Th2, Treg and Th17 cells) in the SITA group and CONT group by flow cytometry at baseline, 6 and 12 months.
The percentage of Treg to CD4+ T cells in the SITA group was significantly lower than that in the CONT group (2.36 ± 0.35% vs 3.92 ± 0.50%, P = 0.016) at baseline. The percentage of Th2 to CD4+ T cells was higher than that of the CONT group at 6 months (4.08 ± 0.58% vs 2.18 ± 0.42%, P = 0.015) and 12 months(5.17 ± 0.92% vs 2.51 ± 0.33%, P = 0.012). At 12 months, the percentage of Th17 to CD4+ T cells was lower in the SITA group than that of the CONT group(0.74 ± 0.57% vs 1.20 ± 0.52%, P = 0.020; Figure 2).
Figure 2.
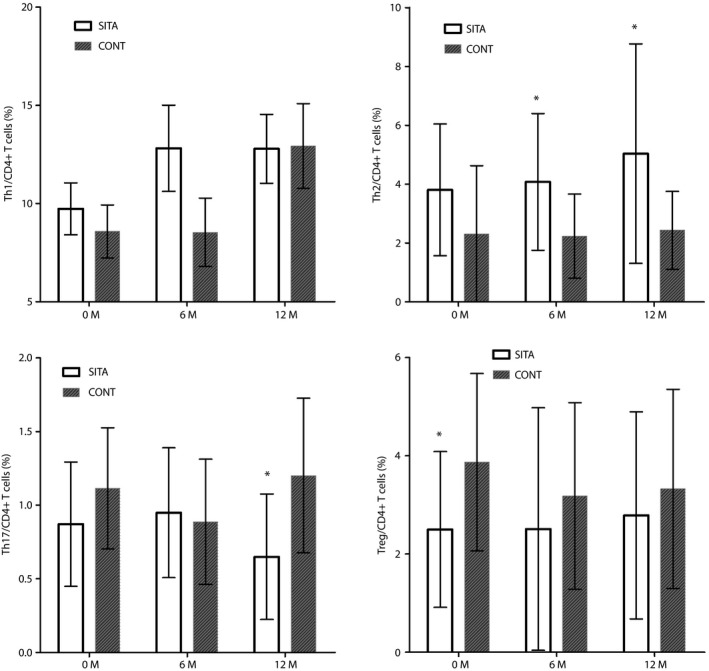
Percentage of T‐cell subsets between the sitagliptin and insulin treatment (SITA) group and insulin alone treatment (CONT) group at baseline, 6 and 12 months. *P < 0.05, compared with the CONT group. Th1, T helper 1 cells; Th2, T helper 2 cells; Th17, T helper 17 cells; Treg, regulatory T cells.
Expression of T‐cell transcription factors
We next extracted RNA from peripheral blood mononuclear cell samples in all 40 LADA patients at baseline, 6 months and 12 months, reversed them into cDNA, and tested the relative mRNA expression of T‐cell transcription (T‐BET, GATA3, RORC and FOXP3) by real‐time polymerase chain reaction.
In Figure 3, no significant difference could be found in the mRNA expression in the SITA group and CONT group at baseline and 6 months, neither difference can be found compared with their baseline, respectively. After the 1‐year visit, no significant difference could be found in these four transcription factors between the SITA group and CONT group. However, the mRNA expression levels of T‐BET (0.29 ± 0.16% vs 0.85 ± 0.24%, P = 0.012) and RORC (0.11 ± 0.07% vs 0.85 ± 0.51%, P = 0.009) in the SITA group were significantly lower when compared with baseline in the SITA group. Whereas the mRNA expression levels of RORC (1.14 ± 0.84% vs 0.19 ± 0.17%, P = 0.004) were also significantly lower than that of its baseline in the CONT group. Although there was a descending tendency in the FOXP3 expression and ascending tendency in the GATA3 expression, the differences were not significant.
Figure 3.
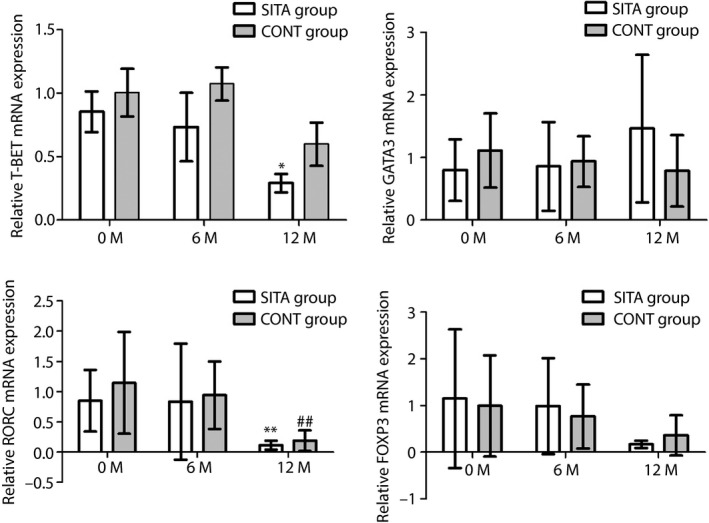
Expression of T‐cell transcription factors at baseline, 6 and 12 months between the sitagliptin and insulin treatment (SITA) group and insulin alone treatment (CONT) group. Real‐time polymerase chain reaction was carried out to test the messenger ribonucleic acid (mRNA) expression of transcription factors (T helper 1 cells = T box expressed in T cells [T‐BET], T helper 2 cells = GATA binding protein 3 [GATA3], regulatory T cells = forkhead box protein 3 [FOXP3] and T helper 17 cells = related orphan receptor C [RORC]) in the SITA group and CONT group at baseline, 6 and 12 months. *P < 0.05, compared with baseline in the SITA group; **P < 0.01, compared with baseline in the SITA group; and ## P < 0.01, compared with baseline in the CONT group.
Discussion
Type 1 diabetes is mainly mediated by T cells, and is associated with loss of immunological tolerance to self‐antigens. LADA is a subclass of autoimmune diabetes. DPP‐4 inhibitors, a novel class of oral hypoglycemic agent, have gained wide acceptance for the treatment of type 2 diabetes. They have also been documented to be protective and effective in autoimmune diabetes, not only in type 1 diabetic animal models10, 11, 12, 13, 14, but also in LADA patients based on small‐scale population studies15, 16, 17, 18, 19. However, it has not been fully understood whether immunological modulation is involved in the disease progression mediated by DPP‐4 inhibition. Therefore, we attempted to elucidate the immunological profile of T‐cell subsets and the main transcription factors with sitagliptin intervention in LADA patients.
We investigated the frequency of T‐cell subsets in LADA patients taking sitaliptin and LADA patients not taking sitaliptin. We found that the percentage of Th2 in the SITA group was statistically higher at 6 months and 12 months, whereas Th17 was dramatically lower than the CONT group after 1‐year follow up. These results confirmed that sitagliptin might alter the subsets of T cells by increasing the protective Th2 and decreasing the pathogenic Th17. This finding shares similarities with another in vitro study21 in which peripheral blood mononuclear cells were extracted from healthy volunteers, confirming sitagliptin inhibition had a suppression effect on Th1, Th2 and Th17 lymphocytes differentiation. The complicated local milieu in the pancreatic islets of Langerhans and the interplay between non‐immune cells and immune cells in vivo might explain this discrepancy. Previous studies22, 23, 24, 25 have shown a decline in Th1 and Th2, lower cytokine secretion, and a low expression of T‐cell‐specific transcription factors in the progression of autoimmune diabetes, suggesting an immune dampening and exhaustion at the onset of autoimmunity. Seldom do clinical trial studies focus on the alteration of the T‐cell phenotype with sitagliptin intervention. Interestingly, the present prospective study observed that although the quantities of Th1 did not significantly decrease, its expression of T‐BET was still obviously decreased in the SITA group. In addition, the percentage of protective Th2 was elevated, and that of the pathological Th17 was decreased. Furthermore, blood glucose was improved, especially postprandial blood glucose, inferring that sitagliptin intervention might alter the quantities of T‐cell subsets in LADA patients and further contribute to better glucose control, resulting in a protective effect on pancreatic β‐cells.
In type 1 diabetes, there was a decreased frequency of Treg24. Defects in Treg number and/or function are a major factor in the progression of type 1 diabetes25. In our previous published study26, we found a lower expression of FOXP3 in LADA patients; a further study27 confirmed that the FOXP3 promoter region was hypermethylated in CD4+ T cells from LADA patients. In the present study, after the 1‐year visit, there was neither a significant alteration in the percentage of Treg nor its corresponding mRNA expression (FOXP3) compared with those in the control group with sitagliptin intervention, though a significant difference of Treg at baseline between these two groups unexpectedly occurred. Sitagliptin did not alter the frequency of Treg and the level of its associated gene expression at 1‐year follow up, and a further long‐term study is required to further confirm this.
Nevertheless, the expression levels of T‐BET and RORC were significantly lower with sitagliptin, suggesting that sitagliptin might downregulate the expression of pathogenic transcription factors. It seems that the percentage of T cells analyzed by fluorescence‐activated cell sorting was unexpectedly not in accordance with the expression of their associated transcription factors. Conflicting results were also found in another type 1 diabetes study28. This unexpected inconsistency might arise from an abnormal translational or post‐translational modulation of protein.
Case reports29, 30 and clinical trials14, 15, 16, 17, 18 in autoimmune diabetes treated with DPP‐4 inhibitor alone or in combination with other drugs showed that sitagliptin significantly improved glycemic control and reduced insulin requirements, accompanied by a good tolerance profile. Although a recent meta‐analysis showed that DPP‐4 inhibitor gives a neutral result for HbA1c31 in type 1 diabetes. The present observed results found that sitagliptin, as an add‐on therapy, seemed to be a better choice to improve the postprandial blood glucose in LADA patients. Although, in regard to C‐peptide and HbA1c, the result from our 1‐year study was not in line with previous studies15, 16, 17, possibly because of the small sample size, different population and relatively short follow‐up period.
In conclusion, the current data first implied that sitagliptin, a DPP‐4 inhibitor, could alter the frequency of CD4+ T‐cell subsets on both a cellular and mRNA level in LADA patients. The downregulation of the expression of pathological mRNAs, including RORC and T‐BET, as well as the decreased Th17 and elevated Th2 cells, might contribute to immune suppression and glycemic control in LADA. A further larger cohort study is warranted to confirm these outcomes from clinic to bench.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
Our work was sponsored by the National Key R&D Program of China (grant numbers 2016YFC1305000, 2016YFC1305001); the National Science and Technology Infrastructure Program (grant number 2015BAI12B13); the National Natural Science Foundation of China (grant numbers 81170725, 81070627, 81500600, 8146168031); and the Key Project of Chinese Ministry of Education (grant number 113050A).
J Diabetes Investig 2019; 10: 375–382
References
- 1. Zhou Z, Xiang Y, Ji L, et al Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic‐based cross‐sectional study. Diabetes 2013; 62: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Li X, Xiang Y, et al Latent autoimmune diabetes in adults with low‐titer GAD antibodies: similar disease progression with type 2 diabetes: a nationwide, multicenter prospective study (LADA China Study 3). Diabetes Care 2015; 38: 16–21. [DOI] [PubMed] [Google Scholar]
- 3. Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev 2012; 28(Suppl 2): 40–46. [DOI] [PubMed] [Google Scholar]
- 4. Yang Z, Zhou Z, Li X, et al Rosiglitazone preserves islet beta‐cell function of adult‐onset latent autoimmune diabetes in 3 years follow‐up study. Diabetes Res Clin Pract 2009; 83: 54–60. [DOI] [PubMed] [Google Scholar]
- 5. Frandsen CS, Dejgaard TF, Madsbad S. Non‐insulin drugs to treat hyperglycaemia in type 1 diabetes mellitus. Lancet Diabetes Endocrinol 2016; 4: 766–780. [DOI] [PubMed] [Google Scholar]
- 6. Scirica BM, Bhatt DL, Braunwald E, et al Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1323. [DOI] [PubMed] [Google Scholar]
- 7. White WB, Cannon CP, Heller SR, et al Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 8. Green JB, Bethel MA, Armstrong PW, et al Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373: 232–242. [DOI] [PubMed] [Google Scholar]
- 9. Zhao Y, Yang L, Wang X, et al The new insights from DPP‐4 inhibtors: their potetial immune modulatory function in autoimmue diabetes. Diabetes Metab Res Rev 2014; 30: 646–653. [DOI] [PubMed] [Google Scholar]
- 10. Kim DH, Lee JC, Lee MK, et al Treatment of autoimmune diabetes in NOD mice by Toll‐like receptor 2 tolerance in conjunction with dipeptidyl peptidase 4 inhibition. Diabetologia 2012; 55: 3308–3317. [DOI] [PubMed] [Google Scholar]
- 11. Jelsing J, Vrang N, van Witteloostuijn SB, et al The DPP4 inhibitor linagliptin delays the onset of diabetes and preserves β‐cell mass in non‐obese diabetic mice. J Endocrinol 2012; 214: 381–387. [DOI] [PubMed] [Google Scholar]
- 12. Tsuji T, Yoshida Y, Fujita T, et al Oral therapy for type1 diabetes mellitus using a novel immunomodulator, FTY720 (fingolimod), in combination with sitagliptin, a dipeptidyl peptidase‐4 inhibitor, examined in non‐obese diabetic mice. J Diabetes Investig 2012; 18: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim SJ, Nian C, Mc Intosh CH. Sitagliptin (MK0431)inhibition of dipeptidyl peptidase IV decreases nonobese diabetic mouse CD4+ T‐cell migration through incretin‐dependent and independent pathways. Diabetes 2010; 59: 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tian L, Gao J, Hao J, et al Reversal of new‐onset diabetes through modulating inflammation and stimulating beta‐cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology 2010; 151: 3049–3060. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Y, Yang L, Xiang Y, et al Dipeptidyl peptidase 4 inhibitor sitagliptin maintains beta‐cell function in patients with recent‐onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab 2014; 99: E876–E880. [DOI] [PubMed] [Google Scholar]
- 16. Buzzetti R, Pozzilli P, Frederich R, et al Saxagliptin improves glycaemic control and C‐peptide secretion in latent autoimmune diabetes in adults (LADA). Diabetes Meta Res Rev 2016; 32: 289–296. [DOI] [PubMed] [Google Scholar]
- 17. Johansen OE, Boehn BO, Grill V, et al C‐peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a 2‐year double‐blind, randomized, controlled study. Diabetes Care 2014; 37: e11–e12. [DOI] [PubMed] [Google Scholar]
- 18. Awata T, Shimada A, Maruyama T, et al Possible long‐term efficacy of sitagliptin, a dipeptidyl peptidase‐4 inhibitor, for slowly progressive type 1 diabetes (SPIDDM) in the stage of non‐insulin‐dependency: an open‐label randomized controlled pilot trial (SPAN‐S). Diabetes Ther 2017; 8: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hari Kumar KV, Shaikh A, Prusty P. Addition of exenatide or sitagliptin to insulin in new onset type 1 diabetes: a randomized, open label study. Diabetes Res Clin Pract 2013; 100: e55–e58. [DOI] [PubMed] [Google Scholar]
- 20. Huang G, Yin M, Xiang Y, et al Persistence of glutamic acid decarboxylase antibody (GADA) is associated with clinical characteristics of latent autoimmune diabetes in adults: a prospective study with 3‐year follow‐up. Diabetes Metab Res Rev 2016; 32: 615–622. [DOI] [PubMed] [Google Scholar]
- 21. Pinheiro MM, Stoppa CL, Valduga CJ, et al Sitagliptin inhibit human lymphocytes proliferation and Th1/Th17differentiation in vitro. Eur J Pharm Sci 2017; 100: 17–24. [DOI] [PubMed] [Google Scholar]
- 22. Ryden A, Ludvigsson J, Fredrikson M, et al General immune dampening is associated with disturbed metabolism at diagnosis of type 1 diabetes. Pediatr Res 2014; 75: 45–50. [DOI] [PubMed] [Google Scholar]
- 23. Foss NT, Foss‐Freitas MC, Ferreira MA, et al Impaired cytokine production by peripheral blood mononuclear cells in type 1 diabetic patients. Diabetes Metab 2007; 33: 439–443. [DOI] [PubMed] [Google Scholar]
- 24. Mysliwska J. Elevated levels of serum IL‐12 and IL‐18 are associated with lower frequencies of CD4(+)CD25 (high) FOXP3 (+)regulatory t cells in young patients with type 1 diabetes. Inflammation 2014; 37: 1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bin Dhuban K, Kornete MS, Mason E, et al Functional dynamics of Foxp3(+) regulatory T cells in mice and humans. Immunol Rev 2014; 259: 140–158. [DOI] [PubMed] [Google Scholar]
- 26. Yang Z, Zhou Z, Huang G, et al The CD4(+) regulatory T‐cells is decreased in adults with latent autoimmune diabetes. Diabetes Res Pract 2007; 76: 126–131. [DOI] [PubMed] [Google Scholar]
- 27. Li Y, Zhao M, Hou C, et al Abnormal DNA methylation in CD4+ T cells from people with latent autoimmune diabetes in adults. Diabetes Res Pract 2011; 94: 242–248. [DOI] [PubMed] [Google Scholar]
- 28. Hamari S, Kirveskoski T, Glumoff V, et al Analyses of regulatory CD4+ CD25+ FOXP3+ T cells and observations from peripheral T cell subpopulation markers during the development of type 1 diabetes in children. Scand J Immunol 2016; 83: 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kandasamy N, Lennox G, Annamalai AK, et al Sitagliptin in glutamic acid decarboxylase antibody‐positive diabetes mellitus. Endocr Pract 2012; 18: e65–e68. [DOI] [PubMed] [Google Scholar]
- 30. Pinheiro MM, Pinheiro FM, Torres MA. Four‐year clinical remission of type 1 diabetes mellitus in two patients treated with sitagliptin and vitamin D3. Endocrinol Diabetes Metab Case Rep 2016; 2016: pii:16–0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo H, Fang C, Huang Y, et al The efficacy and safety of DPP4 inhibitors in patients with type 1 diabetes: a systematic review and meta‐analysis. Diabetes Res Clin Pract 2016; 121: 184–191. [DOI] [PubMed] [Google Scholar]


