Abstract
Today, glucagon‐like peptide‐1 (GLP‐1) receptor agonists are established glucose‐lowering drugs used in the management of type 2 diabetes. Their development emerged from the understanding that a combined islet dysfunction comprising of impaired insulin secretion and exaggerated glucagon secretion is the key defect of hyperglycemia. GLP‐1 was shown to target these defects, and after the discovery that dipeptidyl peptidase‐4 inactivates native GLP‐1, several different dipeptidyl peptidase‐4‐resistant GLP‐1 receptor agonists have been developed. They are administered subcutaneously, but show differences in molecular structure, molecular size and pharmacokinetics, the latter allowing twice‐daily, once‐daily or once‐weekly administration. They have been shown to be efficient in reducing both glycated hemoglobin and bodyweight, and to be safe and highly tolerable. Cardiovascular outcomes trials have shown them to be neutral or beneficial. GLP‐1 receptor agonists are positioned as add‐ons to metformin alone or in combination with oral agents in the clinical paradigm. They are also efficient when combined with insulin, and fixed dose combinations with long‐acting insulin have been developed. Recent development includes a very long administration schedule and oral availability. The research from the first demonstration of the antidiabetic action of GLP‐1 in the early 1990s to the enormously accumulated data today represents a successful and rational development, which has been characterized by focused perseverance to establish this therapy in the management of type 2 diabetes.
Keywords: Glucagon‐like peptide‐1, Treatment, Type 2 diabetes
Introduction
Development of glucagon‐like peptide‐1 (GLP‐1) receptor agonists as a treatment in the management of type 2 diabetes is an example of a rational drug design, which follows characteristic steps required for proper pharmacological development (Figure 1). The process has also engaged many players, in particular scientists, the research‐oriented industry, health authorities and regulators, and the patients. This contribution will focus on the phases of development of GLP‐1 receptor agonists from the idea of a pathophysiology‐directed target based on an endogenous hormone through drug design, clinical studies and positioning in the market.
Figure 1.
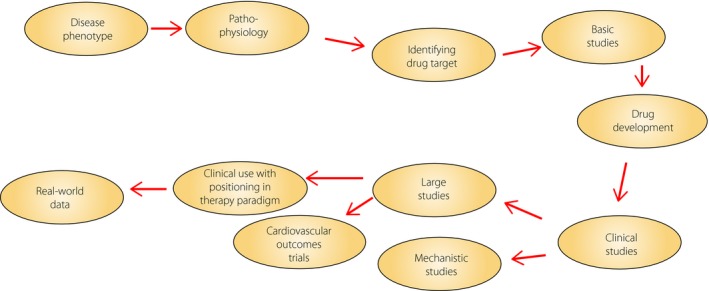
Schematic view of the process of development of novel pharmacotherapies; incretin‐based therapy is a good example of a rational drug design that followed these characteristic steps.
Islet defects in type 2 diabetes
The first step leading to the development of GLP‐1 receptor agonists was carrying out studies establishing that impaired insulin secretion and exaggerated glucagon secretion are the key drivers of hyperglycemia in type 2 diabetes. This is well known today, but in the 1980s – that is, in the beginning of the GLP‐1 area – the key role of the islets for diabetes development was not widely accepted, although clearly shown by islet‐oriented researchers1, 2, 3, 4, 5, 6. In fact, today it is established that this combined islet defect is an early phenomenon during the development of type 2 diabetes, and it has been shown to be present even before the onset of the disease in individuals at risk7. Therefore, a pathophysiology‐directed target for the management of hyperglycemia in type 2 diabetes patients would need to correct these islet defects.
Glp‐1 targeting the islet defects
Coinciding with the understanding that impaired insulin secretion and exaggerated glucagon secretion are drivers of hyperglycemia in the 1980s; it was at the same time shown that GLP‐1 targets these two defects. GLP‐1, which was discovered in 19838, is a gut hormone that is released during meal ingestion9, 10. It is the main gut incretin hormone; that is, the hormone responsible for the augmented insulin release after oral versus intravenous glucose administration at similar glucose levels11. This assures an appropriate insulin response to ingested meals.
Stimulation of insulin secretion by GLP‐1 in humans was first shown by Kreymann et al. in 198712 when they infused GLP‐1 together with glucose and found a potentiation of glucose‐stimulated insulin secretion. The inhibition of glucagon was first shown in animal studies by Ørskov et al. in 198813 and Fridolf et al. in 199114. Indeed, when islet effects of GLP‐1 were characterized in detail in humans, a marked reduction in glucagon after meal ingestion was observed15. In addition to this, GLP‐1 was found to delay gastric emptying, and induce satiety and weight reduction, which add to the glucose‐lowering characteristic of the hormone16. Therefore, GLP‐1 fulfills criteria of being a physiological endogenous factor that has the ability to be antidiabetic through several actions in type 2 diabetes, and these characteristics were evident from the end of the 1980s.
First study in type 2 diabetes
This realization was followed by the first test of GLP‐1 in type 2 diabetes. We thereby infused GLP‐1 or a placebo during meal ingestion in patients with type 2 diabetes, and at the same time, we infused insulin in a variable rate to maintain normoglycemia. We could then calculate the meal insulin requirement during the two tests, and we found that the meal insulin requirement to achieve normoglycemia after meal ingestion was markedly reduced by GLP‐1 in type 2 diabetes when compared with the placebo. This was associated with increased levels of C‐peptide and reduced glucagon levels, as evidence for stimulation of insulin secretion and inhibition of glucagon secretion in the patients17, 18.
We presented this first demonstration of an antidiabetic action of GLP‐1 at the European Association for the Study of Diabetes in 199017 and published the results in 199218. In an editorial accompanying the published article, it was stated that “If these interesting findings can be replicated, GLP‐1 analogs may become useful in the treatment of patients with NIDDM (non‐insulin‐dependent diabetes mellitus)”; that is, type 2 diabetes19. This first study was later followed by several studies reporting that GLP‐1 stimulates insulin secretion and reduces glucose in type 2 diabetes patients. For example, Nathan et al.20 showed in 1992 that GLP‐1 stimulates insulin secretion, Rachman et al.21 showed in 1996 that overnight GLP‐1 infusion nearly normalizes circulating glucose, Gutniak et al.22 showed in 2001 that GLP‐1 suppresses postprandial glucose, and Zander et al.23 showed in 2002 that 6 weeks of GLP‐1 administration improves glycemic and reduces bodyweight.
Dipeptidyl peptidase‐4 responsible for rapid inactivation of glp‐1
A problem for drug development with these early studies was that GLP‐1 had to be given through a continuous subcutaneous or intravenous route to allow for a long‐term effect due to the rapid inactivation of the hormone. Therefore, to really harness the antidiabetic action of GLP‐1 required to overcome the fast inactivation of native GLP‐1. Thus, the native hormone has a half‐life of only a few minutes. It was shown by Mentlein et al.24 that the enzyme dipeptidyl peptidase‐4 (DPP‐4) was responsible for this inactivation. This enzyme rapidly cleaves the two N‐terminal amino acids from the rest of the hormone, which makes GLP‐1 largely inactive. This was further enforced by the finding that inhibition of DPP‐4 raised the endogenous levels of GLP‐125, which is in turn paved the way for DPP‐4 inhibition as a therapy; the first clinical evidence of which was published in 200226.
Several glp‐1 receptor agonists developed
Based on the understanding that DPP‐4‐resistant GLP‐1 receptor agonists needed to be developed, several different GLP‐1 receptor agonists have emerged27, 28. They have different backgrounds, and molecular and kinetics characteristics. Two of them, liraglutide and semaglutide, are true GLP‐1 analogs, meaning that they are modified from the structure of native GLP‐1. Thus, in both of them, the molecular structure in GLP‐1 has been slightly changed and a fatty acid chain has been added, which results in a delayed absorption from the injection site, a high albumin binding and therefore a longer duration. Furthermore, two other GLP‐1 receptor agonists, exenatide and lixisenatide, are based on the peptide exendin derived from the Gila monster (a venomous lizard native to the USA), which has a similar structure as GLP‐1 and retains GLP‐1 receptor activity. Exenatide is the synthetic recombinant form of exendin, and lixisenatide is an exendin molecule that has been prolonged with six lysine residues. Furthermore, two of the GLP‐1 receptor agonists are larger structures based on complex drug engineering using two GLP‐1 molecules. Albiglutide consists of two GLP‐1 molecules that have been coupled and fused to recombinant albumin, and dulaglutide consists of two GLP‐1 molecules, which through two linker peptides have been fused to Fc fragments of immunoglobulin27. All these GLP‐1 receptor agonists are administered subcutaneously, but they differ in other properties. One difference is the structure; exenatide and lixisenatide are based on exendin, whereas the others are based on GLP‐1. Furthermore, they differ in molecular size, as albiglutide and dulaglutide are larger, whereas the others are smaller, with a similar size as native GLP‐1. Moreover, the GLP‐1 receptor agonists differ in pharmacokinetics and can be given twice daily (exenatide), once daily (lixisenatide, liraglutide) or once weekly (semaglutide, albiglutide, dulaglutide and an extended form of exenatide)27.
Clinical studies with glp‐1 receptor agonists
The next step in development of a pharmacotherapy is to examine clinical efficacy, tolerability and safety. Here, all GLP‐1 receptor agonists have gone through extended trials. These extensive programs are called AMIGO (AC2993 Diabetes Management for Improving Glucose Outcomes examining exenatide), LEAD (Liraglutide Effect and Action in Diabetes examining liraglutide), GetGoal (GLP‐1 Agonist AVE0010 in Patients with Type 2 Diabetes Mellitus for Glycemic Control and Safety Evaluation examining lixisenatide), DURATION (Diabetes Therapy Utilization: Researching Changes in A1c, Weight and Other Factors Through Intervention with Exenatide Once Weekly examining an extended form of exenatide), HARMONY (examining albiglutide), AWARD (Assessment of Weekly Administration of LY2189265 [dulaglutide] in Diabetes examining dulaglutide) and SUSTAIN (Trial to Evaluate Cardiovascular and Other Long‐Term Outcomes with Semaglutide in Subjects with Type 2 Diabetes examining semaglutide)28. These programs include several 26–30‐week studies with the GLP‐1 receptor agonists tested in monotherapy, in combination with oral agents and in combination with insulin. The studies have shown that the GLP‐1 receptor agonists efficiently improve glycemia with a reduction in glycated hemoglobin of approximately 0.8–1.5% from baseline levels of 7.5–8.5% and reduce bodyweight by ≈1–5 kg27, 28, 29. The GLP‐1 receptor agonists have also been shown to be safe and highly tolerable with transient gastrointestinal symptoms (nausea and vomiting) as the only consistent adverse event28.
The GLP‐1 receptor agonists have also undergone large cardiovascular outcomes trials, and some of those are already published (LEADER [Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results] for liraglutide, ELIXA [Evaluation of Lixisenatide in Acute Coronary Syndrome] for lixisenatide, EXCSEL [Exenatide Study of Cardiovascular Event Lowering Trial] for exenatide and SUSTAIN 6 for semaglutide). In total, this represents a huge development with thousands of patients. These large trials have shown non‐inferiority compared with other treatments in risk for major acute cardiovascular end‐points, and two GLP‐1 receptor agonists (liraglutide and semaglutide) have in addition shown cardiovascular benefits in these trials28. It should be emphasized that there are several ongoing trials with GLP‐1 receptor agonists, and therefore, in the near future, even more data will accumulate.
An area of special importance is the risk of hypoglycemia during glucose‐lowering therapy. In fact, avoiding hypoglycemia is the key to success for the treatment of diabetes30, and strategies need to be defined to prevent hypoglycemia31. Here, GLP‐1 receptor agonists are powerful, and, as shown in a meta‐analysis, GLP‐1 receptor agonists have largely the same low risk for hypoglycemia as a placebo, even though glycated hemoglobin is reduced by approximately 1%32. One reason for the low risk of hypoglycemia is that the effect is glucose‐dependent, such that the effect to stimulate insulin secretion and inhibit glucagon secretion disappears when glucose levels are reduced. Sustaining glucagon counterregulation to hypoglycemia is also very important to avoid hypoglycemia. This was clearly shown in a study in which the GLP‐1 receptor agonist lixisenatide was added to on‐going insulin in patients with type 2 diabetes33. In a two‐step clamp technique, it was shown that at 3.5 mmol/L glucose, there was slightly lower glucagon with lixisenatide than with a placebo, but when glucose was reduced to 2.5 mmol/L, the glucagon response was completely normal; that is, the glucagon counterregulation to hypoglycemia is sustained, which is a safeguard against hypoglycemia.
Comparison with other therapies
Another important issue in the clinical development of GLP‐1 receptor agonists is their efficacy in relation to other glucose‐lowering therapy. This has been carried out within the various trial programs, which in general have included studies comparing a GLP‐1 receptor agonist with another drug, such as sulfonylurea, usually as an add‐on to metformin. A recent 3‐year trial34 compared a GLP‐1 receptor agonist with two other drugs and also a placebo arm, thereby representing a comprehensive approach. In that study, the efficacy and safety of the GLP‐1 receptor agonist, albiglutide (30–50 mg weekly), were compared with those of DPP‐4 inhibitor sitagliptin (100 mg daily), the sulphonylurea glimepiride (2–4 mg daily) and placebo when added to metformin in a total of ≈1,000 inadequately controlled patients with type 2 diabetes34. It was found that all active treatments reduced glycated hemoglobin compared with a placebo, and the reduction was significantly larger after albiglutide (−0.9%) compared with sitagliptin (−0.4%) and glimepiride (−0.3%) from a baseline of 8.1%. It was also found that hyperglycemic rescue, which was introduced at certain prespecified glucose levels, was lower for albiglutide (26.8%) than for sitagliptin (36.4%), glimepiride (32.7%) or a placebo (59.2%). Gastrointestinal adverse events (nausea and vomiting) were the most common adverse events with albiglutide. Therefore, that study and other studies27, 28, 29 showed efficient glucose‐reducing effects with GLP‐1 receptor agonists associated with bodyweight reduction and gastrointestinal adverse events as most common adverse events.
The next step in development is to position GLP‐1 receptor agonists in relation to other therapies in the glucose‐lowering paradigm. In the position statement by the European Association for the Study of Diabetes and American Diabetes Association, it is suggested that GLP‐1 receptor agonists are one of six options to add to metformin for patients in whom metformin alone is insufficient for adequate glycemic control; the others being sulphonylureas, thiazolidinediones, DPP‐4 inhibitors, sodium–glucose cotransporter 2 inhibitors and insulin35. Also, GLP‐1 receptor agonists might be used as third‐line in combination with metformin and one of sulfonylurea, thiazolidinedione or insulin.
Combination with insulin
Of particular importance is the use of GLP‐1 receptor agonists in combination with insulin36. This is an important combination because of the synergistic physiological effects of the two treatments. Thus, insulin stimulates glucose utilization through actions on muscle and fat tissue in combination with inhibiting hepatic glucose production through liver actions, whereas GLP‐1 stimulates insulin secretion, inhibits glucose secretion and delays gastric emptying37. This results in reduction of both fasting and postprandial glucose, which is achieved together with a low risk of hypoglycemia, and the satiety effect of GLP‐1 results in a lower risk of weight gain compared with treatment with insulin alone. This treatment combination is now also available in fixed dose combinations with insulin degludec with the GLP‐1 receptor agonist, liraglutide38, and insulin glargine with the GLP‐1 receptor agonist, lixisenatide39, respectively. Both these combinations have shown good effect to reduce glucose and to neutralize the increase in bodyweight seen by insulin, and the risk for hypoglycemia is low38, 39.
Novel development
For the near future, two new avenues have opened. One is the potential of very long‐term duration, which has been introduced in the ITCA650, which uses exenatide in a mini‐osmotic pump that can be introduced for up to 1 year40. Another potential is the oral availability of GLP‐1 receptor agonists, which has been introduced in clinical trials with oral semaglutide allowing administration once‐daily as a tablet41, 42.
Conclusions
We have had a unique experience with the development of GLP‐1‐based therapy throughout the past >30 years. This development has been assured through a unique cooperation between several players. Scientists have targeted a drug based on pathophysiology, and industrial developers have developed unique molecular structures that have been tested in clinical trials. Cooperation has been undertaken with healthcare providers and regulators. Through this, patients have received a treatment that not only reduces glucose, but also reduces weight and protects from hypoglycemia, a treatment with easy handling and administration, and a drug with proven effect on not only glycemia and bodyweight, but also on safety and cardiovascular outcomes.
The successful development has required focused efforts and indeed long‐term perseverance – from the first study of GLP‐1 in type 2 diabetes in 1990 to the present day existence of several different GLP‐1 receptor agonists.
Disclosure
The author has received speaking or consultancy fees from GSK, MSD, Novartis, Novo Nordisk, Sanofi and Takeda, which all are companies involved in the development of incretin‐based therapy.
Acknowledgment
This mini review is based on a lecture given at the 61st Annual Meeting of the Japan Diabetes Society, Tokyo, 24–26 May 2018.
J Diabetes Investig 2019; 10: 196–201
References
- 1. Porte D Jr. Banting Lecture 1990. Beta‐cells in type II diabetes mellitus. Diabetes 1991; 40: 166–180. [DOI] [PubMed] [Google Scholar]
- 2. Polonsky KS. Evolution of beta‐cell dysfunction in impaired glucose tolerance and diabetes. Exp Clin Endocrinol Diabetes 1999; 107(suppl 4): S124–S127. [DOI] [PubMed] [Google Scholar]
- 3. Efendic S, Luft R, Wajngot A. Aspects of the pathogenesis of type 2 diabetes. Endocr Rev 1984; 5: 395–410. [DOI] [PubMed] [Google Scholar]
- 4. Unger RH. The Banting Memorial Lecture 1975. Diabetes and the alpha cell. Diabetes 1975; 25: 136–151. [DOI] [PubMed] [Google Scholar]
- 5. Dunning BE, Gerich JE. The role of alpha‐cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007; 28: 253–283. [DOI] [PubMed] [Google Scholar]
- 6. Ahrén B. Glucagon – early breakthroughs and recent discoveries. Peptides 2015; 67: 74–81. [DOI] [PubMed] [Google Scholar]
- 7. Ahrén B. ß‐ and α‐cell dysfunction in subjects developing impaired glucose tolerance. Diabetes 2009; 58: 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bell GI, Sanchew‐Pescador R, Mullenback GT. Hamster proglucagon contains the sequence of glucagon and two related peptides. Nature 1983; 302: 716–718. [DOI] [PubMed] [Google Scholar]
- 9. Ahrén B. Islet G protein‐coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 2009; 8: 369–385. [DOI] [PubMed] [Google Scholar]
- 10. Ahrén B, Carr RD, Deacon CF. Incretin hormone secretion over the day. Vitam Horm 2010; 84: 203–220. [DOI] [PubMed] [Google Scholar]
- 11. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- 12. Kreymann B, Williams G, Ghatei MA, et al Glucagon‐like peptide‐1 7‐36: a physiological incretin in man. Lancet 1987; 2: 1300–1304. [DOI] [PubMed] [Google Scholar]
- 13. Ørskov C, Holst JJ, Nielsen OVC. Effect of truncated glucagon‐like peptide‐1 [proglucagon‐(78‐107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 1988; 123: 2009–2013. [DOI] [PubMed] [Google Scholar]
- 14. Fridolf T, Böttcher G, Sundler F, et al GLP‐1 and GLP‐1(7‐36) amide: influences on basal and stimulated insulin and glucagon secretion in the mouse. Pancreas 1991; 6: 208–215. [PubMed] [Google Scholar]
- 15. Ahrén B, Holst JJ, Mari A. Characteristics of GLP‐1 on beta cell function after meal ingestion in humans. Diabetes Care 2003; 26: 2860–2864. [DOI] [PubMed] [Google Scholar]
- 16. Baggio LL, Drucker DJ. Biology of incretins: GLP‐1 and GIP. Gastroenterology 2007; 132: 131–157. [DOI] [PubMed] [Google Scholar]
- 17. Gutniak M, Ahrén B, Efendic S. Glucagon‐like insulinotropic peptide 1 (7‐36) ‐ new approach to treating diabetes? 26th Assoc for the Study of Diabetes (EASD), Copenhagen 1990. Diabetologia 1990; 33: A73. [Google Scholar]
- 18. Gutniak M, Ørskov C, Holst J, et al Antidiabetogenic effect of glucagon‐like peptide‐1 (7‐36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992; 326: 1316–1322. [DOI] [PubMed] [Google Scholar]
- 19. Ensinck JW, D′Alessio DA. The enteroinsular axis revisited. A novel role for an incretin. N Engl J Med 1992; 326: 1352–1353. [DOI] [PubMed] [Google Scholar]
- 20. Nathan DM, Schreiber E, Fogel H, et al Insulinotropic action of glucagonlike peptide‐1‐(7‐37) in diabetic and nondiabetic subjects. Diabetes Care 1992; 15: 270–2786. [DOI] [PubMed] [Google Scholar]
- 21. Rachman J, Barrow BAS, Levy JC, et al Near‐normalization of diurnal glucose concentrations by continuous administration of glucagon‐like peptide‐1 (GLP‐1) in subjects with NIDDM. Diabetologia 1997; 40: 205–211. [DOI] [PubMed] [Google Scholar]
- 22. Gutniak MK, Svartberg J, Hellström PM, et al Antidiabetogenic action of glucagon‐like peptide‐1 related to administration relative to meal intake in subjects with type 2 diabetes. J Int Med 2001; 250: 81–87. [DOI] [PubMed] [Google Scholar]
- 23. Zander M, Madsbad S, Madsen JL, et al Effect of 6‐week course of glucagon‐like peptide 1 on glycaemic control, insulin sensitivity, and beta‐cell function in type 2 diabetes: a parallel‐group study. Lancet 2002; 359: 824–830. [DOI] [PubMed] [Google Scholar]
- 24. Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl‐peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon‐like peptide‐1(7‐36) amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 1993; 214: 829–835. [DOI] [PubMed] [Google Scholar]
- 25. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon‐like peptide‐1 by human plasma in vitro yields an N‐terminally truncated peptide that is a major endogenous metabolite in vivo . J Clin Endocrinol Metab 1995; 80: 952–957. [DOI] [PubMed] [Google Scholar]
- 26. Ahrén B, Simonsson E, Larsson H, et al Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4‐week study period in type 2 diabetes. Diabetes Care 2002; 25: 869–875. [DOI] [PubMed] [Google Scholar]
- 27. Ahrén B. GLP‐1 receptor agonists in the treatment of type 2 diabetes. Diabetes Manage 2013; 3: 401–413. [Google Scholar]
- 28. Aroda VR. A review of GLP‐1 receptor agonists: evolution and advancement, through the lens of randomised clinical trials. Diabetes Obes Metab 2018; 20(suppl 1): 22–33. [DOI] [PubMed] [Google Scholar]
- 29. Htike ZZ, Zaccardi F, Papamargaritis D, et al Efficacy and safety of glucagon‐like peptide‐1 receptor agonists in type 2 diabetes: a systematic review and mixed‐treatment comparison analysis. Diabetes Obes Metab 2017; 19: 524–536. [DOI] [PubMed] [Google Scholar]
- 30. Ahrén B. Avoiding hypoglycemia: a key to success for glucose‐lowering therapy in type 2 diabetes. Vasc Health Risk Manag 2013; 9: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahrén B. Strategies to mitigate the risk of hypoglycaemia associated with treatment of type 2 diabetes. Treat Strateg Diabetes 2013; 5: 73–76. [Google Scholar]
- 32. Liu SC, Tu YK, Chien MN, et al Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta‐analysis. Diabetes Obes Metab 2012; 14: 810–820. [DOI] [PubMed] [Google Scholar]
- 33. Farngren J, Persson M, Ahrén B. Effect of the GLP‐1 receptor agonist lixisenatide on counter‐regulatory responses to hypoglycemia in subjects with insulin‐treated type 2 diabetes. Diabetes Care 2016; 39: 242–249. [DOI] [PubMed] [Google Scholar]
- 34. Ahrén B, Johnson SL, Stewart M, et al HARMONY 3: 104‐week randomized, double‐blind, placebo and active controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care 2014; 37: 2141–2148. [DOI] [PubMed] [Google Scholar]
- 35. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015; 58: 429–442 &Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 36. Ahrén B. Insulin plus incretin. A glucose‐lowering strategy for type 2‐diabetes. World J Diabetes 2014; 15: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahrén B. Physiological aspects of the combination of insulin and GLP‐1 in the regulation of blood glucose control. Diabetes Metab 2015; 41: 6S3–6S8. [DOI] [PubMed] [Google Scholar]
- 38. Billings LK, Doshi A, Gouet D, et al Efficacy and safety of IDegLira versus basal‐bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care 2018; 41: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 39. Rosenstock J, Aronson R, Grunberger G, et al Benefits of LixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan‐O Randomized Trial. Diabetes Care 2016; 39: 2026–2035. [DOI] [PubMed] [Google Scholar]
- 40. Rosenstock J, Buse JB, Azeem R, et al Efficacy and safety of ITCA650, a novel drug‐device GLP‐1 receptor agonist, in type 2 diabetes uncontrolled with oral antidiabetic drugs: the FREEDOM‐1 trial. Diabetes Care 2018; 41: 333–340. [DOI] [PubMed] [Google Scholar]
- 41. Davies M, Pieber TR, Hartoff‐Nielsen ML, et al Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 2017; 318: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lingvay I, Desouza CV, Lalic KS, et al A 26‐week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care 2018; 41: 1926–1937. [DOI] [PubMed] [Google Scholar]


