Abstract
Aims/Introduction
To determine the current clinical preferences of anti‐vascular endothelial growth factor (VEGF) treatment protocols for diabetic macular edema (DME) in Japan.
Materials and Methods
This was a descriptive cross‐sectional study. Answers to a questionnaire consisting of 16 questions were obtained from 176 of 278 (63.3%) surveyed ophthalmologists.
Results
The results showed that 81.2% preferred intravitreal injections of anti‐VEGF antibodies as the first‐line therapy. The most important indicators for beginning anti‐VEGF therapy were: the best‐corrected visual acuity in 44.3% and the retinal thickness in 30.7%. In the loading phase, 53.4% preferred a single injection, and in the maintenance phase, 75.0% preferred the pro re nata regimen. Financial limitation (85.8%) was reported as the most important difficulty in the treatment. For combination therapy with anti‐VEGF treatment, panretinal photocoagulation, focal photocoagulations and a sub‐Tenon steroid injection were preferred. The contraindications for anti‐VEGF therapy were: prior cerebral infarction (72.7%). Regarding the use of both approved anti‐VEGF agents in Japan, ranibizumab and aflibercept, 39.8% doctors used them appropriately.
Conclusions
Our results present the current clinical preferences of anti‐VEGF treatment for DME in Japan. The best‐corrected visual acuity and the retinal thickness are important indicators to institute this therapy. The majority of the ophthalmologists use anti‐VEGF treatment as first‐line therapy and prefer the 1 + pro re nata regimen.
Keywords: Clinical practice pattern, Diabetic macular edema, Vascular endothelial growth factor
Introduction
Diabetic retinopathy (DR) is a leading cause of blindness, and it is present in over one‐half of patients with diabetes mellitus of >20 years duration1. Diabetic macular edema (DME) is the most common cause of the vision reduction in eyes with DR2. Various surgical and medical therapies have been used to treat DME, and pharmacological therapies; for example, anti‐vascular endothelial growth factor (VEGF) antibodies, have become the first‐line therapy for DME. VEGF has been shown to play a major role in the progression of DME, and anti‐VEGF agents; for example, bevacizumab (Avastin™; Genentech, San Francisco, CA, USA), ranibizumab (Lucentis™; Genentech) and aflibercept (Eylea™; Regeneron Pharmaceuticals, Tarrytown, NY, USA) are commercialized anti‐VEGF agents. The results of many randomized clinical trials (RCTs) have found that treatment with anti‐VEGF agents led to a rapid improvement of vision in eyes with DME. The results of RCTs have shown the superiority of intravitreal anti‐VEGF treatments over existing macular focal/grid laser photocoagulations (PC) and intravitreal triamcinolone acetonide (IVTA) injections3, 4. Thus, anti‐VEGF treatment has become the first‐line therapy for DME. Even though the results of these clinical trials are well known and many ophthalmologists have changed their treatment regimen for DME, it is difficult to rely on the results of RCTs alone, as they have limitations, such as the inclusion criteria, treatment options and initial visual acuities. In addition, two of the anti‐VEGF agents, ranibizumab and aflibercept, have been approved for DME with comparable prices. Different from other countries, sustained‐release steroid agents and bevacizumab have not been approved in Japan. There still exists some skepticism of the results of the RCTs, as they were carried out with specific populations and in specialized environments.
To overcome differences between the RCTs and the real‐world clinical practice, the need for “real‐world evidence (RWE)” has been advocated5. Different from the traditional RCTs, RWE studies are population‐based observational studies, and are less prone to selection with referral biases, which can identify gaps from the RCT results6. For example, the Aspirin Dosing: A Patient‐Centric Trial Assessing Benefits and Long‐Term Effectiveness (ADAPTABLE) study is an ongoing representative large RWE study with 20,000 participants assigned two dose regimens (81 vs 325 mg) of aspirin to prevent heart attacks and strokes7. However, because RWE can be used in multiple research interventions at the levels of health systems, practices and hospitals, appropriate analyses of large numbers using cluster randomization is useful to strengthen the RWE results8. In fact, there are many recent reports on DME treatment based on RWE studies9, 10, 11.
As the first step of translating the RWE results for DME treatment, it is important to understand how physicians use anti‐VEGF agents in the real‐world. For this, a questionnaire‐based survey of ophthalmologists about treatment protocols was carried out in Canada and France12, 13. The results of the real‐world treatment protocols on randomized patients are required to establish general clinical protocols for the treatment of DME in Japan.
Thus, the aim of the present study was to determine the current clinical preferences and trends of anti‐VEGF treatments for eyes with DME in Japan. To accomplish this, we surveyed 176 ophthalmologists in Japan with a questionnaire.
Methods
Study design
This was a descriptive cross‐sectional study. A questionnaire was sent to 278 ophthalmologists in Japan between March 2016 and June 2017. All ophthalmologists were treating DME patients and were informed that their identity would be anonymous.
The protocol for this study was approved by the institutional review board of Mie University Graduate School of Medicine (No. 1598), and it conformed to the principles of Good Clinical Practice and the Helsinki Guidelines. This prospective study was registered at http://www.umin.ac.jp (UMIN000025093).
Questionnaire
The questionnaire consisted of 16 questions divided into three categories: I, General Matters; II, Treatment Practice Pattern; and III, Alternative Therapy (Table 1).
Table 1.
Demographics of respondents
| Classification | Question |
|---|---|
| I. General Matters | |
| (I‐1) Background | (I‐1‐①) Practice years |
| (I‐2) Changes in attitude | (I‐2‐①) Anti‐VEGF therapy changed your therapeutic strategies for DME treatment? |
| (I‐2‐②) Anti‐VEGF is more effective than STTA? | |
| (I‐3) Management before and after Injection | (I‐3‐①) How do you use topical antibiotics? |
| (I‐3‐②) When is the initial examination after injection? | |
| II. Treatment Practice Pattern | |
| (II‐1) Initial DME Treatment | (II‐1‐①) What kind of therapy is used as the initial therapy for DME (multiple choice) |
| (II‐2) Anti‐VEGF Treatment regimen | (II‐2‐①) When did you decide to use anti‐VEGF for DME for the first time? |
| (II‐2‐②) What is the most important assessment indicator for initial therapeutic intervention? | |
| (II‐2‐③) How many anti‐VEGF injections for DME do you perform? (loading phase) | |
| (II‐2‐④) How do you treat anti‐VEGF injections for DME after an effective loading phase? (maintenance phase) | |
| (II‐2‐⑤) Maximum number of anti‐VEGF injections you perform before incorporating other therapy | |
| (II‐2‐⑥) What is an important problem of anti‐VEGF treatment for DME? | |
| III. Alternative Therapy | |
| (III‐1) Combination Therapy | (III‐1‐①) What kind of combination therapy with anti‐VEGF treatment is used for DME? (multiple choice) |
| (III‐1‐②) Which systemic disorder you pay attention with anti‐VEGF treatment for DME? (multiple choice) | |
| (III‐2) Alternative use of anti‐VEGF agents | (III‐2‐①) Do you use two approved anti‐VEGF agents (ranibizumab and aflibercept) properly? |
| (III‐2‐②) On what basis do you distinguish them? | |
DME, diabetic macular edema; STTA, sub‐Tenon triamcinolone acetonide injection; VEGF, vascular endothelial growth factor.
The questions in the General Matters included: I‐1, background (1 question); I‐2, changes in attitude to treatment (2 questions); and I‐3, management before and after injection (2 questions). The questions in Treatment Practice Pattern included: II‐1, initial DME treatment (1 question); and II‐2, anti‐VEGF treatment regimen (6 questions). The questions in Alternative Therapy included: III‐1, use of combination therapy (2 questions); and III‐2, alternative use of anti‐VEGF agents (2 questions).
After the questionnaires were returned, the results were cataloged and analyzed. The percentage of answers to each question was calculated and is presented as the ratio of respondents per question. The comparisons of the demographic characteristics of the respondents were purely descriptive. The percentages in the charts and graphs do not add up to exactly 100%, and the differences are due to the rounding process. Because some of the questions consisted of multiple choices, the distribution of the results can sometimes exceed 100%.
Results
General matters
A total of 176 of 278 surveys (63.3%) were completed and returned from 151 hospitals in 11 of the 47 prefectures of Japan. The length of practice of the respondents ranged from 1 to 42 years: 14 had been in practice for <5 years, 27 for 5–10 years, 60 for 11–20 years and 64 for >21 years (Figure 1a). The analysis showed that 86.4% of the respondents believed that the approval and accessibility of anti‐VEGF treatments changed their therapeutic strategies for treating DME, and 11.4% did not (Figure 1b). In addition, 97.2% believed that anti‐VEGF treatment was more effective than a sub‐Tenon triamcinolone acetonide (STTA) injection, and 2.8% did not (Figure 1c).
Figure 1.
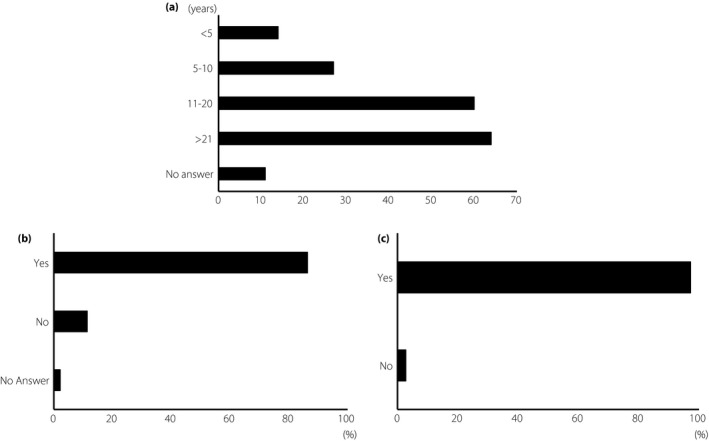
Background and changes in attitude. (a) Practice years are shown. (b) The question on whether anti‐vascular endothelial growth factor treatment changed the therapeutic strategies for diabetic macular edema. (c) The question of whether anti‐vascular endothelial growth factor therapy is superior to sub‐Tenon triamcinolone acetonide injection.
On the use of antibiotics, 72.7% of the respondents used antibiotics pre‐ and post‐injections, with a duration of 3–7 days; 19.3% used antibiotics only post‐injections; and 2.3% used pre‐injections only. In addition, 2.8% did not use any antibiotics during the treatment period, and 2.8% used antibiotics only on the day of the injection (Figure 2a).
Figure 2.
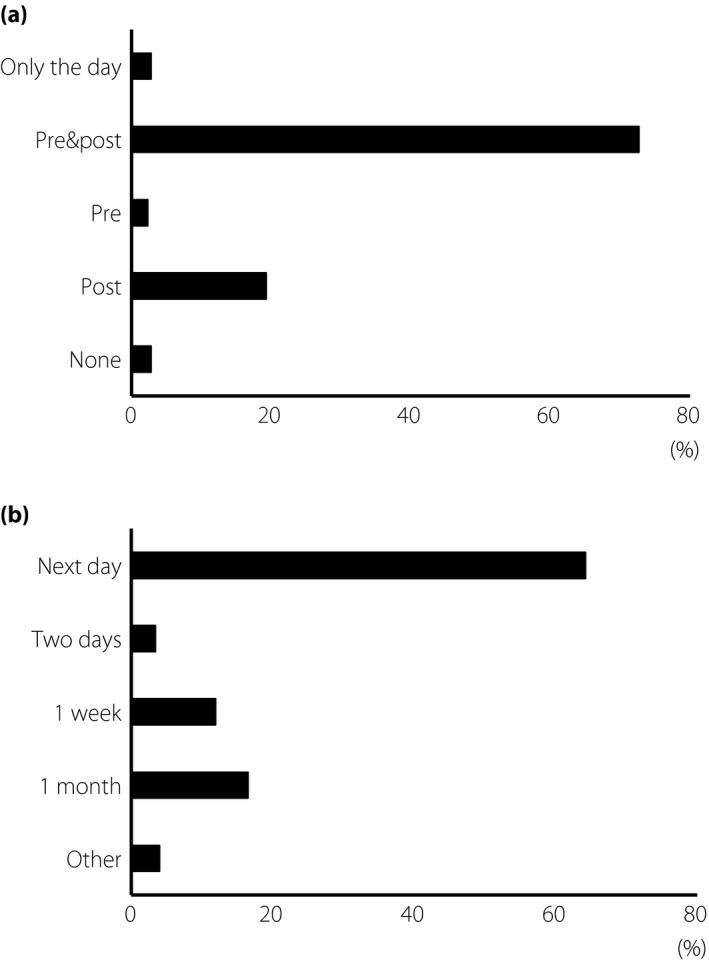
Management before and after injection. (a) The question on how the topical antibiotics were used. Choices of answer were: only the day of injection, both pre‐ and post‐injection, only pre‐injection, only post‐injection and none. (b) The question about the timing of initial examination was also asked. Choices of answer were: the day after the injection, 2 days after the injection, 1 week after the injection, 1 month after the injection and other.
The post‐injection examination protocols were: 64.2% examined their patients on the day after the injections, 11.9% at 1 week and 3.4% at 2 days. Additionally, 16.5% of the respondents examined their patients at 1 month after the injections (Figure 2b).
Treatment practice pattern
The answers for the initial therapy of DME (multiple answers) were: 81.2% used anti‐VEGF treatment, 52.8% used STTA, 49.4% used focal‐PC, 9.7% used grid‐PC, 9.1% used vitrectomy and 7.4% used IVTA.
The time when anti‐VEGF agents were used for the first time: 42.5% used it as the first choice, 40.3% used it when other therapies did not reduce the DME, 13.3% used it at the request of the patients and 3.9% used it on other occasions.
The factors that were used to begin the initial therapeutic intervention were: 44.3% used the visual acuity, 30.7% used the optical coherence tomography findings, and 22.2% used the patients’ complaints (Figure 3a).
Figure 3.
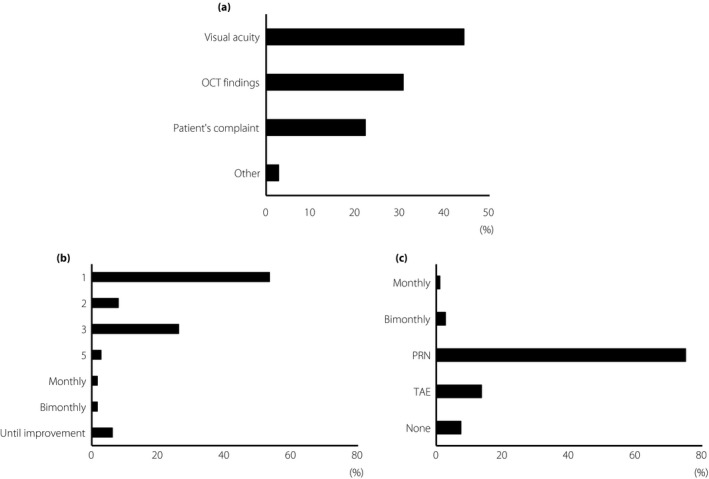
Anti‐vascular endothelial growth factor treatment practice pattern. (a) The question (multiple choice) was about the most important assessment indicator for initial therapeutic intervention. The choices of answer were: visual acuity, optical coherence tomography (OCT) findings, compliance and other. (b) The question about the initial injection numbers in the loading phase. The choices of answer were: one, two, three or five injections, or monthly, bimonthly or until improvement. (c) The question about additional injections after an effective loading phase (maintenance phase). The choices of answer were: monthly injection, bimonthly injection, pro re nata regimen (PRN) injection, treat and extend regimen (TAE) injection, monthly injection until stabilization, no additional injections or other.
The number of injections used during the loading phase was: a single injection by 53.4% of the respondents, two consecutive monthly injections by 8%, three consecutive monthly injections by 26.1%, five consecutive monthly injections by 2.8%, monthly injections by 1.7%, bimonthly injections by 1.7% and monthly injections until an improvement of the DME was detected in 6.2% of the respondents. For all respondents, 46.6% preferred multiple injections (Figure 3b).
During the maintenance phase after a successful loading phase, 75.0% of the respondent used a pro re nata (PRN) regimen, 13.6% used the treat and extend regimen, 2.8% used bimonthly injections, 1.1% preferred monthly injections and 7.4% did not use any additional injections (Figure 3c).
The maximum number of anti‐VEGF injections used before incorporating other therapy was three injections by 37.0% of the respondents, two injections by 18.2%, one injection by 5.5%, four injections by 3.9% and five injections by 3.9%. Finally, 63.0% of the respondents preferred two to five injections. A total of 30.9% of the respondents did not answer the question on the maximum number of injections (Figure 4a).
Figure 4.
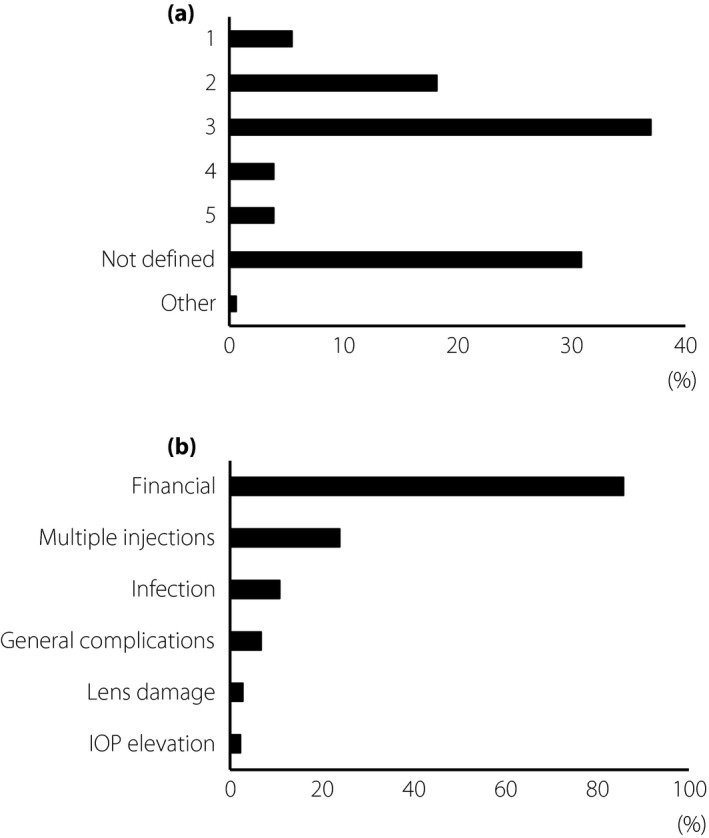
Continuous injections and problems. (a) The question about the maximum number of anti‐vascular endothelial growth factor injections before using other therapies. The choices of answers were: once, twice, three times, four times, five times, not defined and other. (b) The question about important problems of anti‐vascular endothelial growth factor therapy (multiple answers). The choices of answer were: financial, multiple injections, infection, general complications, lens damage, intraocular pressure (IOP) elevation and other.
The results to the question on whether to continue with the anti‐VEGF therapy in both responders and non‐responders showed that 85.8% of the respondents believed that the financial cost was an important factor, and 23.9% believed that the frequency of the injections was an important factor (Figure 4b). Other factors included probability of intraocular infection (10.8%), general complications (6.8%) and other ocular complications; for example, 2.8% for lens damage and 2.3% for intraocular pressure elevation.
Alternative therapy
On the question of combining other therapies with the anti‐VEGF injections, 68.2% of the respondents preferred panretinal PC, 56.8% preferred focal PC and 50.0% preferred STTA. Other answers included IVTA (8.0%), grid PC (5.1%), vitrectomy (1.7%) and no additional treatment required (5.7%; Figure 5a).
Figure 5.
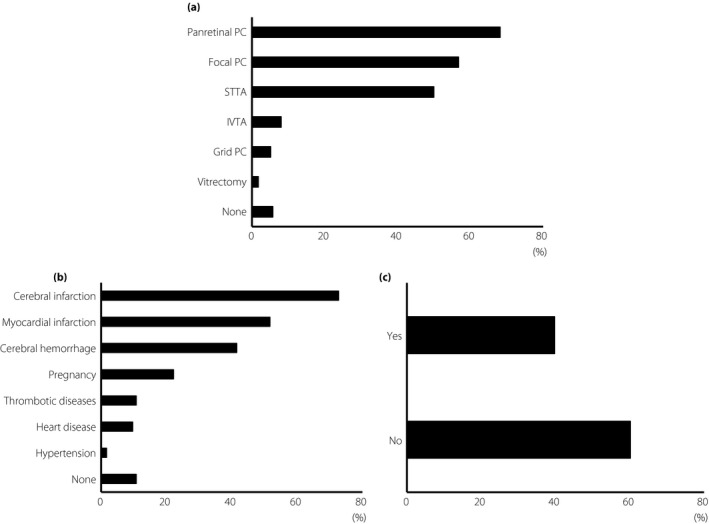
Alternative therapy. (a) The question about combination therapy with anti‐vascular endothelial growth factor injections (multiple choice). The choices of the answers were: panretinal photocoagulation (PC), focal photocoagulation (PC), sub‐Tenon triamcinolone acetonide injection (STTA), intravitreal triamcinolone acetonide injection (IVTA), macular grid photocoagulation (PC), vitrectomy, none or other. (b) The question regarding safety (multiple choice). The choices of answers were: cerebral infarction, myocardial infarction, cerebral hemorrhage, potential pregnancy, thrombotic disease, heart disease, hypertension and none. (c) The question about whether two approved anti‐vascular endothelial growth factor agents, ranibizumab and aflibercept, were used was also asked.
There was one question on the safety of using intravitreal injections of the anti‐VEGF agents to treat the DME: 72.7% believed that a prior cerebral infarction was a contraindication, and 51.7% believed that a prior myocardial infarction was also a contraindication. Other contraindications were cerebral hemorrhage (41.5%), concurrent pregnancy (22.2%), thrombotic diseases (10.8%), heart disease (9.7%) and hypertension (1.7%). In contrast, 10.8% did not mention anything about the history of patients as a determiner of using anti‐VEGF therapy (Figure 5b)
For the question about the preference of ranibizumab or aflibercept, 39.8% of the respondents used them as appropriate (Figure 5c). Among these, we also asked how the respondents selected which was used. Of the respondents, 68.3% reported that the general condition of the eye and patient were the factors, 19.0% replied that the visual acuity or optical coherence tomography findings were the factors, 9.5% replied the age and 3.2% replied with other reasons.
Discussion
The present findings show the methods used to treat DME by 176 ophthalmologists throughout Japan. Multiple factors, including the physicians’ and patients’ background, influenced the practice pattern that can cause differences in the findings from those of RCTs. The present results reflect the current clinical protocols for treatment protocols for DME in Japan, and the results become the first step to translating the real‐world results to the general clinical protocols for the treatment of DME.
In this survey, 176 of 278 ophthalmologists (63.3%) responded to a 16‐item questionnaire. Thus, the present results should be a better representation than that of similar surveys in Canada (55.4% of 61 ophthalmologists requested)12 or France (52% of 95 ophthalmologists requested)13. The total number of members of the Japan Retina and Vitreous Society, who are retinal specialists, was 3,091 in 2017. We sent questionnaires to 278 specialists from 11 of 47 prefectures who participated in J‐CREST. Thus, 9% of all retinal specialists in Japan participated. When we consider such a questionnaire‐based survey from a population of 3,000, an adequate number of participants was estimated to be 100 (margin of error 10%) to 350 (margin of error 5%)14. Because not all retinal specialists treat DME regularly, we can conclude that the number of participants for the present study was statistically adequate.
We asked some general questions. STTA is frequently used for DME treatment in Japan because of its ease of use, low cost and potentially fewer complications compared with IVTA. However, the effectiveness of STTA is limited to only some DME patients, and it is often necessary to switch to anti‐VEGF treatment. This trend changed after 2006, because the effectiveness of bevacizumab was reported15 and ranibizumab was approved in 2014 in Japan. In fact, the present results showed that most of the respondents reported that anti‐VEGF treatment changed their therapeutic strategies for managing DME (86.4%). However, there was an opposite answer from 11.4% of the responders. Although the results of DRCR.net Protocol‐I showed the effectiveness of anti‐VEGF treatment, it is not effective for all eyes with DME, and just 60% of the eyes had their CRT reduced to <250 μm even with continuous anti‐VEGF treatment4. Other therapies were not necessarily ineffective for some cases; STTA is reported to be effective for some patients when combined with focal PC16. The present results and these reports emphasized the difficulty of managing DME effectively.
We also questioned the participants about the use of antibiotics. The guidelines from the USA have stated that pre‐ and postoperative antibiotics had no significant effects on the complications of intravitreal injections17. However, the guidelines from the Japanese Retina and Vitreous Society show that the use of antibiotics and examinations during and after intravitreal injections were left to the ophthalmologists’ discretion18. The prescribing information for the use of aflibercept and ranibizumab from the Japanese manufacturers states that the use of antibiotics during therapy was recommended. From these statements in Japan, most of the participants of our survey chose to use antibiotics pre‐ and post‐injection of the anti‐VEGF agent. In addition, the Japanese guideline states that there is no fixed time for the examinations after treatment, and state that they should examine patients only when some abnormal conditions are present. In our survey, 64.2% of the respondents examined their patients on the day after the anti‐VEGF injection. The present results showed a compliance with the Japanese guidelines on the examination and prescribing protocols. However, because we carried out this survey in various prefectures, including urban and rural areas, the ease of access to the examination venues was most likely different, which may might affected the answers.
Our survey showed that 63.0% of the ophthalmologists used more than one injection of the anti‐VEGF agents as the maximum number, and most frequently used three injections (37.0%). However, it is still not clear how many injections during the loading phase are best for DME treatment. From RCTs, monthly injection was most frequently carried out in the Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus (RISE) and A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus (RIDE) studies19, and most of the other RCT protocols stated multiple loading injections; three initial consecutive injections in the Safety and Efficacy of Ranibizumab in Diabetic Macular Edema With Center Involvement (RESOLVE)20 and Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema (RESTORE) Studies21, four initial consecutive injections in the DRCR.net Protocol‐I3, five initial consecutive injections in the Intravitreal Aflibercept Injection in Vision Impairment due to DME (VIVID‐DME) and Study of Intravitreal Aflibercept Injection in Patients with DME (VISTA‐DME) study22, and monthly injections until stabilization in the DRCR.net protocol‐T23. The results from these RCT studies showed that these loading injections were sufficient. In addition, subgroup analysis from the DRCR.net protocol‐I showed that eyes with suboptimal early vision response after an initial three consecutive injections had poorer long‐term (3 years) visual outcomes than eyes with pronounced early responses. These results show the importance of the initial three consecutive injections, and also that anti‐VEGF treatment is not necessarily appropriate for all cases. From this, it can be inferred that the initial consecutive injections are important to rule out the non‐responders24. In addition, different from the RCTs, there were differences in the demographics of the patients, including their insurance plans. It is still important to establish adequate loading injection numbers when considering both the results of the RCTs and real‐world practices. Although a meta‐analysis of 21 randomized cohort studies25 and the results of 10 RCTs26 reported that the frequency of related Antiplatelet Trialists’ Collaborations events was not so high, the prescribing information for allibercept and ranibizumab did mention concern about thromboembolic events (Lucentis™ prescribing information from Genentec, https://www.gene.com/patients/medicines/lucentis). Some clinical studies also reported that the concentration of the anti‐VEGF drugs differed between the agents during anti‐VEGF treatment for age‐related macular degeneration27, 28, 29. This implies that there might be differences in the risk for thromboembolic events between the two anti‐VEGF agents. Although it is still not known whether intravitreal injection of anti‐VEGF agents affected the systemic VEGF, this lack of information creates a concern in Japanese physicians when they carry out anti‐VEGF treatments.
In Japan, two anti‐VEGF agents have been approved for use in patients with DME, and they have comparable prices. The DRCR.net Protocol‐T23 reported that there might exist different effectiveness between these two agents. Among our respondents, 39.8% used the two approved agents appropriately, and among them, 68.3% used them according to the general complications and 19.0% used them based on the differences in their effectiveness. Alternative uses of the approved anti‐VEGF agents are effective for some patients, because each agent has a different pharmacological aspect.
The rationale for combination therapy is that it might improve the effectiveness of a single therapy or might reduce the number of injections that would reduce the chances of complications and lessen the financial burdens. Because sustained‐release steroid agents have not been approved in Japan, the treatment patterns in Japan are different from other countries. From our survey, just 5.7% of the respondents used anti‐VEGF monotherapy, which indicates that physicians are aware of the difficulties in managing DME with only anti‐VEGF therapy. Thus, some type of combination therapy including PC or STTA needs to be considered, although their effectiveness has not been fully determined.
Comparing the present results with the previous surveys in other countries, there are similar trends. From the real‐world patient data in Denmark and the USA, the frequency of use of anti‐VEGF agents is increasing, especially after governmental approval11, 30. From the European Union survey, the average injection numbers of anti‐VEGF agents were low, which might indicate the difficulty of frequent injections except in RCTs31. Ogura et al.32 reported on the clinical practice patterns of 83 retinal specialists in Japan. Similar to the present results, 76.3% of the ophthalmologists preferred the PRN regimen, but 50.0% responded that the treat and extend regimen was ideal. Although the results tended to be similar to the present results, they differed from our survey because the respondents were mainly retinal specialists practicing at university hospitals or general hospitals. Our survey included not only university hospitals, but also small primary and private clinics. The present results complement earlier reports, and we can determine the Japanese general clinical treatment protocol for DME from both types of results. However, it is necessary to carry out similar surveys frequently, because the trends also change with time, and the introduction of new drugs and procedures.
Different from other retinal disease treated with anti‐VEGF agents; that is, age‐related macular degeneration, retinal vein occlusion and myopic choroidal neovascularization, there exist differences between the BCVA and retinal thickness for the treatment protocol for DME33. In our respondents, 44.3% used anti‐VEGF based on the BCVA, and 30.7% based on the optical coherence tomography findings. These results reflect a unique aspect of DME in the actual clinical practice. Second, most ophthalmologists prefer one‐time injection in the loading phase and PRN for the maintenance phase. Therefore, the results indicated that the 1 + PRN regimen is preferred in Japan.
The present study had several limitations. First, the study design was not based on clinical patient visits. Owing to the indirect inclusions, the study could be susceptible to physicians’ bias. Second, the real‐world trend from our survey does not necessarily mean that they are the most effective treatment. Although our results showed that the 1 + PRN regimen is preferred, it is still unclear whether the 1 + PRN regimen is effective. We must realize the differences between RCT and real‐world trends, and the present results are not from patient data, but generally, retrospective studies using RWE are considered to be of less statistical and clinical value than prospective RCT results. However, this weakness might be lessened by using many participants34.
In conclusion, the present study showed the Japanese trend of anti‐VEGF treatment. It will be important to continue to survey new indications of drugs to receive both high‐quality and cost‐effective care from analysis of real‐world trends.
Disclosure
Masahiko Sugimoto has received financial support from Alcon Pharma and Bayer, and royalties from Alcon Pharma, Kowa Pharma, Senjyu Pharma, Daiichi Yakuhin Sangyo, Bayer and Wakamoto Pharma. The other authors declare no conflict of interest.
Acknowledgments
We thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute of the University of Miami for critical discussion and English correction. We also thank Bayer Yakuhin Ltd. (Osaka, Japan) for the grant support with this study.
J Diabetes Investig 2019; 10: 475–483
Clinical Trial Registry University Hospital Medical Information NetworkUMIN000025093
References
- 1. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010; 376: 124–136. [DOI] [PubMed] [Google Scholar]
- 2. Moss SE, Klein R, Klein BEK. The incidence of visual loss in a diabetic population. Ophthalmology 1988; 95: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 3. Diabetic Retinopathy Clinical Research Network , Elman MJ, Aiello LP, et al Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117: 1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen QD, Shah SM, Khwaja AA, et al Two‐year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. READ‐2 Study Group. Ophthalmology 2010; 117: 2146–2151. [DOI] [PubMed] [Google Scholar]
- 5. Sherman RE, Anderson SA, Dal Pan GJ, et al Real‐world evidence – what is it and what can it tell us? N Engl J Med 2016; 375: 2293–2297. [DOI] [PubMed] [Google Scholar]
- 6. Booth CM, Tannock IF. Randomised controlled trials and population‐based observational research: partners in the evolution of medical evidence. Br J Cancer 2014; 110: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnston A, Jones WS, Hernandez AF. The ADAPTABLE trial and aspirin dosing in secondary prevention for patients with coronary artery disease. Curr Cardiol Rep 2016; 18: 81. [DOI] [PubMed] [Google Scholar]
- 8. Anderson ML, Califf RM, Sugarman J. Ethical and regulatory issues of pragmatic cluster randomized trials in contemporary health systems. Clin Trials 2015; 12: 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egan C, Zhu H, Lee A, et al The United Kingdom diabetic retinopathy electronic medical record users group, report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol 2017; 101: 75–80. [DOI] [PubMed] [Google Scholar]
- 10. Ziemssen F, Feltgen N, Holz FG, et al Demographics of patients receiving Intravitreal anti‐VEGF treatment in real‐world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmol 2017; 17: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vorum H, Olesen TK, Zinck J, et al Real‐world evidence of use of anti‐VEGF therapy in Denmark. Curr Med Res Opin 2016; 32: 1943–1950. [DOI] [PubMed] [Google Scholar]
- 12. Schweitzer KD, Eneh A, Gale J. Practice patterns of Canadian vitreoretinal specialists in diabetic macular edema treatment. Can J Ophthalmol 2011; 46: 227–231. [DOI] [PubMed] [Google Scholar]
- 13. Qu‐Knafo L, Fajnkuchen F, Sarda V, et al French practice patterns in the management of diabetic macular edema. J Fr Ophtalmol 2016; 39: 521–526. (French) [DOI] [PubMed] [Google Scholar]
- 14. Singh AS, Masuku MB. Sampling technique & determination of sample size in applied statistics research: an overview. Int J Econom, Commerce Manage 2014; 11: 1–22. [Google Scholar]
- 15. Haritoglou C, Kook D, Neubauer A, et al Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006; 26: 999–1005. [DOI] [PubMed] [Google Scholar]
- 16. Tunc M, Onder HI, Kaya M. Posterior sub‐tenon's capsule triamcinolone injection combined with focal laser photocoagulation for diabetic macular edema. Ophthalmology 2005; 112: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 17. Avery RL, Bakri SJ, Blumenkranz MS, et al Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina 2014; 34(Suppl 12): S1–S18. [DOI] [PubMed] [Google Scholar]
- 18. Ogura Y, Takahashi K, Iida T. Guidelines for intravitreal injection for macular diseases Nippon Ganka Gakkai Zasshi. J Jpn Ophthalmol Soc 2016; 120: 87–90. [PubMed] [Google Scholar]
- 19. Nguyen QD, Brown DM, Marcus DM, et al Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- 20. Massin P, Bandello F, Garweg JG, et al Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12‐month, randomized, controlled, double‐masked, multicenter phase II study. Diabetes Care 2010; 33: 2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitchell P, Bandello F, Schmidt‐Erfurth U, et al The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625. [DOI] [PubMed] [Google Scholar]
- 22. Brown DM, Schmidt‐Erfurth U, Do DV, et al Intravitreal aflibercept for diabetic macular edema: 100‐Week results from the VISTA and VIVID studies. Ophthalmology 2015; 122: 2044–2052. [DOI] [PubMed] [Google Scholar]
- 23. Diabetic Retinopathy Clinical Research Network , Wells JA, Glassman AR, et al Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372: 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gonzalez VH, Campbell J, Holekamp NM, et al Early and long‐term responses to anti‐vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol 2016; 172: 72–79. [DOI] [PubMed] [Google Scholar]
- 25. Thulliez M, Angoulvant D, Le Lez ML, et al Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor monoclonal antibodies: systematic review and meta‐analysis. JAMA Ophthalmol 2014; 132: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 26. Kitchens JW, Do DV, Boyer DS, et al Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology 2016; 123: 1511–1520. [DOI] [PubMed] [Google Scholar]
- 27. Avery RL, Castellarin AA, Steinle NC, et al Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014; 98: 1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Sawada T, Sawada O, et al Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age‐related macular degeneration. Am J Ophthalmol 2014; 158: 738–744. [DOI] [PubMed] [Google Scholar]
- 29. Yoshida I, Shiba T, Taniguchi H, et al Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age‐related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2014; 252: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 30. Parikh R, Ross JS, Sangaralingham LR, et al Trends of anti‐vascular endothelial growth factor use in ophthalmology among privately insured and Medicare advantage patients. Ophthalmology 2017; 124: 352–358. [DOI] [PubMed] [Google Scholar]
- 31. Adelman R, Parnes A, Michalewska Z, et al Strategy for the management of diabetic macular edema: the European vitreo‐retinal society macular edema study. Biomed Res Int 2005; 2015: 352487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogura Y, Shiraga F, Terasaki H, et al Clinical practice pattern in management of diabetic macular edema in Japan: survey results of Japanese retinal specialists. Jpn J Ophthalmol 2017; 61: 43–50. [DOI] [PubMed] [Google Scholar]
- 33. Diabetic Retinopathy Clinical Research Network , Browning DJ, Glassman AR, et al Relationship between optical coherence tomography‐measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology 2007; 114: 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Lusignan S, Crawford L, Munro N. Creating and using real‐world evidence to answer questions about clinical effectiveness. J Innov Health Inform 2015; 22: 368–373. [DOI] [PubMed] [Google Scholar]


