Abstract
The paper provides the issues regarding the body composition change by sodium–glucose cotransporter 2 inhibitors.
To date, diabetes treatment has focused on the prevention of chronic complications as a result of microangiopathy and cardiovascular diseases, as well as acute complications with diabetic ketoacidosis and diabetic hyperosmolar hyperglycemic coma. Recently, the prevention of sarcopenia, a dysnutrition state accompanying aging or obesity in patients with diabetes, has become a new target of diabetes treatment.
Sarcopenia is characterized by reduced skeletal muscle mass and decreased muscle functions (i.e., muscular strength and performance) that are accompanied by a decrease in physical activity. It is a serious problem owing to the risk of falling and bone fractures, and the state‐of‐life prognosis, including risk of aspiration pneumonia from reduced swallowing ability or frailty. Frailty refers to a state wherein the vulnerability of the entire body is elevated, usually resulting from various pathological conditions, such as a decrease in mental and/or physical activity. Frailty can also occur from repeated severe hypoglycemia, associated with diabetes treatment. Once in the state of frailty, a patient can experience dysphagia as a result of muscle weakness in swallowing and exacerbation of anorexia, which can lead to further malnutrition. In this way, frailty based on sarcopenia forms a vicious cycle (Figure 1). Therefore, it is important to cut off this cycle through effective therapeutic interventions1. Sarcopenia and frailty have, however, few early diagnostic methods, such as biomarkers, and therapeutic treatment so far. For the definition and diagnostic criteria of sarcopenia, consensus by the European Working Group on Sarcopenia in Older People was announced in 20102. The body composition of an average Asian person is apparently different from that of a European person; hence, the diagnostic criteria of sarcopenia for Asian people should be independently developed. With this perspective, consensus by the Asian Working Group on Sarcopenia in Older People was subsequently announced in 20143. In these consensuses, sarcopenia was originally determined based on the data obtained by dual‐energy X‐ray absorption method. In recent years, the bioelectrical impedance method has gained popularity as a simplified method, although its evaluation remains to be established owing to accuracy errors.
Figure 1.
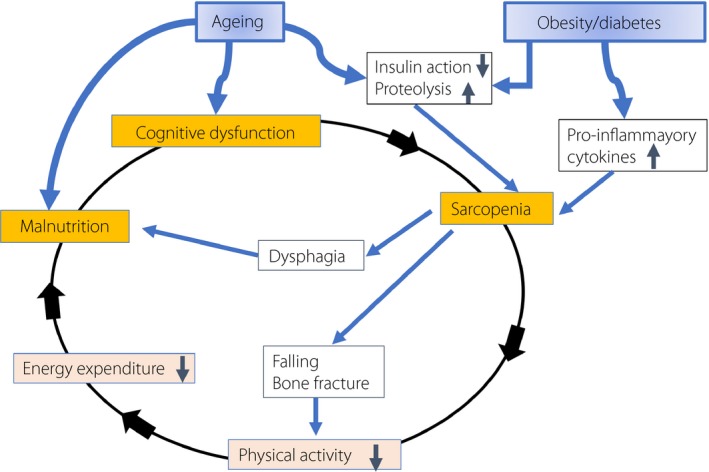
The frailty cycle. Hormonal and nutritional change with aging causes malnutrition. In addition, a loss or decrease in function of insulin causes proteolysis in skeletal muscle. These conditions both lead to the frailty cycle, a vicious circle.
In addition to age‐related or primary sarcopenia, dysnutrition based on metabolic syndrome with visceral fat accumulation and decreased muscle mass, regardless of age, has been recognized as secondary sarcopenia. It has been shown that patients with poorly controlled glycemia of diabetes have significantly less muscle volume4. Furthermore, the possibility that this muscle loss might be exacerbated even by diabetes treatment has been indicated when measured as total lean mass5. It has been recently clarified that sodium–glucose cotransporter 2 (SGLT2) inhibitors show excellent effects in treating type 2 diabetes not only by correcting hyperglycemia and reducing the risk of cardiovascular events, but also by reducing the body fat mass, including visceral fat. Symptoms based on insulin resistance should be therefore further alleviated by this decrease in visceral fat mass. The mechanism for such a reduction in fat mass with the use of SGLT2 inhibitor is believed to be a result of lipolysis in the adipose tissue due to activation of gluconeogenesis (Figure 2). There is a concern, however, that the activation of the gluconeogenic system should induce not only lipolysis in the adipose tissue, but also proteolysis in the skeletal muscle that supplies amino acids to the liver as a substrate and can therefore lead to sarcopenia.
Figure 2.
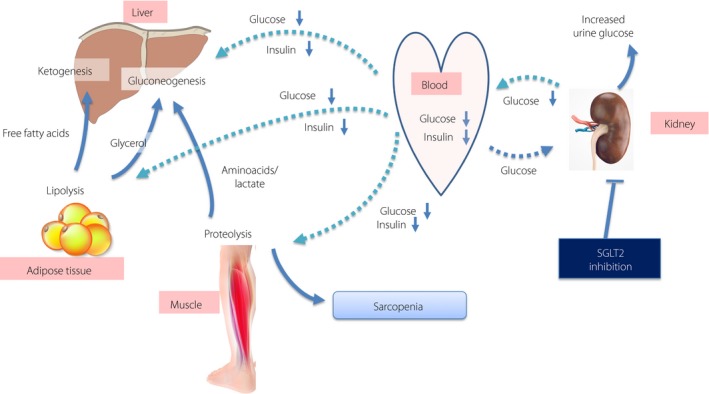
Sarcopenia and sodium–glucose cotransporter 2 (SGLT2) inhibitors. Decreased serum insulin concentration causes a gluconeogenesis in liver and proteolysis in skeletal muscle. This process could cause sarcopenia if its extent is severe.
To address this issue, we carried out a clinical study6 using luseogliflozin, an SGLT2 inhibitor, for patients with Japanese type 2 diabetes who had an average body mass index of 28 kg/m2. The mean change in the lean body mass was −0.79 kg at week 24 compared with that at baseline, and it was −0.99 kg at week 52 of treatment, which is a significant decrease. In addition, we measured the skeletal muscle index (SMI), which estimates the whole‐body skeletal muscle mass more accurately than the total lean body mass. We did not find any significant change at week 24 by SMI, albeit a significant decrease of −0.151 kg/m2 muscle mass was detected at week 36, followed by a decrease of −0.155 kg/m2 at week 52 with no further decline. The proportion of the body composition was, however, favorably changed. Unlike the changes in bone mineral content, which did not detect any significant change at week 52, SMI was considered to be most likely influenced by the use of an SGLT2 inhibitor, because the decrease in SMI became significant after 36 weeks from the initiation of SGLT2 inhibitor treatment. A report on the other study with canagliflozin, another SGLT2 inhibitor, was also made in which SMI also showed similar trends7.
As mentioned earlier, it is an extremely important finding in clinical diabetes practice that the reduction of skeletal muscle mass can occur during treatment with SGLT 2 inhibitors, although the reduction is small. This finding raises questions on the effective means of preventing muscle loss and sarcopenia.
It is estimated that diabetes mellitus might soon become a highly frequent disease among the elderly and individuals with restricted physical activity. Thus, establishing new guidelines for diet therapy and exercise applicable to type 2 diabetes is critical. In fact, determining the nutritional content of a diet that can promote and induce insulin secretion after meals is urgent, and so is investigating the nutritional balance and types of exercise required to maintain skeletal muscles. In this step, we are actively seeking such molecular research against sarcopenia for publication in our journal.
DISCLOSURE
The author received lecture fees from Taisho Toyama Pharmaceutical Co., Ltd., and research support, consulting fees and lecture fees from Taisho Pharmaceutical Co., Ltd. and Novo Nordisk Pharma. The sponsors had no role in the research design, data collection, data analysis, data interpretation and report preparation.
References
- 1. Xue QL, Bandeen‐Roche K, Varadhan R, et al Initial manifestations of frailty criteria and the development of frailty phenotype in the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci 2008; 63: 984–990. [DOI] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen LK, Liu LK, Woo J, et al Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 4. Park SW, Goodpaster BH, Strotmeyer ES, et al Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007; 30: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 5. Bolinder J, Ljunggren Ö, Kullberg J, et al Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 6. Sasaki T, Sugawara M, Fukuda M. Sodium‐glucose cotransporter 2 inhibitor‐induced changes in body composition and simultaneous changes in metabolic profile: 52‐week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig 2019; 10: 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cefalu WT, Leiter LA, Yoon KH, et al Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]


