Abstract
Aims/Introduction
To investigate the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes mellitus, with a focus on hypoglycemia.
Materials and Methods
Type 2 diabetes mellitus patients who started sitagliptin therapy and were followed for 52 weeks were enrolled in the Impact of Sitagliptin on Diabetes Mellitus in Japanese Elderly Patients study. The frequency of hypoglycemia and knowledge of hypoglycemia were analyzed using a questionnaire.
Results
In total, 5,130 patients (aged 73.8 ± 6.1 years) were analyzed. A significant reduction in glycated hemoglobin (−0.7 ± 1.1%, P < 0.001) and glycoalbumin levels (−2.2 ± 3.8%, P < 0.001) was observed at week 52. The percentage of patients with hypoglycemia did not increase from the baseline (3.3%) to week 52 (2.8%) of sitagliptin administration. Hypoglycemia incidence was significantly higher for combination therapy with insulin (odds ratio 17.75, P < 0.001) or sulfonylurea (odds ratio 2.22, P < 0.001). The increase in sitagliptin dose for combination therapy with antidiabetic drug(s) increased the percentage of patients with hypoglycemia (5.6% in sitagliptin increased subgroup, 2.4% in sitagliptin maintained subgroup, P < 0.01). The awareness of hypoglycemia symptoms and attitude to carry glucose as a countermeasure to prevent hypoglycemia increased during the study.
Conclusions
Sitagliptin did not increase the percentage of patients with hypoglycemia among elderly patients with type 2 diabetes mellitus. However, hypoglycemia occurred more frequently in add‐on therapy to sulfonylurea or when the sitagliptin dose was increased in combination therapy, showing that sitagliptin should be used with caution.
Keywords: Hypoglycemia, Sitagliptin, Sulfonylurea
Introduction
The aging population in Japan is rapidly increasing, resulting in an aging society. A national survey in Japan reported that the prevalence of diabetes mellitus in Japan reached 10 million, and approximately two‐thirds of patients were aged ≥65 years and approximately half of them were aged ≥75 years1. These findings show that most of the patients with diabetes mellitus in real‐world clinical settings in Japan are elderly. Elderly patients with diabetes mellitus are more prone to hypoglycemia. Hypoglycemia is one of the biggest risk factors for frailty in elderly patients with diabetes mellitus2. It has been shown that severe hypoglycemia is a risk factor for cardiovascular events and cognitive impairment, which lower the quality of life of patients and induce death3, 4, 5, 6. Most of the patients transported by ambulance owing to severe hypoglycemia were elderly7, and this has become a serious social issue in Japan.
Recently, dipeptidyl peptidase‐4 (DPP‐4) inhibitors have become the most commonly used oral hypoglycemic agents in Japan7. A survey on hypoglycemia in elderly Japanese patients with type 2 diabetes mellitus carried out by the Japan Physicians Association showed that DPP‐4 inhibitors were the most commonly prescribed oral antidiabetic drugs, followed by sulfonylurea (SU)8. Although DPP‐4 inhibitors are known to have a lower risk of hypoglycemia in monotherapy, cases of severe drug‐induced hypoglycemia were reported in dual therapy with SU9. ‘The Committee on Proper Usage of Incretin and Sulfonylurea Drugs’ recommended decreasing the dosage of SU when a DPP‐4 inhibitor was administered as an add‐on therapy to SU10. As SU is still widely prescribed to elderly patients in Japan, the present study investigated the efficacy and safety of sitagliptin, the first DPP‐4 inhibitor approved for clinical use, in elderly patients with type 2 diabetes mellitus, with a focus on hypoglycemia.
Methods
Study design
The Impact of Sitagliptin on Diabetes Mellitus in Japanese Elderly Patients (SMILE) study was designed as a multicenter, prospective, observational, single‐arm study. This study was registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN000009332), a non‐profit organization in Japan that meets the requirements of the International Committee of Medical Journal Editors. The study protocol was approved by the ethics review board of each participating institution. This study was carried out according to the Declaration of Helsinki and current legal regulations in Japan. The processes of data collection and management were carried out by third‐party entities for data without bias. All personal information was anonymized. Written informed consent was obtained from all participants after a full explanation of the present study.
Study population
The present study was carried out at 1,502 clinical sites in Japan, which mainly consisted of members of the Japan Physicians Association. Outpatients with type 2 diabetes mellitus were approached to participate in this study from December 2012 to November 2014. The inclusion criteria were as follows: (i) patients with type 2 diabetes mellitus; (ii) male and female patients aged ≥65 years and <90 years; (iii) patients with inadequate plasma glucose control by diet and exercise or with additional hypoglycemic agents; and (iv) patients who started sitagliptin therapy for the first time. The exclusion criteria were as follows: (i) patients with a history of hypersensitivity against sitagliptin; (ii) patients who had used incretin‐related drugs in the past 3 months; (iii) patients with a history of serious ketosis, diabetic coma or precoma in the past 6 months; (iv) patients with serious infections, those who were scheduled for surgery or underwent a surgery, or those who suffered serious trauma; (v) patients with type 1 diabetes mellitus; (vi) patients who used glinides, which were not covered under insurance for use with sitagliptin; and (vii) patients who were considered unsuitable subjects by the attending physician. Regarding exclusion criterion (vi), although glinides were not covered under insurance for use with sitagliptin at the start of this study, they were covered during the study. Therefore, the study protocol was revised to include patients who used glinides.
Study drug administration
Sitagliptin (12.5–100 mg) was administered once daily to patients who met the aforementioned criteria, depending on the patient's condition for 52 weeks. The dose of sitagliptin was determined by the physician according to the package insert of sitagliptin. Dose change or additional administration of any other hypoglycemic agents was allowed during the study, according to the patients’ condition.
Observation
Observation was carried out for 52 weeks. Clinical data and biomarkers were collected at baseline (week 0) and week 52 after the start of sitagliptin therapy. Diabetic complications were diagnosed by the attending physicians according to the Treatment Guide for Diabetes 201011. All adverse events were reported during the study. A questionnaire on hypoglycemia was given before and after sitagliptin therapy. The physician completed the questionnaire to describe the absence or presence of hypoglycemic symptoms in each participant during the past 1 month before the baseline or week 52 based on medical interview and medical records, which is defined as ‘physician‐recognized hypoglycemia’ in the present study. In this study, hypoglycemia did not necessarily require the measurement of plasma glucose level, and the severity of hypoglycemia was not determined. The questionnaire for patients also contained the following items: (i) the presence or absence of symptoms; (ii) knowledge regarding hypoglycemia (‘Do you know what hypoglycemia is?’); (iii) knowledge regarding hypoglycemia, such as treatment for hypoglycemia, symptoms, causes and susceptible time during the day; and (iv) whether the patient carried glucose or an equivalent item as a countermeasure for hypoglycemia.
Study outcomes
The end‐points of the present study were as follows: (i) the percentage of patients with hypoglycemia based on physician‐recognized hypoglycemia; (ii) the relationship between the percentage of patients with hypoglycemia and characteristics of patients, use of concomitant antidiabetic drugs, complications, and glycemic control; (iii) the relationship between the percentage of patients with hypoglycemia and hypoglycemic symptoms; and (iv) the assessment of the knowledge and attitude of patients regarding hypoglycemia.
Statistical analysis
The reported values were expressed as n, % or mean ± standard deviation in the text. Statistical analyses were carried out using two‐sided tests with 5% significance level. Two‐sample t‐test and analysis of variance (anova) were applied to continuous variables for two‐group comparisons and for comparing more than two groups, respectively. The χ2‐test or Fisher's exact test were applied to nominal variables. In case a significant difference among groups was detected by anova, multiple comparisons were carried out with multiple testing corrections by Tukey's method. In case a significant difference among groups was detected with the χ2‐test or Fisher's exact test, multiple comparisons were carried out with Bonferroni correction. Logistic regression analysis was carried out to test the relationship between the percentage of patients with hypoglycemia and the use of concomitant antidiabetic drugs; the former was set as the objective variable and the latter as the explanatory variable. Logistic regression analysis was further carried out to test the relationship between the percentage of patients with hypoglycemia and hypoglycemic symptoms, and the former was set as the objective variable and the latter as the explanatory variable. Multiple logistic regression analysis was carried out to test the relationship between the percentage of patients with hypoglycemia and risk factors. First, by using univariate logistic regression analysis, variables that had a P‐value <0.1 were detected. By using these factors as explanatory variables, multiple logistic regression analysis was carried out.
Results
Clinical characteristics
In total, 6,012 patients with type 2 diabetes mellitus were enrolled at 1,502 clinical sites during the study period. Among them, 882 patients were excluded from the analysis due to serious violations of the prespecified inclusion/exclusion criteria or protocol, duplicated registration, incomplete case report forms, withdrawal, or no visit after enrollment. Finally, 5,130 patients were included in the full analysis set. The characteristics of full analysis set patients were as follows: 51.7% men and 48.3% women, mean age 73.8 ± 6.1 years, body mass index of 24.2 ± 3.6 kg/m2 and glycated hemoglobin (HbA1c) of 7.5 ± 1.2%. The patients were divided into the following subgroups according to the criteria of the elderly in Japan: 65–74 years (young‐old; 57.7%), 75–84 years (old‐old; 36.3%) and ≥85 years (oldest‐old; 6.0%). The major diabetic complications were as follows: diabetic retinopathy (11.3%), nephropathy (15.2%) and neuropathy (12.6%). SU was the most commonly prescribed drug (24.7%) at baseline, followed by metformin (14.9%). Even in the oldest‐old subgroup (aged 85–89 years), SU was prescribed to 21.6% of the patients. The mean dose of SU at baseline was 1.5 ± 1.7 mg/day of glimepiride, if other SU drugs were converted to glimepiride at a dose of glimepiride 1 mg for gliclazide 40 mg or glibenclamide 0.625 mg. The use of alpha‐glucosidase inhibitor (α‐GI), pioglitazone, glinide and insulin was 13.0, 7.7, 1.1 and 9.0%, respectively, at baseline, and no significant difference was observed among the different age subgroups. The percentage of full analysis set patients untreated with antidiabetic drugs at baseline was 52.7%.
Effect of sitagliptin on clinical laboratory values
HbA1c and glycoalbumin values at baseline were 7.5 ± 1.2% and 20.3 ± 5.0%, respectively. The changes in HbA1c and glycoalbumin from baseline to week 52 of sitagliptin administration were −0.7 ± 1.1% and −2.2 ± 3.8%, respectively (both P < 0.001; Table 1). A similar improvement in HbA1c and glycoalbumin was observed in all age subgroups. The bodyweight, body mass index, systolic blood pressure, diastolic blood pressure and hepatic function biomarkers (alanine transaminase and gamma‐glutamyl transpeptidase) also showed significant reduction from baseline to week 52 (all P < 0.001).
Table 1.
Changes in clinical laboratory values from baseline
| Baseline | Change at week 52 from baseline | P‐value | |
|---|---|---|---|
| HbA1c (%) | 7.5 ± 1.2 | −0.7 ± 1.1 | <0.001 |
| GA (%) | 20.3 ± 5.0 | −2.2 ± 3.8 | <0.001 |
| FPG (mg/dL) | 141.2 ± 43.6 | −17.7 ± 35.3 | <0.001 |
| PPG (mg/dL) | 187.7 ± 73.2 | −32.6 ± 70.9 | <0.001 |
| Bodyweight (kg) | 59.8 ± 11.2 | −0.3 ± 2.5 | <0.001 |
| BMI (kg/m2) | 24.2 ± 3.6 | −0.1 ± 1.0 | <0.001 |
| SBP (mmHg) | 133.8 ± 16.2 | −2.0 ± 15.4 | <0.001 |
| DBP (mmHg) | 73.5 ± 10.5 | −1.3 ± 10.1 | <0.001 |
| AST (IU/L) | 25.3 ± 13.6 | −0.3 ± 11.1 | 0.095 |
| ALT (IU/L) | 23.6 ± 17.2 | −1.7 ± 15.4 | <0.001 |
| γGTP (mg/dL) | 41.5 ± 63.1 | −3.9 ± 34.8 | <0.001 |
| Urine Alb/Cre ratio (mg/g Cr) | 69.1 ± 292.5 | 4.6 ± 226.4 | 0.61 |
Data are shown as mean ± standard deviation. Dose of sitagliptin (n) at baseline: 12.5 mg (15 patients), 25 mg (943 patients), 50 mg (4,107 patients) and 100 mg (65 patients). γGTP, gamma‐glutamyl transpeptidase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; GA, glycoalbumin; PPG, postprandial plasma glucose; SBP, systolic blood pressure; Urine Alb/Cre ratio, urine albumin/creatinine ratio.
Relationship between hypoglycemia and use of antidiabetic drugs
Table 2 presents the percentage of patients with hypoglycemia before baseline and at week 52 in patients treated or untreated with antidiabetic drugs. The percentage of patients with hypoglycemia among those treated with insulin, metformin, α‐GI and SU was 18.1, 5.2, 3.5 and 3.1%, respectively, and it was higher than that in patients untreated with any antidiabetic drugs (all P < 0.001) at baseline. No significant change in the percentage of patients with hypoglycemia was observed from baseline to week 52 in any subgroups of patients treated with other antidiabetic drugs. However, it significantly decreased from baseline (1.4%) to week 52 (0.5%) among patients untreated with any antidiabetic drugs (P = 0.0079).
Table 2.
Percentage of patients with hypoglycemia at baseline and week 52 of sitagliptin treatment among patients treated or untreated with other antidiabetic drugs
| Antidiabetic drug | Baseline | Week 52 | P‐value (0 week vs 52 weeks) | ||||
|---|---|---|---|---|---|---|---|
| Percentage of patients with hypoglycemia (%) | n | P‐value (vs untreated) | Percentage of patients with hypoglycemia (%) | n | P‐value (vs untreated) | ||
| Sulfonylurea | 3.1 | 35/1140 | <0.001 | 4 | 42/1153 | <0.001 | 0.25 |
| Glinide | 3.8 | 2/52 | 0.17 | 3.9 | 3/76 | 0.013 | 1 |
| α‐GI | 3.5 | 21/601 | <0.001 | 3.3 | 18/542 | <0.001 | 0.86 |
| Metformin | 5.2 | 36/697 | <0.001 | 3.5 | 24/188 | <0.001 | 0.12 |
| Pioglitazone | 2.5 | 9/356 | 0.1 | 3.5 | 11/312 | <0.001 | 0.46 |
| Insulin | 18.1 | 73/404 | <0.001 | 17.2 | 61/344 | <0.001 | 0.76 |
| Untreated | 1.4 | 32/2339 | – | 0.5 | 10/1841 | – | 0.0079 |
Data are shown as % and n. α‐GI, alpha‐glucosidase inhibitor.
The effect of antidiabetic drugs on the percentage of patients with hypoglycemia receiving sitagliptin treatment was further investigated with logistic regression analysis (Table 3). Insulin therapy had the greatest effect on the percentage of patients with hypoglycemia at baseline (odds ratio [OR] 11.59, P < 0.001), followed by metformin (OR 1.55, P = 0.036); however, no statistically significant effect was detected in treatment with SU, glinide, α‐GI and pioglitazone. After 52‐week treatment with sitagliptin, combination therapy with insulin and sitagliptin had the greatest effect (OR 17.75, P < 0.001). Combination therapy with SU and sitagliptin also had a significant effect (OR 2.22, P < 0.001), whereas combination therapy with sitagliptin and glinide, α‐GI, metformin, and pioglitazone had no significant effect on the percentage of patients with hypoglycemia.
Table 3.
Effect of antidiabetic drugs on the percentage of patients with hypoglycemia at baseline and week 52 of sitagliptin treatment
| Antidiabetic drug | Baseline | Week 52 | ||||||
|---|---|---|---|---|---|---|---|---|
| FAS (n = 4,514) | FAS (n = 3,876) | With insulin (n = 354) | Without insulin (n = 3,522) | |||||
| Odds ratio | P‐value | Odds ratio | P‐value | Odds ratio | P‐value | Odds ratio | P‐value | |
| Sulfonylurea | 0.98 | 0.92 | 2.22 | <0.001 | 1.10 | 0.79 | 4.01 | <0.001 |
| Glinide | 1.66 | 0.51 | 2.05 | 0.26 | 0.83 | 0.86 | 4.14 | 0.07 |
| α‐GI | 0.68 | 0.13 | 0.74 | 0.28 | 0.72 | 0.35 | 0.73 | 0.49 |
| Metformin | 1.55 | 0.036 | 1.10 | 0.71 | 1.18 | 0.64 | 0.93 | 0.84 |
| Pioglitazone | 0.73 | 0.37 | 1.59 | 0.18 | 0.54 | 0.42 | 2.18 | 0.043 |
| Insulin | 11.59 | <0.001 | 17.75 | <0.001 | – | – | – | – |
Data are shown as odds ratio. α‐GI, alpha‐glucosidase inhibitor; FAS, full analysis set.
The mean insulin dose was 22.0 ± 17.0 U/day (n = 463) at baseline and 22.1 ± 14.9 U/day (n = 395) at week 52 of sitagliptin therapy, and no significant change was detected from baseline to week 52. Stratified analysis based on the presence or absence of hypoglycemia showed no significant difference in the mean insulin dose between patients with hypoglycemia (23.5 ± 15.1 U/day, n = 73) and those without hypoglycemia (21.8 ± 14.1 U/day, n = 330) at baseline. The mean insulin dose in patients with hypoglycemia (27.0 ± 15.8 U/day, n = 61) was significantly higher than that in patients without hypoglycemia (20.8 ± 14.3 U/day, n = 293) at week 52 of combination therapy with insulin and sitagliptin (P = 0.003).
Most of the insulin users were also treated with other oral hypoglycemic agents. Among patients treated with the combination therapy of sitagliptin and insulin at week 52, no other oral hypoglycemic agents had a significant effect on the percentage of patients with hypoglycemia. Among patients untreated with insulin, combination therapy with sitagliptin and SU had a significant effect on the percentage of patients with hypoglycemia (OR 4.01, P < 0.001), followed by combination with pioglitazone (OR 2.18, P = 0.043; Table 3). Combination therapy with sitagliptin and glinide showed a trend towards an increase in hypoglycemia (OR 4.14, P = 0.07).
The stratified analysis of SU dose showed that SU dose had no significant effect on the percentage of patients with hypoglycemia in combination therapy with sitagliptin. The percentage of patients with hypoglycemia was 6.4% (16/250), 2.3% (10/437), 4.3% (9/211) and 4.5% (7/155) among those treated with glimepiride at doses <1.0, 1.0–1.5, 1.0–3.0 and >3.0 mg, respectively, at week 52 (P = 0.58), when the dose of other SU drugs was converted to that of glimepiride. The effect of sitagliptin dose on the percentage of patients with hypoglycemia was analyzed. The dose of sitagliptin had no significant effect on the percentage of patients with hypoglycemia. The percentage of patients with hypoglycemia was 20.0% (2/10), 6.7% (17/253), 4.3% (71/1658) and 7.0% (8/144) among patients treated with sitagliptin at doses of 12.5, 25, 50 and 100 mg, respectively, at week 52 (P = 0.73). However, the dose of sitagliptin was increased in some patients during the study. In patients receiving sitagliptin monotherapy, no significant difference was detected in the percentage of patients with hypoglycemia between the subgroups in which sitagliptin dose was increased (1.2%) or maintained (0.4%) during the study (P = 0.31; Fig. 1). In contrast, in patients treated with sitagliptin and other concomitant antidiabetic drug(s), the percentage of patients with hypoglycemia was significantly higher in the subgroup in which sitagliptin dose was increased (5.6%) than in the subgroup in which sitagliptin dose was maintained (2.4%; P < 0.01).
Figure 1.
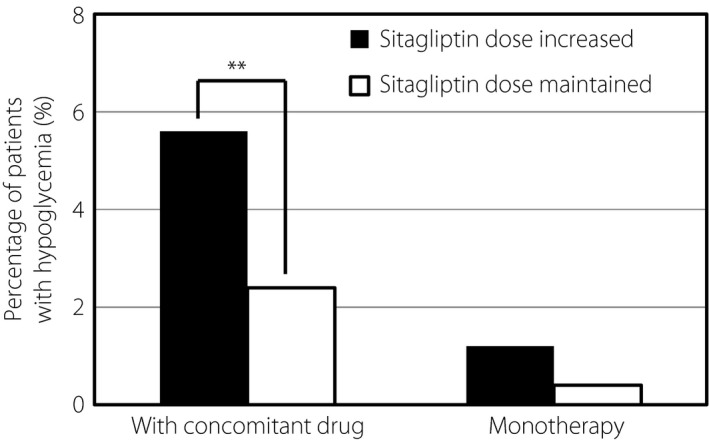
Effect of sitagliptin dose on the percentage of patients with hypoglycemia. Hypoglycemia was defined as hypoglycemia recognized by the physician during the past 1 month. The percentage of patients with hypoglycemia among patients treated with sitagliptin and other concomitant antidiabetic drugs or among patients treated with sitagliptin monotherapy is shown. *P < 0.05, **P < 0.01, ***P < 0.001.
Relationship between hypoglycemia and glycemic control
The mean HbA1c at baseline in patients who had or did not have hypoglycemia before baseline was 7.6 ± 1.1% (n = 151) and 7.5 ± 1.2% (n = 4,653), respectively. No significant difference in mean HbA1c was detected between the two subgroups (P = 0.32). At week 52 of sitagliptin therapy, the mean HbA1c in patients with or without hypoglycemia was 7.2 ± 0.8% (n = 101) and 6.7 ± 0.8% (n = 3,508), respectively. HbA1c improved in both subgroups from baseline to week 52, but the mean HbA1c was significantly lower in patients without hypoglycemia than in patients with hypoglycemia (P < 0.001).
The recent guideline for diabetes treatment in the elderly issued by the Japan Diabetes Society12 indicated the glycemic control target. In patients with diabetes mellitus who use insulin or SU, HbA1c should be ≥6.5% in the elderly aged 65–74 years, and ≥7.0% in elderly aged ≥75 years. Among the patients who used insulin or SU in the present study, 136 patients aged 65–74 years had HbA1c <6.5%, and 245 patients aged ≥75 years had HbA1c <7.0% at week 52 of sitagliptin therapy. The percentage of patients with hypoglycemia among patients who used insulin or SU was 0 and 4.1%, respectively (Table 4). The percentage of patients with hypoglycemia was not higher among patients whose HbA1c was lower than the lower limits indicated in the guidelines.
Table 4.
Percentage of patients with hypoglycemia between glycated hemoglobin subgroups in patients treated with insulin or sulfonylurea
| Age (years) | Week | HbA1c | Percentage of patients with hypoglycemia (%) | n | P‐value |
|---|---|---|---|---|---|
| 65–74 | 52 | <6.5% | 0 | 0/136 | <0.001 |
| ≥6.5% | 9.0 | 55/608 | |||
| ≥75 | 52 | <7.0% | 4.1 | 10/245 | 0.11 |
| ≥7.0% | 7.4 | 20/269 |
Data are shown as n and %. HbA1c, glycated hemoglobin.
Relationship between hypoglycemia and other risk factors
Figure 2 shows the relationship between the percentage of patients with hypoglycemia and diabetic complications at baseline. The percentage of patients with hypoglycemia was higher among patients with diabetic retinopathy, nephropathy or neuropathy than among patients without these complications (all P < 0.001). A similar trend was also detected at week 52 of sitagliptin therapy. The relationship between the percentage of patients with hypoglycemia and risk factors was further investigated by multiple logistic regression analysis. The duration of diabetes (≥20 years; OR 2.488, P = 0.0028) and use of insulin (OR 10.935, P < 0.0001) had significant effects on the percentage of patients with hypoglycemia, and diabetic nephropathy (OR 1.578, P = 0.0658) and use of SU (OR 1.559, P = 0.0697) showed significant trends (Table 5).
Figure 2.
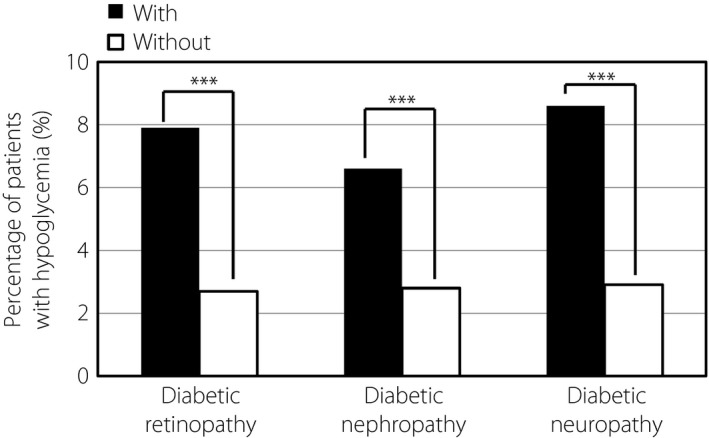
Effect of diabetic complications on the percentage of patients with hypoglycemia. Hypoglycemia was defined as hypoglycemia recognized by the physician during the past 1 month. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 5.
Multiple logistic regression analysis to determine the risk factors for hypoglycemia
| Variables | Odds ratio | P‐value |
|---|---|---|
| BMI (kg/m2) | 0.969 | 0.3147 |
| SBP (mmHg) | 0.985 | 0.0702 |
| DBP (mmHg) | 0.995 | 0.7022 |
| HbA1c (NGSP, %) | 1.036 | 0.7002 |
| Duration of diabetes | ||
| ≥5 years, <10 years | 1.477 | 0.7683 |
| ≥10 years, <20 years | 1.008 | 0.1002 |
| ≥20 years | 2.488 | 0.0028 |
| Diabetic complications | ||
| Retinopathy | 1.41 | 0.1969 |
| Nephropathy | 1.578 | 0.0658 |
| Neuropathy | 1.025 | 0.9281 |
| Sulfonylurea | 1.559 | 0.0697 |
| Insulin | 10.935 | <0.0001 |
Data are shown as odds ratio. BMI, body mass index; DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; NGSP, National Glycohemoglobin Standardization Program; SBP, systolic blood pressure.
Relationship between hypoglycemia and hypoglycemic symptoms
Table 6 summarizes the frequent hypoglycemic symptoms in patients with hypoglycemia. At baseline, cold sweat was the most common symptom (OR 15.22, P < 0.001), followed by intense hunger (OR 7.45, P < 0.001), tremulousness (OR 3.04, P < 0.001), and feeling languid (OR 1.86, P = 0.007). Similar trends in the incidence of hypoglycemic symptoms were observed at week 52 of sitagliptin therapy, although slight changes were seen in the order of hypoglycemic symptoms.
Table 6.
Hypoglycemic symptoms in patients with hypoglycemia
| Week | Hypoglycemic symptoms | Type of symptoms | Odds ratio | P‐value |
|---|---|---|---|---|
| 0 | Cold sweat | Sympathetic nerve | 15.22 | <0.001 |
| (n = 4,514) | Intense hunger | Parasympathetic nerve | 7.45 | <0.001 |
| Tremulousness | Sympathetic nerve | 3.04 | <0.001 | |
| Feeling languid | Central nerve | 1.86 | 0.007 | |
| 52 | Cold sweat | Sympathetic nerve | 17.70 | <0.001 |
| (n = 3,876) | Tremulousness | Sympathetic nerve | 6.71 | <0.001 |
| Intense hunger | Parasympathetic nerve | 5.49 | <0.001 | |
| Lightheadedness | Central nerve | 2.40 | 0.012 | |
| Palpitation | Sympathetic nerve | 2.41 | 0.022 |
Data are shown as odds ratio.
Assessment of knowledge and attitude of patients regarding hypoglycemia
At baseline, 70.8% of patients aged 65–74 years answered that they had knowledge of hypoglycemia, but awareness of hypoglycemia was lower in the elderly subgroups (Fig. 3a). Figure 3b shows the content of knowledge of hypoglycemia in patients who answered that they had knowledge of hypoglycemia at baseline. The most common knowledge in any age subgroup was the treatment for hypoglycemia, followed by symptoms. The awareness of susceptible time during the day or cause of hypoglycemia tended to be low in all age subgroups. The awareness increased from the baseline to week 52 of sitagliptin therapy in all age subgroups.
Figure 3.
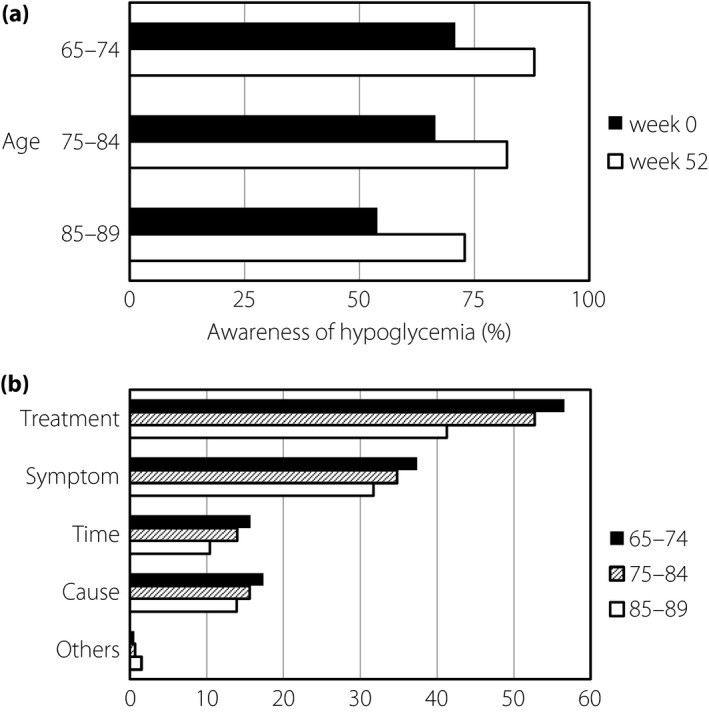
Knowledge of hypoglycemia. (a) Awareness of hypoglycemia in different age subgroups. (b) Content of knowledge of hypoglycemia.
As a countermeasure to prevent hypoglycemia, 71.2% of insulin users always carried or intended to carry glucose at baseline (Fig. 4a). Whereas just 37.8% of SU users prepared glucose at baseline, this ratio increased to 45.0% from baseline to week 52. The ratio of carrying glucose increased from baseline (28.4%) to week 52 (38.9%) in the 65–74 years subgroup, but it did not change in the elderly subgroups during the study (Fig. 4b).
Figure 4.
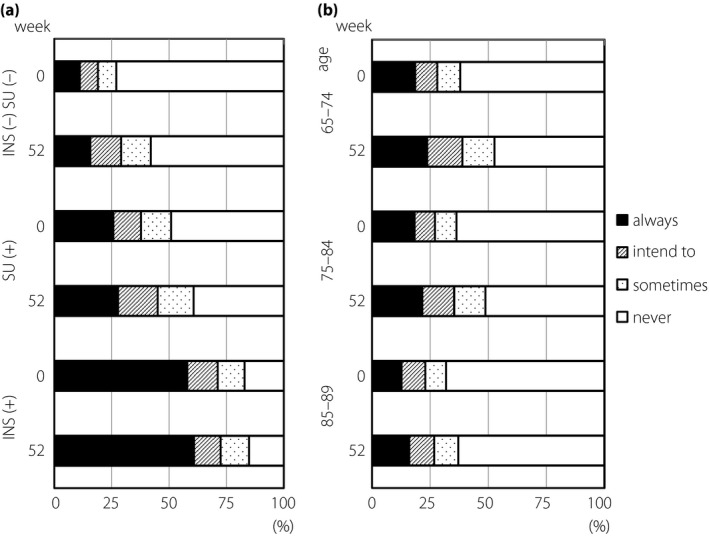
Attitude to carry glucose as a countermeasure to prevent hypoglycemia. (a) Attitude of patients who were treated with/without insulin (INS) and sulfonylurea (SU). (b) Attitude of patients in different age subgroups.
Discussion
In the present study, we aimed to investigate the efficacy and safety of sitagliptin in elderly patients with type 2 diabetes mellitus, with a focus on hypoglycemia. The add‐on therapy of sitagliptin with any antidiabetic drugs, including insulin, did not show a significant increase in the percentage of patients with hypoglycemia. Although SU had no significant effect on the percentage of patients with hypoglycemia at baseline, the combination therapy of sitagliptin and SU for 52 weeks showed a significant impact on the percentage of patients with hypoglycemia. In addition, when sitagliptin dose was increased in combination therapy, the percentage of patients with hypoglycemia increased. These findings showed that sitagliptin should be used with caution.
In case of add‐on therapy to SU, sitagliptin should be administered with much caution to patients with hypoglycemia. Our previous study showed that SU is still prescribed to >30% of elderly patients with type 2 diabetes mellitus8. We also showed that SU was most commonly prescribed to elderly patients (24.7%), even to the oldest‐old subgroup (85–89 years; 21.6%). The dose of SU should be <2 mg/day of glimepiride in combination therapy with sitagliptin, as announced previously10. In the present study, the mean dose of SU was 1.5 ± 1.7 mg/day of glimepiride both at baseline and week 52 of sitagliptin administration. The dose of SU had no significant effect on hypoglycemia, when the dose of other SU drugs was converted to that of glimepiride. These results suggested that sitagliptin should be used in combination with SU with caution in regard to hypoglycemia, even if the dose of SU is low. Although dose reduction in SU has been recommended, when DPP‐4 inhibitor is administered as an add‐on therapy to SU10, the dose of SU was not modified after the start of sitagliptin administration in the present study. This might have contributed to the increase in OR for hypoglycemia in this study. The dose of sitagliptin should be increased with caution, because increasing the sitagliptin dose increased the percentage of patients with hypoglycemia in combination therapy with other antidiabetic drugs. In the present study, insulin had the highest OR for hypoglycemia both at baseline and at week 52, and the OR for hypoglycemia further increased at week 52 of sitagliptin treatment. In contrast, previous randomized controlled trials in Korea13 and Japan14 reported that the addition of sitagliptin to insulin decreased the frequency of hypoglycemia. Because sitagliptin shows an effect to suppress the amplitude of glucose excursion15, it might prevent hypoglycemia on infusion of external insulin. Although previous trials showed that the daily dose of insulin decreased after the administration of sitagliptin13, 14, the insulin dose did not change in the present study. This might contribute to the increase of the OR for hypoglycemia after the administration of sitagliptin. Indeed, the insulin dose for patients with hypoglycemia was significantly higher than that for patients without hypoglycemia. The suppression of glucose excursion amplitude by sitagliptin administration to drug‐naïve patients at baseline might contribute to the decrease in the percentage of patients with hypoglycemia.
The treatment guidelines for diabetes issued by the Japan Diabetes Society12, announced after the present study, indicated the targets of glycemic control, including the lower limits, to avoid severe hypoglycemia when insulin or SU is used, based on the age and frailty of elderly patients. A recent survey of treatment‐related severe hypoglycemia by the Japan Diabetes Society16 showed that 93.9% of causal agents of severe hypoglycemia were insulin and SUs, and the median HbA1c value at onset of severe hypoglycemia was 6.8% in patients with type 2 diabetes mellitus. Although HbA1c in patients treated with insulin or SU in the present study was lower than the recommended lower limits in the current guidelines, the percentage of patients with hypoglycemia did not increase in the subgroups and the patients were safely controlled (Table 5). However, dose reduction or withdrawal of insulin and SU should be considered in such patients in future according to the current guidelines. The percentage of patients with hypoglycemia was higher in patients with any diabetic complications than in patients without complications. Impairment of renal function is a known risk factor for hypoglycemia6. We could not assess the relationship between the frequency of hypoglycemia and renal function, because we did not measure estimated glomerular filtration rate in the present study. However, multiple logistic regression analysis showed that the duration of diabetes (≥20 years) had a significant effect on hypoglycemia, and diabetic nephropathy showed a trend toward higher OR, although it was not statistically significant. These findings suggest that further caution is required in regard to hypoglycemia in patients with diabetic nephropathy.
Although the awareness of hypoglycemia tended to be lower in the elderly subgroups, it tended to increase from baseline to week 52 of the administration of sitagliptin in all age subgroups. The attitude of patients to carry glucose was improved in the 65–74 years subgroup. This might be because the awareness and recognition of hypoglycemia in patients were improved through the questionnaire and medical interview with the attending physicians. The improvement of attitude was less in the elderly subgroups, probably because elderly patients tend to have a stronger status quo bias and a change in attitude is more difficult. These findings suggest that tenacious, repeated and easy‐to‐understand indications are required for elderly patients in clinical practice.
There were certain limitations to the present study. First, this was an observational, single‐arm study without a placebo or control group. Second, more than half of the participating patients in this study were treated with antidiabetic drugs. Third, the definition of hypoglycemia in this study was based on hypoglycemic symptoms and not on plasma glucose measurement, because the majority of the patients who were treated with oral hypoglycemic agents or even some of the patients treated with insulin did not carry out self‐monitoring of blood glucose. Fourth, we did not measure creatinine levels in this study; therefore, the relationship between the frequency of hypoglycemia and glomerular filtration rate could not be analyzed. Fifth, we did not distinguish the types of institutions, such as rural versus urban area, hospital versus clinic or diabetes specialist versus non‐specialist. However, the majority of the institutions that participated were clinics or small‐scale hospitals. Sixth, the relationship between the frequency of hypoglycemia and renal function was not assessed, because we did not measure the estimated glomerular filtration rate in the present study.
Sitagliptin, a DPP‐4 inhibitor, did not increase the percentage of patients with hypoglycemia among elderly patients with type 2 diabetes mellitus. However, hypoglycemia occurred more frequently with add‐on therapy to SU or when the sitagliptin dose was increased in the combination therapy, showing that sitagliptin should be used with caution.
Disclosure
MF received lecturer's fees from Ono Pharmaceutical Co., Ltd. and Mitsubishi Tanabe Pharma Corporation. The other authors declare no conflict of interest.
Acknowledgments
This study was carried out using the research fund of the Japan Physicians Association. No other external funder supported this study.
J Diabetes Investig 2019; 10: 383–391
Clinical Trial Registry University Hospital Medical Information Network Clinical Trial RegistryUMIN000009332
References
- 1. Ministry of Health, Labour and Welfare of Japan . The National Health and Nutrition Survey, 2016. (Japanese). Available from: http://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html Accessed October 31, 2017.
- 2. Abdelhafiz AH, Rodriguez‐Manas L, Morley JE, et al Hypoglycemia in older people ‐ a less well recognized risk factor for frailty. Aging Dis 2015; 6: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitmer RA, Karter AJ, Yaffe K, et al Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009; 301: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laiteerapong N, Karter AJ, Liu JY, et al Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care 2011; 34: 1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonds DE, Miller ME, Bergenstal RM, et al The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340: b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee AK, Lee CJ, Huang ES, et al Risk factors for severe hypoglycemia in Black and White adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2017; 40: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7(Suppl 1): 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukuda M, Doi K, Sugawara M, et al Survey of hypoglycemia in elderly people with Type 2 Diabetes mellitus in Japan. J Clin Med Res 2015; 7: 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwakura T, Sasaki S, Fujiwara Y, et al Clinical analysis of 135 Type 2 diabetes patients with severe drug‐induced hypoglycemia. J Japan Diab Soc 2012; 55: 857–865 (Japanese). [Google Scholar]
- 10. Recommendation of the Committee on proper usage of incretin and sulfonylurea drugs (Japanese). Available from: http://www.fa.kyorin.co.jp/jds/uploads/photos/797.pdf Accessed February 15, 2018.
- 11. Japan Diabetes Society . Treatment Guide for Diabetes 2010. Tokyo: Bunkodo, 2010. (Japanese). [Google Scholar]
- 12. Japan Diabetes Society . Treatment Guide for Diabetes 2016–2017. Tokyo: Bunkodo, 2016. (Japanese). [Google Scholar]
- 13. Hong ES, Khang AR, Yoon JW, et al Comparison between sitagliptin as add‐on therapy to insulin and insulin dose‐increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab 2012; 14: 795–802. [DOI] [PubMed] [Google Scholar]
- 14. Sato S, Saisho Y, Kou K, et al Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 diabetes: the EDIT randomized trial. PLoS ONE 2015; 10: e0121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimoda S, Iwashita S, Ichimori S, et al Efficacy and safety of sitagliptin as add‐on therapy on glycemic control and blood glucose fluctuation in Japanese type 2 diabetes subjects ongoing with multiple daily insulin injections therapy. Endocr J 2013; 60: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 16. Namba M, Iwakura T, Nishimura R, et al The current status of treatment‐related severe hypoglycemia in Japanese patients with diabetes mellitus: a report from the committee on a survey of severe hypoglycemia in the Japan Diabetes Society. J Diabetes Investig 2018; 9: 642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]


