Abstract
Aims/Introduction
Vitamin D3 deficiency can lead to male hypogonadism in diabetes mellitus, but the target organs and the mechanism driving the disorder are unclear. This experiment was designed to study the relationship between vitamin D3 deficiency and hypogonadism in diabetes mellitus.
Materials and Methods
Rats with streptozotocin‐induced diabetes were randomly divided into four groups and treated with different doses of vitamin D3: blank (no vitamin D3), low (0.025 μg/kg/day), high (0.1 μg/kg/day), high (0.1 μg/kg/day) and with bisphenol A diglycidyl ether (peroxisome proliferator‐activated receptor gamma inhibitor 30 mg/kg/day). They were compared with wild‐type rats.
Results
After 12 weeks, the vitamin D3 supplements had partially restored testicular pathological changes, as shown by reduced testicular fibrosis related to downregulation transforming growth factor beta 1 and apoptosis related to downregulation of nuclear factor kappa B, but not the pituitary gland. The expression of peroxisome proliferator‐activated receptor gamma, which can inhibit transforming growth factor beta 1 and nuclear factor kappa B, was significantly increased after treatment with vitamin D3.
Conclusions
These results suggest that treatment with vitamin D3 can improve testicular function in diabetic rats through the peroxisome proliferator‐activated receptor gamma/transforming growth factor beta 1/nuclear factor kappa B signaling pathway.
Keywords: 1,25(OH)2D3; Diabetes; Hypogonadism
Introduction
Diabetes mellitus is a multisystemic syndrome. In recent years, male hypogonadism caused by diabetes mellitus has begun to attract attention1, 2. Multiple metabolic disorders caused by diabetes mellitus are related to the deterioration of gonad function, including the disorder of glucose metabolism caused by insulin secretion defects; in addition, insulin resistance leads to testosterone synthesis disorder1, 3 and hyperglycemia‐induced testicular and epididymal microvascular disease, which limit the ability of the testes to produce sperm and cause epididymis recession4, 5.
The active form of vitamin D3, 1α, 25‐dihydroxy vitamin D3 (1,25‐[OH]2D3), exerts its effects through the vitamin D receptor (VDR)6. In the classical pathway, vitamin D3 binds to its nuclear receptor to regulate calcium and phosphorus metabolism. Recent studies have found that vitamin D3 can affect a variety of disease processes, including diabetes, prediabetes and autoimmune conditions, through more than one pathway7, 8, 9. With the discovery of the expression of the VDR in the central and peripheral reproductive systems, researchers have realized that vitamin D3 might have an impact on the gonadal axis and play a role in hypogonadism in diabetes mellitus8, 10.
Therefore, we studied the relationship between vitamin D3 and diabetes mellitus‐driven hypogonadism. A previous study from our group showed that vitamin D3 supplement or treatment can partially improve testicular function in men with diabetes mellitus11. However, many questions remain. It is not clear if vitamin D3 deficiency affects the central reproductive organs or gonads; the pathway through which vitamin D3 acts in this disease state is also unknown.
Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) is an important component of the nuclear receptor superfamily. In addition, the activation of PPAR‐γ signaling can improve testicular function12, and vitamin D3 can regulate the expression of PPAR‐γ13. Therefore, we speculated that vitamin D3, the PPAR‐γ pathway and testicular function could be linked. In the present study, we investigated whether the central reproductive organs or gonads were affected by vitamin D3 supplementation, and studied the pathway that regulates its biological effects, with the aim of improving clinical diagnosis and treatment.
Methods
Materials
Streptozotocin (STZ) was purchased from Sigma‐Aldrich (St. Louis, Missouri, USA). The following rabbit anti‐rat polyclonal antibodies were used: anti‐VDR (Abcam, Cambridge, Massachusetts, USA), anti‐PPAR‐γ (Santa Cruz Biotechnology, Santa Cruz, California, USA), anti‐transforming growth factor beta 1 (TGF‐β1), anti‐nuclear factor kappa B (NF‐κB), anti‐phospho‐NF‐κB, anti‐B‐cell lymphoma 2 (Bioworld Technology, St. Louis Park, Minnesota, USA), anti‐Bcl‐2‐associated X protein (Bax), anti‐inhibitor of NF‐κB alpha and anti‐β‐actin (Sangon Biotech, Shanghai, China).
Animals and treatment
Specific‐pathogen‐free, 8‐week‐old male Sprague–Dawley rats (200 ± 20 g) were purchased from the Experimental Animal Center of Henan Province, China. All animal studies were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Zhengzhou University, and fully complied with the university guidelines for the care and use of laboratory animals.
After 12 h of fasting, 25 rats were injected once, intraperitoneally, with a solution of 60 mg STZ/kg to induce diabetes. Their blood glucose (BG) levels were measured to validate the induction of diabetes (BG > 16.7 mmol/L) 72 h after STZ injection. Five rats that received an injection of diluent buffer alone served as the normal control group (NC group). The diabetic rats were randomly divided into four groups of six rats each: untreated diabetic rats, rats treated with a low dose of active vitamin D3 (LVD; 0.025 μg/kg/day), rats treated with a high dose of active vitamin D3 (HVD; 0.1 μg/kg/day), and rats treated with a high dose of active vitamin D3 (0.1 μg/kg/day) and the PPAR‐γ inhibitor bisphenol A diglycidyl ether (BADGE; 30 mg/kg/day; Sigma‐Aldrich). The vitamin D3 and BADGE doses were determined by consulting the literature and previous experiments11, 14. During the following 12 weeks, each vitamin D3 group received 1,25‐(OH)2D3 dissolved in 0.1 mL peanut oil daily, at the doses described above, by oral gavage. The BADGE group received a daily intraperitoneal injection of BADGE (30 mg/kg) in 10% dimethyl sulfoxide in phosphate‐buffered saline. The remaining groups received intraperitoneal injections of the same dilution of dimethyl sulfoxide in phosphate‐buffered saline. The NC and diabetic groups received daily 0.1 mL peanut oil blanks.
Tissue collection
At the end of 12 weeks, after weighing the rats and testing their BG levels, the rats were anesthetized with chloral hydrate and blood samples were collected from them to test serum electrolyte and hormone levels. Finally, the rats were killed by decapitation. The testes and pituitary were dissected for use in biochemical studies. The testes were weighed after removal from the rats. Some of the tissues were stored in liquid nitrogen for real‐time polymerase chain reaction (PCR) and western blotting analysis, and others were fixed in 10% buffered formalin or 4% buffered glutaraldehyde for histological assessments by light microscopy or electron microscopy, respectively.
Sperm count and proportion of deformed sperm
The right epididymis of each animal was minced, dipped in 4 mL phosphate‐buffered saline, and then incubated in a water bath at 37°C for 10 min. A total of 10 μL of the sperm suspension was dropped on dry SpermBlue slides (MICROPTIC, Barcelona, Spain), and the slides were heated for 15 s. Then, the total number of sperm and that of deformed sperm were calculated using a hemocytometer under a light microscope.
Light and transmission electron microscopy
The pathology of the testes and pituitaries in hematoxylin–eosin, Masson trichome (JianCheng Bioengineering Institute, Nanjing, China) and terminal deoxynucleotidyl transferase dUTP nick‐end labeling (Roche, Basel, Switzerland) stained sections was examined by light microscopy. The testes and pituitary glands were dissected into small cubes (1 mm3), fixed in 4% pre‐cooling glutaraldehyde and then fixed using osmium acid, dehydrated with an acetone gradient, soaked in epoxy resin, embedded, sliced, and stained with uranium acetate and citric acid lead.
The ultrastructure of the testes and pituitary glands was examined with an H‐7500 transmission electron microscope (Hitachi, Tokyo, Japan).
Enzyme‐linked immunosorbent assay
Serum was separated from blood by centrifugation at 3000 g for 5 min at 4°C, supernatants were collected, and levels of 1,25‐(OH)2D3, testosterone, follicle‐stimulating hormone (FSH), luteinizing hormone (LH), insulin and C‐peptide were measured using enzyme‐linked immunosorbent assay kits (AMEKO, Shanghai, China), according to the manufacturer's instructions, in three duplicate wells. The optical density of each well was measured with a microplate reader. The standard curve was prepared using GraphPad 5 software (GraphPad Software Inc., La Jolla, California, USA), and the concentrations of the hormones were then calculated.
Ribonucleic acid extraction and quantitative reverse transcription polymerase chain reaction
Total ribonucleic acid (RNA) was extracted from the testes with TRIzol™ (TaKaRa Bio, Shiga, Japan). Complementary deoxyribonucleic acid was synthesized with the M‐MuLV First‐Strand cDNA Synthesis Kit (Sangon Biotech). The expression of the target genes was assessed using SGExcel UltraSYBR Mixture Kit (Sangon Biotech) on the Applied Biosystems® 7500 FAST System (Foster City, California, USA). The relative target gene expression was normalized to the expression of rat β‐actin. The method was used to calculate the values. The primers were obtained from Sangon Biotech, and the primer details are available on request (Table 1).
Table 1.
Primer sequences for quantitative reverse transcription polymerase chain reaction
| Gene symbol | Forward primer (5′–3′) | Reverse primer (3′–5′) |
|---|---|---|
| VDR | CCATTCAGGACCGCCTATCC | AGGAGAGGGAGCGGTATTGT |
| PPAR‐γ | TGTGGACCTCTCTGTGATGG | AGCTCTTGTGAACGGGATGT |
| TGF‐β | ATGACATGAACCGACCCTTC | TTCTCTGTGGAGCTGAAGCA |
| β‐actin | TACAACCTCCTTGCAGCTCC | GGATCTTCATGAGGTAGTCAGTC |
PPAR‐γ, peroxisome proliferator‐activated receptor gamma; TGF‐β1, transforming growth factor beta 1; VDR, vitamin D receptor.
Protein extraction and western blotting
Total protein was extracted from the testes with a protein extraction reagent (CWBio, Beijing, China). Western blotting was carried out, as previously described11. The membranes were exposed to anti‐VDR (1:100), anti‐PPAR‐γ (1:100), anti‐TGF‐β1 (1:500), anti‐NF‐κB (1:500), anti‐phospho‐NF‐κB (1:500), anti‐B‐cell lymphoma 2 (1:500), anti‐Bax (1:1,000), anti‐inhibitor of NF‐κB alpha (1:500) and anti‐β‐actin (1:1,000). After incubation with the horseradish peroxidase‐conjugated secondary antibody, the membranes were treated with enhanced chemiluminescence substrate (Life Technologies, Carlsbad, California, USA) and exposed to X‐ray film. The relative optical density of each target protein was determined and normalized to β‐actin levels using ImageJ software (public domain; National Institutes of Health, Bethesda, Maryland, USA).
Immunofluorescence and immunohistochemistry
Immunofluorescent and immunohistochemical staining was carried out as previously described11, 15, 16. Primary antibodies against VDR (1:1,000), anti‐PPAR‐γ (1:50), anti‐TGF‐β1 (1:50), FSH (1:10) and LH (1:10) were used. The distributions and localizations of the target proteins were visualized by light and fluorescence microscopy. Immunofluorescence and immunohistochemical signals were quantified with ImageJ (National Institutes of Health).
Statistical analysis
The data were analyzed with SPSS 21.0 software (IBM, Armonk, New York, USA). Statistical differences between the experimental groups were determined by one‐way analysis of variance, followed by the Bonferroni test for comparisons. Results are expressed as mean ± standard deviation, and P < 0.05 is the standard used for statistical significance.
Results
General conditions of the animals
Unlike those in the NC group, the rats in the diabetic group showed symptoms such as polyuria, polydipsia and polyphagia. Differences can also be seen in their BG levels and bodyweights. The BG levels were significantly higher and the bodyweights were significantly lower in the diabetic groups than in the NC group (P < 0.05). However, there were no observed significant differences in the BG levels or bodyweights among the diabetic, LVD, HVD and BADGE groups (P > 0.05). The serum levels of calcium in the diabetic groups were lower than in the NC group, although the differences were not statistically significant (P > 0.05). The levels of phosphate were not statistically different among the groups (P > 0.05; Table 2).
Table 2.
Physiological parameters of all treatment groups at 12 weeks
| Group | No. rats | BG (mmol/L) | BW (g) | Serum calcium (mmol/L) | Serum phosphate (mmol/L) |
|---|---|---|---|---|---|
| NG | 5 | 6.60 ± 0.40 | 441.3 ± 21.8 | 2.53 ± 0.11 | 2.67 ± 0.40 |
| DM | 5 | 30.30 ± 0.60† | 235.0 ± 23.6† | 2.50 ± 0.08 | 3.02 ± 1.13 |
| HVD | 5 | 29.57 ± 3.33† | 234.3 ± 40.3† | 2.66 ± 0.02† , ‡ | 2.80 ± 0.26 |
| LVD | 5 | 28.13 ± 4.72† | 273.7 ± 44.6† | 2.64 ± 0.05† | 2.22 ± 0.23 |
| BADGE | 5 | 29.97 ± 2.99† | 229.3 ± 11.9† | 2.58 ± ±0.12‡ | 2.78 ± 0.35 |
Data are presented as mean ± standard deviation. † P < 0.05 vs untreated diabetes model (DM) group; ‡ P < 0.05 vs normal controls with no treatments (NC) group. BADGE, bisphenol A diglycidyl ether; BG, blood glucose; BW, bodyweight; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3.
Effects of vitamin D3 on hormones
Compared with the NC group, the serum level of vitamin D3 in the diabetic group was lower (P < 0.05; Figure 1a), but could be increased by vitamin D3 treatment. Similarly, the level of testosterone was lower (P < 0.05; Figure 1b) in the diabetic group, and higher in the LVD and HVD groups than in the NC group; on treatment with BADGE, the level of testosterone decreased to the level in the diabetic group.
Figure 1.
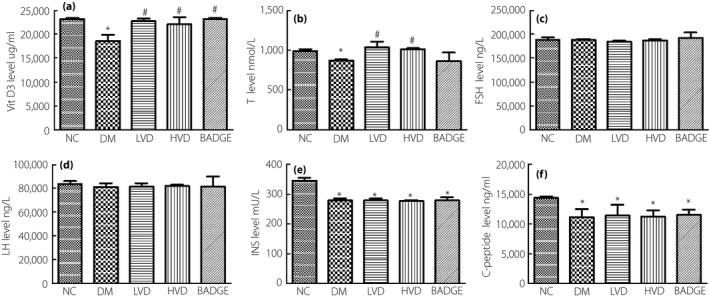
The level of vitamin D3 (VitD3), testosterone (T), follicle‐stimulating hormone (FSH), luteinizing hormone (LH), insulin (INS) and C‐peptide in each group. (a) Serum VitD3 level; (b) serum T level; (c) serum FSH level; (d) serum LH level; (e) serum INS level; and (f) serum C‐peptide level. The data are shown as mean ± standard error of the mean vs the normal controls with no treatments (NC) group, *P < 0.05 vs NC group; # P < 0.05 vs the untreated diabetes model (DM) group. BADGE, bisphenol A diglycidyl ether; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3.
As FSH and LH are important for the regulation of gonadal function, which can reflect the condition of the central reproductive organs, we tested the effects of vitamin D3 on their serum levels. We found that the levels of FSH and LH did not statistically differ among the groups (P > 0.05; Figure 1c,d). This result suggests that diabetes might have no effect on pituitary function.
In order to clarify the effect of vitamin D3 on pancreas function, we measured the insulin and C‐peptide levels in the experimental animals. We found that, compared with the levels in the NC group, the serum levels of insulin and C‐peptide in the diabetic, LVD, HVD and BADGE groups were lower; however, we did not observe a statistical difference in the levels with vitamin D3 treatment (P > 0.05; Figure 1e,f). This result suggests that the protective effects of vitamin D3 on testicular function in diabetic rats are not caused by the regulation of insulin and C‐peptide levels, but are caused by the action of vitamin D3 directly on the testes.
Spermatogenesis in diabetic rats improved after vitamin D3 treatment
Spermatogenesis by the testis is an important index to evaluate the gonad function of male rats. We observed that, compared with the counts in the NC group, the sperm counts were significantly lower in the diabetic group (P < 0.05; Figure 2a,b), and the proportion of deformed sperm was significantly higher (P < 0.05; Figure 2c). After treatment with vitamin D3, the total counts and activity of sperm were higher and the proportion of deformed sperm was lower in the LVD and HVD groups than in the diabetic group (P < 0.05; Figure 2a–c). However, the PPAR‐γ inhibitor, BADGE, antagonized the protective effects of vitamin D3 (P < 0.05; Figure 2a–c).
Figure 2.
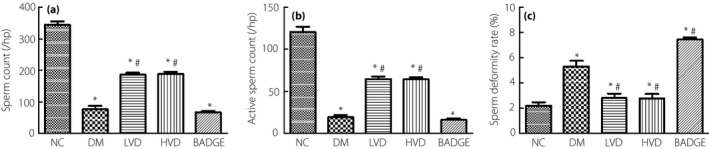
The level of sperm count, active sperm count and sperm deformity rate in each group. (a) Sperm count; (b) active sperm count; and (c) sperm deformity rate. The data are shown as mean ± standard error of the mean vs the normal controls with no treatments (NC) group, *P < 0.05 vs NC group; # P < 0.05 vs the untreated diabetes model (DM) group. BADGE, bisphenol A diglycidyl ether; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3.
Spermatogenesis of testis is an important index to evaluate the gonad function of male rats. We observed that compared with group NC, the sperm count decreased significantly in group DM (P < 0.05; Figure 2a,b), and the malformation rate increased significantly (P < 0.05; Figure 2c). However, after treatment with vitamin D3, the total counts and activity of sperm increased and the malformation rate decreased in group LVD and HVD (P < 0.05; Figure 2a–c). However, the PPAR‐γ inhibitor, BADGE, can antagonize the protective effects after treatment with vitamin D3, reduce the total sperm count and viability, and increase the malformation rate (P < 0.05; Figure 2a–c).
Pathological lesions in diabetic rat testes were alleviated by vitamin D3 treatment
Hematoxylin–eosin staining showed that the spermatogenic and Leydig cells were arranged loosely, and the seminiferous tubules were degenerating in the diabetic group, in comparison with their status in the NC group. The loose arrangement of the testicular germ cells and the degree of degeneration of the seminiferous tubules were ameliorated in the LDV and HDV groups, but exacerbated in the BADGE group (Figure 3a). Our transmission electron microscopy results showed that the spermatogonia in the diabetic group were sparser with a greater extent of nuclear condensation and fragmentation than in the NC group. In addition, the mitochondria and endoplasmic reticulum were swollen in the diabetic group compared with the NC group. Consistent with hematoxylin–eosin staining, the LVD and HVD groups were lighter than the diabetic group, and the BADGE group was the heaviest (Figure 3b).
Figure 3.
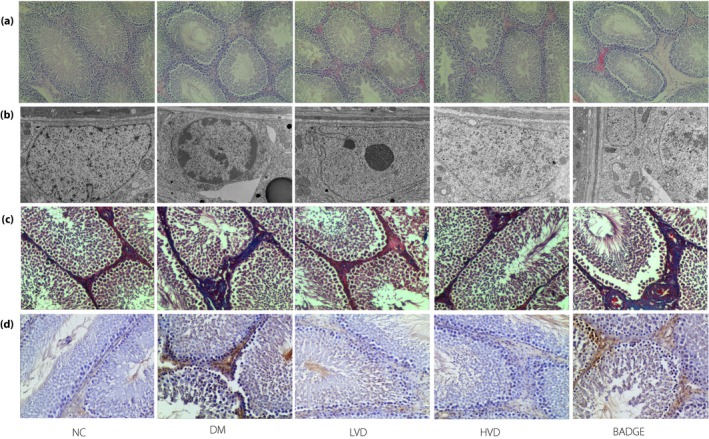
The pathological lesions in each group. (a) Hematoxylin–eosin staining (magnification: ×100); (b) transmission electron microscope (magnification: ×6,000); (c) Masson staining (magnification: ×200); and (d) terminal deoxynucleotidyl transferase dUTP nick‐end labeling staining (magnification: ×200). BADGE, bisphenol A diglycidyl ether; DM, untreated diabetes model; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3; NC, normal controls with no treatments.
Expression of TGF‐β1 and the degree of fibrosis were decreased in diabetic rat testes after treatment with vitamin D3
Tissue fibrosis is known to lead to testicular dysfunction. To determine the degree of fibrosis in the testicular tissues of the rats, we carried out Masson's trichrome staining. The staining showed significantly more fibrosis in the diabetic group than in the NC group. The degree of fibrosis was significantly decreased after treatment with vitamin D3, but the greatest degree of fibrosis was observed in the BADGE group (Figure 3c). Furthermore, TGF‐β1, a major driver of fibrosis, was significantly increased at the messenger RNA (mRNA) and protein levels in the diabetic and BADGE groups compared with the NC group. The TGF‐β1 levels were significantly lower in the LVD and HVD groups than in the diabetic group (all P < 0.05; Figures 4c,d,g and 5c,f).
Figure 4.
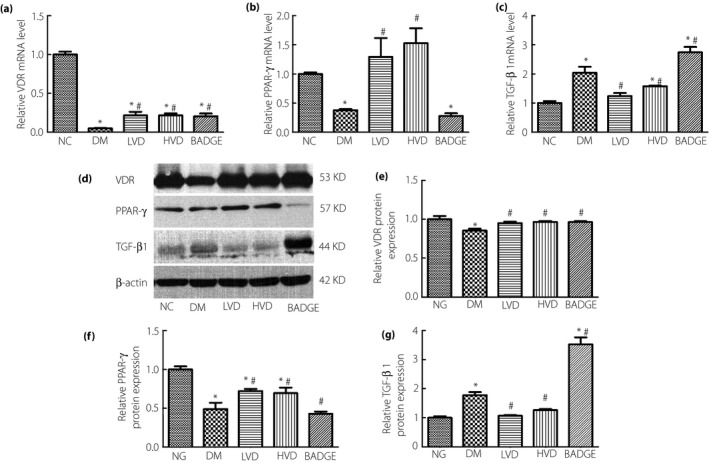
The level of vitamin D receptor (VDR), peroxisome proliferator‐activated receptor gamma (PPAR‐γ) and transforming growth factor beta 1 (TGF‐β1) in each group. (a) VDR messenger ribonucleic acid (mRNA) level; (b) PPAR‐γ mRNA level; (c) TGF‐β1 mRNA level; (d) the protein levels of VDR, PPAR‐γ and TGF‐β1 detected by western blotting; (e) relative VDR protein level; (f) relative PPAR‐γ protein level; (g) and relative TGF‐β1 protein level. The data are shown as mean ± standard error of the mean vs the normal controls with no treatments (NC) group, *P < 0.05 vs NC group; # P < 0.05 vs the untreated diabetes model (DM) group. BADGE, bisphenol A diglycidyl ether; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3.
Figure 5.
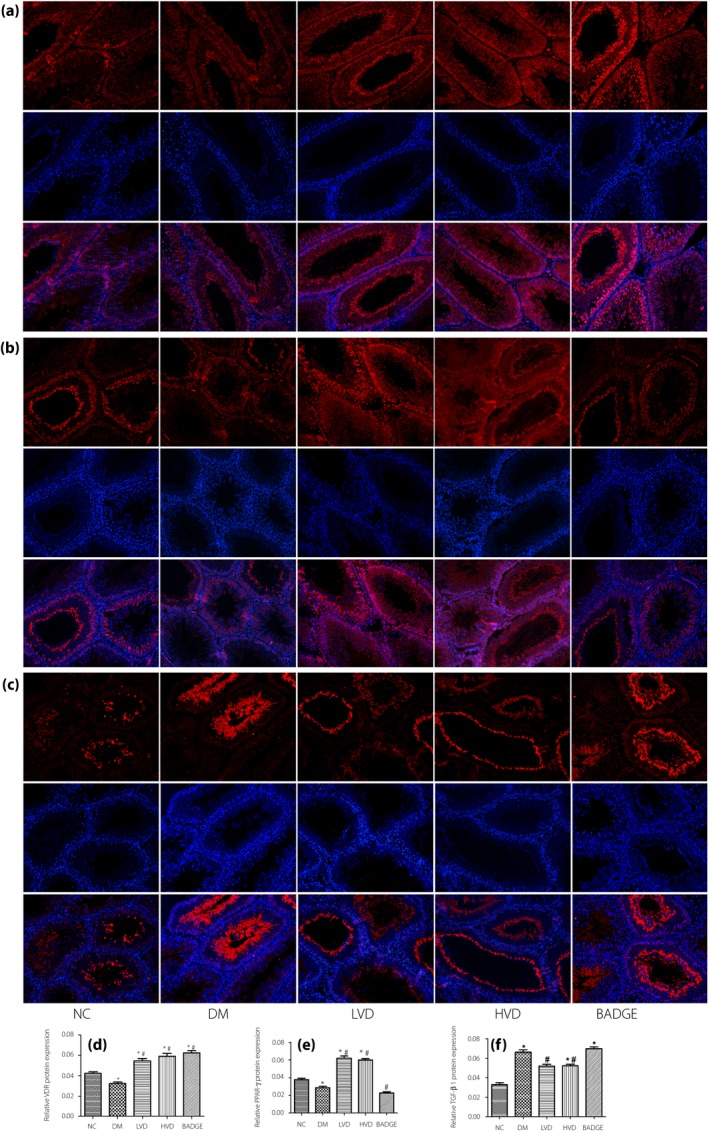
The immunofluorescence result of vitamin D receptor (VDR), peroxisome proliferator‐activated receptor gamma (PPAR‐γ) and transforming growth factor beta 1 (TGF‐β1) in each group, the first line is each protein expression, the second line is 4′,6‐diamidino‐2‐phenylindole images and the third line is merged images (magnification: ×200). (a) VDR protein expression; (b) PPAR‐γ protein expression; (c) TGF‐β1 protein expression; (d) relative VDR protein level; (e) relative PPAR‐γ protein level; and (f) relative TGF‐β1 protein level. The data are shown as mean ± standard error of the mean vs the normal controls with no treatments (NC) group, *P < 0.05 vs NC group; # P < 0.05 vs the untreated diabetes model (DM) group. BADGE, bisphenol A diglycidyl ether; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3.
NF‐κB expression and apoptosis were decreased in diabetic rat testes after vitamin D3 treatment
Apoptosis is a major cause of testicular dysfunction, so we investigated if apoptosis was increased, using terminal deoxynucleotidyl transferase dUTP nick‐end labeling, in diabetic rats. We found that the degree of apoptosis in the testicular tissues of diabetic rats was higher than in the control group. The degree of apoptosis decreased after treatment with vitamin D3 (Figure 3c). Previous studies showed that the NF‐κB signaling pathway is a major regulator of apoptosis17. Western blotting showed that the levels of NF‐κB and Bax were significantly increased in the diabetic group compared with in the NC group. After treatment with vitamin D3, the levels of NF‐κB and Bax were significantly lower than in the diabetic group. The levels of NF‐κB and Bax were highest in the BADGE group (all P < 0.05; Figure 6a,e,f). In contrast, we found that the levels of phospho‐NF‐κB, IκBα, and B‐cell lymphoma 2 were significantly lower in the diabetic and BADGE groups than in the NC group. Compared with their levels in the diabetic group, the levels of these proteins were significantly lower after treatment with vitamin D3 (all P < 0.05; Figure 6b–e,g,h).
Figure 6.
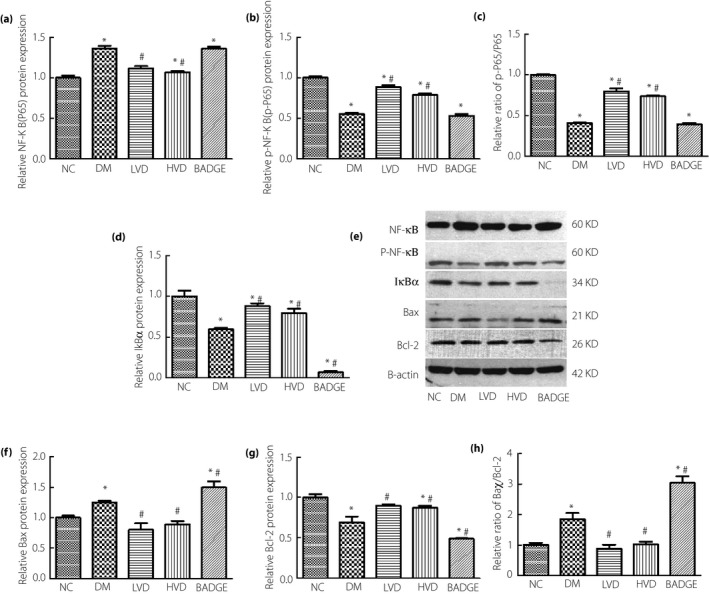
The level of nuclear factor kappa B (NF‐κB; P65), phospho‐NF‐κB (p‐NF‐κB; p‐P65), inhibitor of NF‐κB alpha (IκBα), Bcl‐2‐associated X protein (Bax) and B‐cell lymphoma 2 (Bcl‐2) in each group. (a) Relative NF‐κB (P65) protein level; (b) relative p‐NF‐κB (p‐P65) protein level; (c) relative ratio of p‐NF‐κB (p‐P65)/NF‐κB (P65); (d) relative IκBα protein level; (e) the protein levels of NF‐κB (P65),p‐NF‐κB (p‐P65), IκBα, Bax and Bcl‐2 detected by western blotting; (f) relative Bax protein level; (g) relative Bcl‐2 protein level; and (h) relative ratio of Bax/Bcl‐2. The data are shown as mean ± standard error of the mean vs the normal controls with no treatments (NC) group, *P < 0.05 vs NC group; # P < 0.05 vs the untreated diabetes model (DM) group. BADGE, bisphenol A diglycidyl ether; HVD, model with high‐dose vitamin D3; LVD, model with low‐dose vitamin D3.
Levels of VDR and PPAR‐γ were increased in diabetic rat testes after treatment with vitamin D3
Peroxisome proliferator‐activated receptor gamma is upstream of TGF‐β1 and NF‐κB, and vitamin D3 can regulate the expression of PPAR‐γ. Vitamin D3 signaling through VDR can promote PPAR‐γ expression. Therefore, we examined the expression of VDR and PPAR‐γ in the testes of the experimental animals. The quantitative reverse transcription polymerase chain reaction, western blotting, and immunofluorescence results showed that the relative mRNA and protein levels of VDR and PPAR‐γ were significantly lower in the diabetic group than in the NC group. The relative mRNA and protein levels of VDR and PPAR‐γ were significantly increased in the LVD and HVD groups compared with in the diabetic group. The BADGE group had the lowest relative mRNA and protein levels of VDR and PPAR‐γ (all P < 0.05; Figures 4a,b,d–f and 5a,b,d,e).
Vitamin D3 has no significant effect on pituitary function in diabetic rats
The present results suggest that the serum levels of LH and FSH did not significantly change after vitamin D3 treatment. However, changes in hormone levels can be a function of morphological changes. Therefore, we examined if the pituitary tissues were altered in morphology, structure or molecular composition to determine if any changes upstream of hormone production had occurred. Hematoxylin–eosin staining showed no significant difference in the morphology of the pituitary cells among the groups. Transmission electron microscopy analysis showed no significant differences in the number and size of the gonadotroph endocrine granules among the groups. Immunohistochemistry showed that VDR protein expression in the pituitary cells did not vary statistically among the groups. The pituitary gonadotropic cells were scattered along the far side of the pituitary gland, and the expression of FSH and LH was not statistically different among the groups (all P > 0.05; Figure 7).
Figure 7.
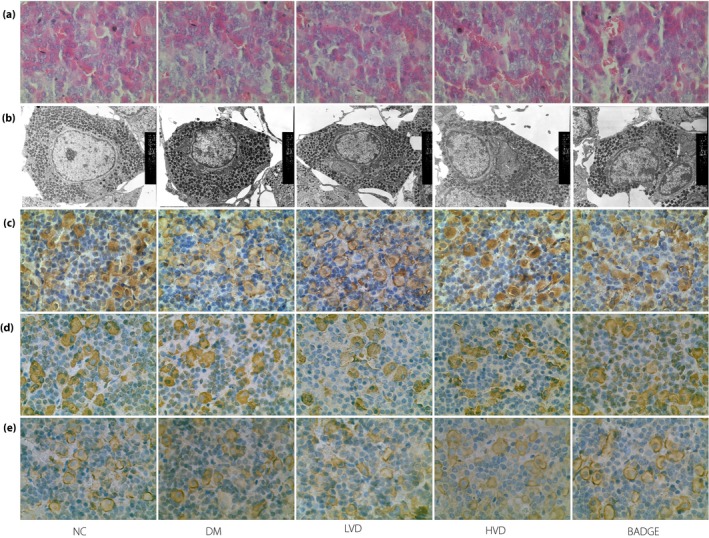
The pathological lesions and immunochemistry results of vitamin D receptor (VDR), follicle‐stimulating hormone (FSH), luteinizing hormone (LH) in each group. (a) Hematoxylin–eosin staining (magnification: ×400); (b) transmission electron microscope (magnification: ×6,000); (c) VDR protein expression; (d) FSH protein expression; and (e) LH protein expression.
Discussion
The present study focused on the mechanism of vitamin D3‐mediated protection of gonadal function and its site of action in diabetic rats. We found that vitamin D3 protects gonad function by acting on the testes, not the pituitary, through PPAR‐γ/TGF‐β1/NF‐κB signaling. At the same time, the protective effect of vitamin D3 on testicular function in diabetic rats was not related to the dose of vitamin D3.
Vitamin D is a multifunctional signaling molecule with a well‐established role in the regulation of calcium homeostasis and bone health. With increasing research, the role of vitamin D in the regulation of non‐calcium/phosphorus pathways in diabetes mellitus and its complications has been gradually recognized18. Our previous study showed that treatment with vitamin D3 can improve testicular function in diabetic rats, which led us to investigate the mechanism of male hypogonadism caused by vitamin D3 deficiency in diabetes mellitus.
Testicular tissue fibrosis and apoptosis cause testicular damage and impair function19, 20, so we analyzed the degrees of fibrosis and apoptosis in the testicular tissues of diabetic rats. We found that vitamin D3 reduced fibrosis and apoptosis in the testes of diabetic rats. Previous studies reported that activation of the TGF‐β pathway was associated with fibrosis in the testes21, 22. The present data suggest that TGF‐β1 mRNA and protein levels were increased in diabetic rat testes, but decreased after treatment with vitamin D3. Therefore, we conclude that vitamin D3 can improve testicular function through modulation of the TGF‐β1 signaling pathway.
Activation of the NF‐κB/Bax pathway can cause apoptosis17, 23. The inactive form of NF‐κB resides in the cytoplasm and associates or links with its natural biological inhibitor, IκB24. NF‐κB can enter the nucleus and cause apoptosis after it dissociates from inhibitor of NF‐κB. Consistent with previous studies, the present data showed that NF‐κB p65, phospho‐IκBα and Bax levels were significantly higher in diabetic rats than in the LVD and HVD groups.
Expression of PPAR‐γ, a member of the nuclear receptor superfamily, can improve testicular function and regulate the expression of TGF‐β1 and NF‐κB12, 25. Both PPAR‐γ and VDR can interact with the retinoid X receptor7, 12. Given that vitamin D3 can also regulate the expression of PPAR‐γ13, we assessed the expression of PPAR‐γ in our model. We found that the decrease in PPAR‐γ levels in the testes of diabetic rats was antagonized by vitamin D3. Thus, we conclude that vitamin D3 can improve testicular function by activating PPAR‐γ. To validate this conclusion, we utilized the PPAR‐γ inhibitor, BADGE26, 27, and found that testicular function was decreased, and fibrosis and apoptosis were increased in the BADGE group. We identified a possible mechanism through which PPAR‐γ inhibits fibrosis and apoptosis in the testes.
VDR mRNA and protein are expressed in the human pituitary gland, suggesting a particular role for vitamin D3 in the central reproductive organs28. We hypothesized that vitamin D3 might improve gonadal function by improving pituitary function in diabetic rats. We found that the serum FSH and LH concentrations were similar in each group, as was the immunohistochemical staining for VDR, FSH and LH. Thus, in our experiments, vitamin D3 had no apparent effect on pituitary function in diabetic rats. The present experimental results are consistent with some previous studies29, 30. Our results could be due to the difficulty of exogenous vitamin D3 passing through the blood–brain barrier or because vitamin D receptor is only expressed in other cells in the pituitary gland, in addition to the gonadotroph. Further study is required to resolve the effects of vitamin D3 on the individual types of pituitary cells.
In summary, the present findings showed that vitamin D3 can alleviate fibrosis and apoptosis in the testicular tissue of diabetic rats, likely through PPAR‐γ/TGF‐β1/NF‐κB signaling. However, over the period of the study, diabetes did not affect the pituitary gonadotroph in rats. These findings provide new insights into the role of vitamin D3 in diabetes, and indicate that vitamin D3 supplementation could be a new adjunctive therapy for testicular dysfunction in people with diabetes.
Disclosure
The authors declare is no conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81570746 and no. 81770812 to G Qin), and the National Natural Science Foundation of China (grant no. 81600647 to F Guo). The authors express their heartfelt thanks to Dr Bo Zhang and Yi Song (Institute of Clinical Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China) for providing necessary facilities.
J Diabetes Investig 2019; 10: 261–271
References
- 1. Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 2013; 9: 479–493. [DOI] [PubMed] [Google Scholar]
- 2. Kapoor D, Aldred H, Clark S, et al Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 2007; 30: 911–917. [DOI] [PubMed] [Google Scholar]
- 3. Fujitani Y, Ueno T, Watada H. Autophagy in health and disease. 4. The role of pancreatic beta‐cell autophagy in health and diabetes. Am J Physiol Cell Physiol 2010; 299: C1–C6. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Zhang Z, Guo W, et al Sulforaphane reduction of testicular apoptotic cell death in diabetic mice is associated with the upregulation of Nrf2 expression and function. Am J Physiol Endocrinol Metab 2014; 307: E14–E23. [DOI] [PubMed] [Google Scholar]
- 5. Hajizadeh MR, Eftekhar E, Zal F, et al Mulberry leaf extract attenuates oxidative stress‐mediated testosterone depletion in streptozotocin‐induced diabetic rats. Iran J Med Sci 2014; 39: 123–129. [PMC free article] [PubMed] [Google Scholar]
- 6. Zanatta L, Zamoner A, Zanatta AP, et al Nongenomic and genomic effects of 1alpha,25(OH)2 vitamin D3 in rat testis. Life Sci 2011; 89: 515–523 [DOI] [PubMed] [Google Scholar]
- 7. Nandi A, Sinha N, Ong E, et al Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig 2016; 25: 15–28. [DOI] [PubMed] [Google Scholar]
- 8. Bouillon R, Carmeliet G, Verlinden L, et al Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008; 29: 726–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verstuyf A, Carmeliet G, Bouillon R, et al Vitamin D: a pleiotropic hormone. Kidney Int 2010; 78: 140–145. [DOI] [PubMed] [Google Scholar]
- 10. Blomberg JM, Nielsen JE, Jorgensen A, et al Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod 2010; 25: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 11. Ding C, Wang Q, Hao Y, et al Vitamin D supplement improved testicular function in diabetic rats. Biochem Biophys Res Commun 2016; 473: 161–167. [DOI] [PubMed] [Google Scholar]
- 12. Abd ETA, Shahin NN, AbdelMohsen MM. Protective effect of Satureja montana extract on cyclophosphamide‐induced testicular injury in rats. Chem Biol Interact 2014; 224: 196–205. [DOI] [PubMed] [Google Scholar]
- 13. Park S, Kim DS, Kang S. Vitamin D deficiency impairs glucose‐stimulated insulin secretion and increases insulin resistance by reducing PPAR‐gamma expression in nonobese Type 2 diabetic rats. J Nutr Biochem 2016; 27: 257–265. [DOI] [PubMed] [Google Scholar]
- 14. Li G, Xu Z, Hou L, et al Differential effects of bisphenol A diglicydyl ether on bone quality and marrow adiposity in ovary‐intact and ovariectomized rats. Am J Physiol Endocrinol Metab 2016; 311: E922–E927. [DOI] [PubMed] [Google Scholar]
- 15. Huang F, Wang Q, Ma X, et al Valsartan inhibits amylin‐induced podocyte damage. Microvasc Res 2016; 106: 101–109. [DOI] [PubMed] [Google Scholar]
- 16. Jiang Z, Wang J, Li X, et al Echinacoside and Cistanche tubulosa (Schenk) R. wight ameliorate bisphenol A‐induced testicular and sperm damage in rats through gonad axis regulated steroidogenic enzymes. J Ethnopharmacol 2016; 193: 321–328. [DOI] [PubMed] [Google Scholar]
- 17. Hu W, Zhou PH, Rao T, et al Adrenomedullin attenuates interleukin‐1beta‐induced inflammation and apoptosis in rat Leydig cells via inhibition of NF‐kappaB signaling pathway. Exp Cell Res 2015; 339: 220–230. [DOI] [PubMed] [Google Scholar]
- 18. Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol 2018; 175: 177–189. [DOI] [PubMed] [Google Scholar]
- 19. Wang YJ, Yan J, Zou XL, et al Bone marrow mesenchymal stem cells repair cadmium‐induced rat testis injury by inhibiting mitochondrial apoptosis. Chem Biol Interact 2017; 271: 39–47. [DOI] [PubMed] [Google Scholar]
- 20. Soomro MH, Shi R, She R, et al Molecular and structural changes related to hepatitis E virus antigen and its expression in testis inducing apoptosis in Mongolian gerbil model. J Viral Hepat 2017; 24: 696–707. [DOI] [PubMed] [Google Scholar]
- 21. Monsivais D, Matzuk MM, Pangas SA. The TGF‐beta family in the reproductive tract. Cold Spring Harb Perspect Biol 2017; 9: a022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiraishi K, Yoshida K, Fujimiya T, et al Angiotensin II dependent testicular fibrosis and effects on spermatogenesis after vasectomy in the rat. J Urol 2003; 170: 2104–2108. [DOI] [PubMed] [Google Scholar]
- 23. Zhao H, Liu J, Song L, et al Oleanolic acid rejuvenates testicular function through attenuating germ cell DNA damage and apoptosis via deactivation of NF‐kappaB, p53 and p38 signalling pathways. J Pharm Pharmacol 2017; 69: 295–304. [DOI] [PubMed] [Google Scholar]
- 24. Shih RH, Wang CY, Yang CM. NF‐kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 2015; 8: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mangoni M, Sottili M, Gerini C, et al A PPAR gamma agonist protects against oral mucositis induced by irradiation in a murine model. Oral Oncol 2017; 64: 52–58. [DOI] [PubMed] [Google Scholar]
- 26. Liang X, Xing W, He J, et al Magnolol administration in normotensive young spontaneously hypertensive rats postpones the development of hypertension: role of increased PPAR gamma, reduced TRB3 and resultant alleviative vascular insulin resistance. PLoS One 2015; 10: e120366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yin Y, Hou G, Li E, et al PPARgamma agonists regulate tobacco smoke‐induced Toll like receptor 4 expression in alveolar macrophages. Respir Res 2014; 15: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perez‐Fernandez R, Alonso M, Segura C, et al Vitamin D receptor gene expression in human pituitary gland. Life Sci 1997; 60: 35–42. [DOI] [PubMed] [Google Scholar]
- 29. Ahangarpour A, Oroojan AA, Khorsandi L, et al Effects of betulinic acid on the male reproductive system of a streptozotocin‐nicotinamide‐induced diabetic mouse model. World J Mens Health 2016; 34: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahangarpour A, Oroojan AA, Heidari H, et al Effects of hydro‐alcoholic extract from Arctium lappa L. (Burdock) root on gonadotropins, testosterone, and sperm count and viability in male mice with nicotinamide/streptozotocin‐induced type 2 diabetes. Malays. J Med Sci 2015; 22: 25–32. [PMC free article] [PubMed] [Google Scholar]


