Abstract
Diabetic foot ulceration has been a serious issue over the past decades in Asia, causing economic and social problems. Therefore, it is important to identify and reduce the risk factors of diabetic foot. Cigarette smoking has been reported to be associated with diabetes and its macrovascular complications, but the relationship between smoking and diabetic foot ulcers is still unclear. In the present review, we summarize the effects of cigarette smoking on diabetic foot ulcers with respect to peripheral neuropathy, vascular alterations and wound healing. One underlying mechanism of these impacts might be the smoking‐induced oxidative stress inside the cells. At the end of this review, the current mainstream therapies for smoking cessation are also outlined. We believe that it is urgent for all diabetic patients to quit smoking so as to reduce their chances of developing foot ulcers and to improve the prognosis of diabetic foot ulcers.
Keywords: Cigarette smoking, Diabetic foot ulcers, Oxidative stress
Introduction
As the global prevalence of diabetes is increasing, diabetic foot ulcers have become one common complication of diabetes, with a lifetime incidence 25% in diabetes patients1, posing enormous medical, social and economic burden. The situation of diabetic foot ulcerations in Asia remains severe. Amputations are common and specialist foot‐care clinics are rare2. As a result, the diabetes foot epidemic is rapidly emerging, and the prevalence of diabetic foot is reported to increase to approximately 5.5%3. It is urgent for clinical practitioners to use systemic methods, including identification and reduction of risk factors, optimization of metabolic control (e.g., blood glucose, blood pressure and cholesterol), patient podiatric education and so on, to avoid the onset of foot ulcerations, and reduce limb amputation rate and related mortality. As is reported, the risk factors for diabetic foot include foot insensitivity to the monofilament, past history of amputation or foot ulcer, insulin use, Charcot deformity, reduced skin oxygenation and foot perfusion, increased bodyweight, poor vision, hammer/claw toe deformity, cigarette smoking and so on4. Cigarette smoking has been decreasing, but still remains a serious global problem5. According to the global data, east Asian countries have accounted for the highest percentage (38%) among smokers all over the world6. This risk factor correlates with many chronic diseases, such as cardiovascular disease, diabetes, cancer and lung diseases. The associations between cigarette smoking and risks of pre‐diabetes, gestational diabetes, type 2 diabetes, coronary artery disease or even mortality in diabetes patients have been confirmed by many studies7, 8, 9, 10, 11, 12. Active smokers had a much lower mean age at amputation compared with non‐smokers13, and smoking cessation improved amputation‐free survival in diabetes patients14. Although secondhand smoke was not significantly associated with peripheral artery disease, exposure to passive smoke is independently associated with the risk of type 2 diabetes15. The aforementioned all indicate a role of cigarette smoking in diabetic foot. However, how smoking affects the development of diabetic foot ulcers is still not fully understood. Here, we provide a review in regard to the role of cigarette smoking in the development of diabetic foot ulcers and explore to what extent smoking might affect diabetes patients with foot complications.
We declare that the protocol for the project has been approved by the ethics committee of Mingci Cardiovascular Hospital, and that it conforms to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, October 2013).
Smoking and diabetic peripheral neuropathy
Diabetic peripheral neuropathy is one of the most common complications in diabetic foot ulcers, accounting for approximately 30% in diabetes patients, and >50% in type 2 diabetes patients aged >60 years16, 17. The presence of peripheral neuropathy is found in 78% of patients with foot ulcerations18. There are many types of nerve injury in diabetic patients, including distal symmetrical polyneuropathy, small‐fiber predominant neuropathy, autonomic neuropathy, diabetic amyotrophy, mononeuritis multiplex and so on, among which distal symmetrical polyneuropathy affects the longest nerve fibers first, and small‐fiber sensory neuropathy mainly affects pain and temperature sensations, both closely related to the onset of foot ulceration19, 20. The pathology of diabetic neuropathy includes axonal degeneration, demyelination of the nerve fibers, proliferation of Schwann cells, remyelination, hypertrophy of the basal lamina and so on20. The distal symmetrical sensory loss is often the first component in the pathway to foot ulceration, and the inability to feel pain in the case of trauma might subsequently facilitate chronic plantar ulcers to grow18. In addition, diabetic autonomic neuropathy causes arteriovenous shunting, neuropathic edema and dry skin in the foot. Motor neuropathy is named “claw foot,” and characterized by clawing of the toes and plantar flexion of the metatarsal heads. These autonomic and motor neural alterations are all common causes of callus and ulceration formation18.
Mechanisms of diabetic peripheral neuropathy
Oxidative stress is believed to be the ultimate mechanism of cellular damage in diabetic neuropathy. It is characterized by high levels of sustained generation of reactive oxygen species (ROS), including ozone, superoxide, hydrogen peroxide, singlet oxygen and organic peroxides in cells21, 22. The nervous system is especially vulnerable to oxidative damage23. Hyperglycemia, hyperlipidemia and impaired insulin sensitivity could activate various signaling pathways leading to oxidative stress in nerve fibers. Excessive ROS then causes demyelination and axon damage24, leading to diabetic neuropathy19.
Effects of cigarette smoking on diabetic peripheral neuropathy
Cigarette smoke might exacerbate diabetic neuropathy partly through the mechanism of oxidative stress. Cigarette smoking has been confirmed an independent risk factor for diabetic neuropathy25. As a source of free radicals and oxidants, cigarette smoke could induce cellular oxidative stress in many organs, including the nervous system and blood vessels, leading to cellular damage and even apoptosis (Figure 1)26, 27.
Figure 1.
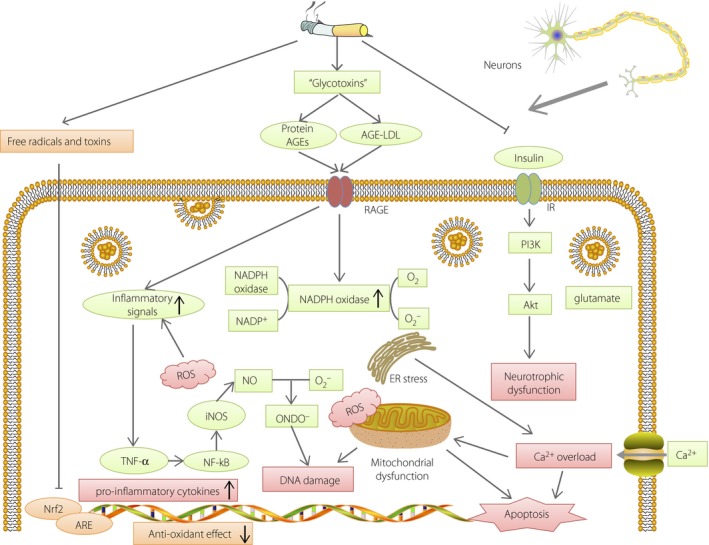
Mechanisms of cell damage in peripheral neurons caused by cigarette smoking. Cigarette smoking induces formation of advanced glycation end‐products (AGEs) and inhibits insulin signaling and the NF‐E2‐related factor 2 (Nrf2)–anti‐oxidant responsive element (ARE) pathway, causing oxidative stress, endoplasmic reticulum stress, mitochondrial dysfunction, deoxyribonucleic acid (DNA) damage and apoptosis in peripheral neurons. ER, endoplasmic reticulum; iNOS, nitric oxide synthase; LDL, low‐density lipoprotein; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; RAGE, receptor for advanced glycation end‐products; ROS, reactive oxygen species; TNF‐α, tumor necrosis factor‐α.
In vitro and in vivo evidence shows that cigarette smoke contains “glycotoxins.” These glycotoxins are highly reactive glycation products that can rapidly induce advanced glycation end‐products (AGE) formation outside the cells28, 29, 30. The increased modified proteins and lipids in the circulation of smokers bind to the receptor for AGE, which activates nicotinamide adenine dinucleotide phosphate oxidase and expression of pro‐inflammatory cytokines and chemokines, inducing oxidative stress. The excessive ROS caused by cigarette smoking results in the production of nitric oxide synthase and an overload of glutamate in the synapses, and the consequent influx of Ca2+ leads to mitochondrial dysfunction, deoxyribonucleic acid damage, inflammation and even apoptosis31, 32, 33.
In addition, cigarette smoking also worsens neuropathy by inducing insulin resistance. Smoking is a risk factor for impaired insulin secretion, and chronic cigarette smokers are insulin resistant and hyperinsulinemic34. The increased insulin receptor substrate‐1ser636 phosphorylation in smokers could return to normal after smoking cessation. This effect might partly come from nicotine through the mechanistic target of rapamycin pathway in skeletal muscle cells35. In adipocytes, another important cell type in insulin resistance, nicotine was proved to activate adenosine monophosphate‐activated protein kinase α2, increasing circulating free fatty acid and inducing insulin resistance36. In neurons, nicotine‐derived nitrosamine ketone could inhibit the insulin receptor and Akt pathway, leading to loss of insulin‐mediated neurotropism and neuronal dysfunction37.
Cigarette smoking also inhibits the NF‐E2‐related factor 2–anti‐oxidant responsive element (Nrf2‐ARE) pathway38, 39. Nrf2 is a transcription factor recognized as the master regulator of the cellular response to oxidants, and upregulates the expression of the ARE‐regulated genes in various cell types. When smoking occurs, the anti‐oxidative effects exerted by the Nrf2‐ARE pathway are weakened, exposing astrocytes and neurons to more damage, including oxidative glutamate toxicity and calcium disturbance40.
Smoking and diabetic vascular disease
Diabetic vascular disease has been found in approximately 30% of patients with foot ulcers, and includes macrovascular and microvascular disease.
In peripheral arterial disease, mechanisms of atherosclerosis formation have been reported by numerous studies41, 42, 43, 44. Severe atherosclerotic plaques in peripheral arteries result in ischemia in the foot tissues, resulting in tissue loss and slowness of the wound healing process18, 45. Smoking, as a very important risk factor, is closely related to the development of vascular plaques and mortality46, 47. A recent review has elaborated the roles of smoking on all stages of atherogenesis, as well as pathological atherothrombosis formation48.
Microvascular mechanisms of diabetic foot ulcers
Some researchers say that diabetic foot ulcers are more of a “small‐vessel disease”49. As an important component of diabetic neuropathy, the microcirculatory changes seem to play a more crucial role in the pathogenesis of foot ulcers. Microvascular structure in the skin consists of capillaries and thermoregulatory arteriovenous shunt flow. Although occlusive lesions are not found in diabetic microcirculation, other structural abnormalities could be detected, including thickening of the capillary basement membrane50, decrease in luminal and capillary size51, and reduction in capillary density52, resulting in reduction of the vascular elasticity and impairment of normal transport through the capillary walls. Apart from the structural changes, functional ischemia also exists, as a result of maldistribution of blood flow between the capillaries and the arteriovenous shunts. Both the structural and functional deficiencies lead to compromised vasorelaxation in case of stress, reduced blood flow in nutrient capillaries and lower tissue PO2 53.
Effects of cigarette smoking on diabetic microangiopathy
Cigarette smoking exerts effects on diabetic microangiopathy. Due to the high requirement of experimental techniques in studying microcirculation, studies in this field are relatively few. It is reported that chronic smokers have a weakened vasodilation in response to different stimuli in skin microvasculature, mainly endothelium‐dependent, further decreasing the already reduced blood flow in diabetic microcirculation. This impaired vasoreactivity is associated with the duration and intensity of smoking54, 55, 56. A recent in vivo study showed that this diminished acetylcholine‐induced vasodilation in skin caused by cigarette smoking is a result of reduced nitric oxide‐ and cyclooxygenase‐dependent vasodilation57.
In addition, smoking also causes ROS generation in the leukocytes. The activated leukocytes and/or platelets aggregate in the circulation and adhere to the microcirculation endothelium58, 59, implicating the presence of local inflammation as well.
In addition, acute smoking also activates the adrenergic system and causes vasoconstriction, further decreasing blood flow and tissue oxygen tension60, 61, 62. The reduction of blood flow is rather substantial: by approximately 50% in healthy people and by >10% in people with diabetes63. Even passive inhalation of cigarette smoke could cause an immediate and prolonged reduction in capillary blood flow velocity in healthy people64. However, this acute reduction could be recovered by stopping smoking61. Anti‐oxidant vitamin C is shown to improve impaired endothelium‐dependent responses in chronic smokers65, indicating the important role of oxidative stress caused by cigarette smoking.
Smoking and wound healing
Normal Wound Healing Process
Normal wound healing can be divided into four phases: coagulation, inflammation, migration‐proliferation and remodeling. Vascular injury in the wound triggers the formation of blood clots, and then induces the inflammatory cells including neutrophils and monocytes to migrate into the wound. These immune cells produce a large amount of pro‐inflammatory cytokines, such as interleukin (IL)‐1, IL‐8 and tumor necrosis factor‐α, and proteolytic enzymes. Monocytes then differentiate into macrophages, and remove the microorganisms (if infected), the debris and necrotic cells together with neutrophils. After this, the proliferative phase starts with the migration and proliferation of fibroblasts in the wound, and formation of the extracellular matrix and granulation tissue. At day 4–5 after wounding begin the proliferative and remodeling phases. In the normal proliferative process, fibroblasts migrate and proliferate in the wound area by the induction of platelet‐derived growth factor and transforming growth factor‐β, producing a great number of matrix proteins, forming the extracellular matrix66. Collagen is a very important type of protein, and provides strength and support for the whole extracellular matrix. Ultimately, the healing process is ended by the remodeling of the extracellular matrix and the maturation of the scar66.
Wound healing in diabetic foot ulcers
Wound healing is already impaired in diabetic foot ulcers, and the risk of amputation is higher than for healthy people. In diabetic patients, the sensory, motor and autonomic neuropathies combined with aforementioned microcirculatory and macrovascular deficiencies are believed to be the intrinsic factors for the wound healing process being impaired, whereas infection, callus formation and high plantar pressure are the extrinsic factors, though the extrinsic factors are partly attributable to the intrinsic factors67. Diabetes could also cause impaired angiogenesis in the wound, probably as a result of the diabetes‐induced overproduction of ROS68, despite the fact that low levels of ROS, such as H2O2, actually promote angiogenesis69, 70.
Effects of cigarette smoking on wound healing in diabetic foot ulcers
How cigarette smoking affects the wound healing process has always been a strong research hot spot. It is reported that smoking is associated with a higher incidence of serious postoperative complications71, and preoperative smoking cessation of >3 weeks decreases the occurrence of impaired wound healing72 and reduces postoperative morbidity from 52% to 18%73, implicating the important role of smoking cessation on improving cutaneous wound healing. In fact, the effects of smoking on wound healing have been found in each phase of wound healing (Figure 2)74, 75.
Figure 2.
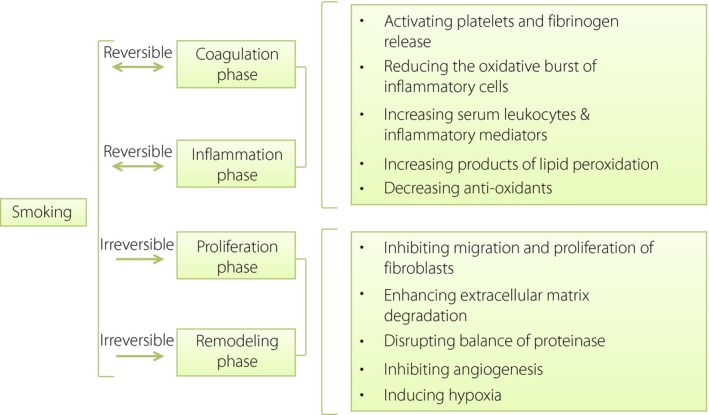
Effects of cigarette smoking on wound healing. The effects of smoking on wound healing have been found in each phase of wound healing, further worsening the impaired wound healing in diabetic patients.
Coagulation and inflammation phases
In the coagulation phase, smoking promotes hemostatic clot formation by activating platelets and fibrinogen release76. In the inflammatory phase, inflammation and proliferation of wound healing are delayed in smokers77, and the immune system is impaired78. Smoking could increase the infiltration of neutrophils, but reduce the chemotaxis of monocyte‐macrophages into the wound area79. Increased levels of protease were also observed in smokers, leading to connective tissue degradation80, 81. In addition, ROS production and release exist in the normal healing process. A transient oxidative burst by neutrophils and monocyte macrophages in the wound has the function of attacking microorganisms82, 83, and might be involved in promoting migration of polymorphonuclear leukocytes to the wound area22, angiogenesis69 and intracellular signaling pathways22. Cigarette smoking could reduce the oxidative burst of inflammatory cells and weaken the ability of neutrophils to phagocytize the pathogens79. This is due to the cytotoxic effects of cigarette smoking, partly through suppression of caspase‐3 activity and the modification of membrane receptors (L‐selectin and CD18)84, 85.
Systemically, smoking causes chronic inflammation and increases serum polymorphonuclear leukocytes by 20–30% by stimulating the hematopoietic system in the bone marrow86. Chronic smoking could lead to increased products of lipid peroxidation, decreased anti‐oxidants and increased inflammatory mediators, including C‐reactive protein and cytokines in the circulation87, exacerbating the systemic inflammatory responses caused by the wound.
Migration–proliferation and remodeling phases
In vitro studies show that cigarette smoke extracts inhibit migration and proliferation of fibroblasts88, 89, and reduce the synthesis of collagen89, 90. In addition, the balance of proteinase is also disrupted in smokers. Increased levels of matrix metalloproteinase (MMP)‐890 and MMP‐1 messenger ribonucleic acid91in skin tissues, and higher circulating MMP‐9 levels81, 92 are found in smokers, resulting in enhanced extracellular matrix degradation, which could also be part of the mechanisms for the decrease of collagen.
Solid evidence shows that low levels of nicotine could enhance angiogenesis in the setting of inflammation, ischemia, atherosclerosis and neoplasia93, 94. However, the effect of nicotine alone could not be generalized to the effects of cigarette smoking. As a matter of fact, in vitro, cigarette smoking inhibits endothelial migration and capillary‐like tube formation95, and in vivo, smoking caused a reduction of capillary density in ischemic muscles by inhibiting vascular endothelial growth factor through decreased expression of hypoxia‐inducible factor‐1α96, both showing the detrimental influence of cigarette smoking on angiogenesis and granulation tissue formation. In addition, bone marrow‐derived circulating endothelial progenitor cells (EPCs) could be isolated from the blood and play crucial roles by differentiating into mature endothelial cells in areas of neovascularization97. A reduction of circulating EPC numbers and functions was observed in diabetes98, 99, and one study showed that decreased expression of an anti‐oxidant enzyme, MnSOD, was responsible for the EPC dysfunction in diabetic mice100, showing a role of ROS overproduction by mitochondria in this disorder. Studies showed that smokers demonstrated a decreased number of EPCs, impaired differentiation and function of EPCs, and an increase of ROS production in EPCs101. Smoking cessation could also upregulate the circulating EPC levels in chronic smokers102. This evidence shows the adverse impacts of smoking on the already impaired angiogenesis in diabetic ulcers.
Finally, as mentioned above, smoking reduces tissue blood flow and oxygen supply18, 60, 75, 103. Smoking leads to microvascular alterations, such as impaired vasodilation, stimulation of sympathetic nervous activity by nicotine and atherosclerosis of the blood vessels in the lower extremities, but other possible mechanisms might be carbon monoxide, another component of cigarette smoking, could bind to hemoglobin with a much higher affinity and cyanide interferes with cellular oxygen metabolism104. Although acute hypoxia might initiate the healing, promote fibroblast proliferation and stimulate collagen synthesis, chronic hypoxic conditions caused by smoking exert the opposite effects by diminishing fibroblast activity, inhibiting collagen synthesis and interfering with angiogenesis105, 106. Neutrophils were also shown in vitro to lose their bacterial killing capacity at a pO2 level <40 mmHg107. During the re‐epithelialization phase, a proliferative burst of keratinocytes occurs in an oxygen‐dependent manner, stimulated by various cytokines and chemokines (e.g., epidermal growth factor, transforming growth factor‐α, keratinocyte growth factor, hepatocyte growth factor, insulin‐like growth factor‐1, IL‐1 and IL‐6). Hypoxia induced by smoking also inhibits this process and leads to delayed closure of the wound108.
Smoking Cessation
Smoking cessation and diabetic foot ulcers
Smoking appears to increase the risk of diabetic foot amputation109. However, direct evidence is very limited for the effect of smoking cessation on lower leg lesions and amputation in diabetes. It is reported that effective smoking intervention programs before surgery showed a significant reduction of postoperative morbidity73, 110, indicating a beneficial effect of smoking cessation in promoting postoperative wound healing. Transient hypoxia and oxidative stress seem to be reversed by smoking abstinence, though the detrimental effects of smoking on proliferation and remodeling in the diabetic wound might be prolonged and irreversible75. As is known, smoking cessation is also a very cost‐effective intervention for patients with atherosclerosis111, 112, slowing the progression into ischemia of the lower extremities.
Clinically, several therapies have already been adopted to increase the success rate of smoking cessation. These include behavioral interventions, nicotine replacement therapy (NRT), bupropion, electronic cigarettes and varenicline (Table 1).
Table 1.
Recommended cessation treatments for smokers
| Mode of action | Efficacy (vs placebo) | Recommended dosage | Duration of therapy | Major side‐effects | Cautions | |
|---|---|---|---|---|---|---|
| Behavior interventions | Psychotherapy | Increasing quit rate by 40–60% | – | – | None | – |
| NRT | Desensitizing the nicotinic acetylcholine receptors and preventing them to resensitize | Increasing quit rate by 50–70% |
|
|
|
Not recommended for:
|
| Bupropion |
|
Increasing quit rate by >80% | 150 mg twice a day | 12 weeks |
|
Cautions for smokers with:
|
| Varenicline | Selective α4β2 nicotinic receptor partial agonist | More than double the quit rate | 1.0 mg twice a day | 12 weeks and may be continued for another 12 weeks if cessation is achieved |
|
Not recommended for:
|
| E‐cigarette | Heating a liquid containing nicotine etc. to generate a vapor | No efficacy | Not recommended | – | – | – |
NRT, nicotine replacement therapy; E‐cigarette, electronic cigarette.
Behavioral interventions
A recent Cochrane review showed that individual behavioral counseling of >10 min significantly increases the likelihood of cessation by 40–60% than a minimal contact control (brief advice, usual care or provision of self‐help materials)113. Additional telephone follow‐up counseling sessions are also reported to double the quit rate at 12‐month follow up114. A specialized counseling intervention has proved more effective for helping patients cease smoking than a usual state quit‐line115. Smoking cessation counseling is associated with a higher likelihood of attempting to quit, even for patients who are less engaged during medical encounters116. What is good about behavioral interventions is that they are absolutely safe and have no side‐effects. They are an appropriate choice for pregnant smokers to improve maternal and child health outcomes117, 118. Thus, individual behavioral counseling should be suggested wherever the counseling is available to all smokers.
Despite the efficacy of behavioral interventions for smokers, Tindle et al.119 have recently made their comments, stressing the importance of pharmacotherapy for smoking cessation treatment, even without behavioral counseling.
Nicotine replacement therapy
Nicotine replacement therapies could assist quitters by alleviating the discomfort in the process of cigarette withdrawal, and have been approved by Food and Drug Administration (FDA) in the USA to treat tobacco dependence. They come in different forms: gum, inhalers, lozenges, nasal sprays and transdermal patches. The absorption time of each form is different: the inhalers are the fastest (peak concentrations usually within seconds), and the transdermal patches the slowest (peak concentrations within hours). When nicotine is released continuously and slowly in the brain, it could desensitize the nicotinic acetylcholine receptors and prevent them from resensitizing, thus reducing both the pleasurable symptoms (e.g., improved mood and enhanced vigilance) and the withdrawal symptoms (e.g., anxiety, irritability and impaired concentration). Randomized controlled trials (RCTs) show that NRTs significantly increase the quit rate by 50–70% compared with a placebo. For smokers not ready to stop, NRTs could assist twice as many quitters to achieve 6 months of abstinence as those who take a placebo. A dosage of 4‐mg gum rather than 2‐mg gum showed an increased quit rate in highly dependent smokers. Doses of 22‐mg and 44‐mg transdermal nicotine therapy produced similar effects during a 26‐week follow‐up period, and the 44‐mg patch dose produced more adverse effects, such as vomiting, nausea and erythema with edema at the patch site120, 121, 122, 123, 124. Combined use of nicotine patches and chewing gum together has the highest quit rate (95.2%) after 4 weeks and at 12‐month follow up (62.5%)125.
Theoretically, nicotine might cause cardiovascular disease due to its hemodynamic effects of sympathetic neural stimulation and systemic catecholamine release, but it was presumed to be safe and the advantages outweigh the disadvantages126. However, a recent cohort study127 suggested that treatment with NRT over 4 weeks results in an increase in cardiovascular events in a follow‐up period of 52 weeks, which prompts more studies with long‐term follow up to clarify this concern. Furthermore, whether the NRTs should be prescribed to the critically ill patients in the intensive care units is also still questionable and in need of further studies128. NRT is generally not recommended for pregnant smokers, and is classified by the FDA as category D (transdermal nicotine) and category C (shorter‐acting forms). Furthermore, among smokers who are motivated to stop smoking, quitting abruptly after 2 weeks of precessation NRT is shown to be more effective than gradually reducing cigarettes per day after the same precessation NRT129.
Bupropion
Bupropion, a dopamine‐uptake inhibitor, is also a FDA‐approved smoking cessation and antidepressant medication. During the search for a smoking cessation medication that does not contain nicotine, a RCT in 1997 reports that administration of a sustained‐release form of bupropion for 7 weeks could increase the quit rate by >80% at 1‐year follow up. The dosage of 300 mg (150 mg twice a day) was recommended due to its greatest efficacy on abstinence and less occurrence of weight gain. Use of sustained‐release bupropion alone or in combination with a nicotine patch resulted in significantly higher long‐term quit rates than treatment of either the nicotine patch alone or placebo, also indicating the great efficacy of bupropion130. Bupropion was recommended to start at least 7 days before the quitting date to achieve steady‐state plasma levels, and the treatment is recommended for 7–12 weeks131. As for safety, no increased risks of neuropsychiatric or heart and circulatory problems have been found in the bupropion studies. As quitters with a history of depression are at higher risk of developing a new episode of major depression for at least 6 month132, bupropion seems a better choice than NRT for smokers with a history of depression133.
Electronic cigarettes
An electronic cigarette, or e‐cigarette, is a small electronic device that resembles the feeling of cigarette smoking. It works by heating a liquid to generate a vapor, and the liquid usually contains nicotine, propylene glycol, glycerine and flavorings. The e‐cigarette products vary in engineering with regard to nicotine concentrations in the solution, volumes of solution in the product, different carrier compounds, additives and flavors, and battery voltage. A study says e‐cigarettes are “modestly effective” at assisting smokers to quit134. However, the other evidence to date is not strong enough to prove that e‐cigarettes are efficacious for smoking cessation. Therefore, no e‐cigarette products are currently approved by the FDA as smoking cessation therapy. Although e‐cigarettes are likely to be much less toxic than cigarette smoking, they still produce lower levels of the toxic chemicals including 1,2‐propanediol, glycerin, aluminum and seven polycyclic aromatic hydrocarbons, considered probable carcinogens. Therefore, for the protection of public health, e‐cigarettes are also prohibited in some public places where cigarettes are not allowed135. Furthermore, a cross‐sectional study reported that use of e‐cigarettes is associated with increased odds of cigarette smoking and decreased odds of abstinence from conventional cigarettes among USA adolescents, showing that the use of e‐cigarettes might even encourage conventional cigarette use in younger people136. Therefore, e‐cigarette currently should not be prescribed to smokers for smoking cessation, and more randomized clinical trials were still required to clarify the effects of e‐cigarettes on the smoking cessation rate.
Varenicline
Varenicline is another FDA‐approved smoking cessation medication. It is a selective α4β2 nicotinic receptor partial agonist, showing approximately 30–60% of the efficacy of nicotine and at the same time blocking the nicotine response in vivo, which is the ideal profile for reducing nicotine dependence and minimizing the potential side‐effects through activation of nicotinic receptors137. Many RCTs have confirmed the efficacy of varenicline in smoking cessation. A review concludes that varenicline more than doubles the smoking cessation success compared with a placebo138. Furthermore, varenicline shows its superiority over any form of NRT monotherapy and bupropion, and is as effective as combination NRT138, 139. For every 100 patients treated with varenicline, compared with NRT, approximately additional five patients would be abstinent from cigarettes 2 years after treatment140. However, other recent clinical trials comparing varenicline with combination NRT and NRT monotherapy showed no significant difference in the long‐term quit rates, although varenicline might enhance the success rate in the short and medium term141, 142. The efficacy of varenicline increases as the dose is higher, and the dosage of 1.0 mg twice daily provides the highest continuous quit rates, and shows a good safety and tolerability profile143. However, long‐term surveillance for the safety of varenicline is still required in the aspect of adverse neuropsychiatric and cardiac events.
Combination therapies
Some researchers advocate combination therapy for the purpose of maximizing the efficacy, which includes the pharmacotherapy plus behavioral interventions and the combination drug therapies.
A Cochrane review in 2012 concludes that combining behavioral support with medication might increase the chances of smoking cessation success by 70–100% compared with the chance of success when receiving advice only144. A recent RCT also showed that adopting nicotine lozenges plus phone counseling significantly increased tobacco abstinence rates compared with either intervention alone145. Other studies also showed the efficacy of NRT plus behavior therapy among smokers with a psychotic disorder146 and among pregnant women147.
Combination NRT is also recommended by many guidelines, and usually consists of the use of long‐lasting nicotine transdermal patch and short‐acting forms including an inhaler, gum, lozenges or nasal sprays. The combination could be sequential or concurrent. The rationale for the sequential therapy is that the patch could provide a stable level of nicotine to achieve and remain cessation, and the after use of short‐acting forms is expected to deal with the emergent craving. Furthermore, the rationale for concurrent combination therapy is that the sensory stimulation and acute nicotine delivery might provide a higher level of craving relief148. Clinical studies have shown that concurrent combination NRT significantly increased sustained abstinence rates149, 150 and is as effective as using varenicline alone138. In addition, the combination use of nicotine drugs is generally considered safe and no significant adverse effects have occurred, but the safety of the combined use of drugs on pregnant smokers is still unclear and caution should be taken148.
Besides NRT, combination use of other medications might also yield great efficacy. For instance, combined use of varenicline and bupropion, compared with monotherapy, might increase prolonged abstinence151, 152. Bupropion plus nicotine patch has also been shown to be well tolerated and significantly improve the short‐term quit rate in smokers with schizophrenia153. However, another clinical trial reported that NRT plus bupropion was not more effective than NRT alone133. More relevant clinical trials are still required to clarify the efficacy of combined use of NRT and bupropion.
Conclusions
Cigarette smoking has been known to correlate with diabetes. The present review shows that smoking has extensive effects on all stages of diabetic foot ulcerations, both the onset and healing processes (Figure 3). Smoking can exacerbate diabetic peripheral sensory, autonomic and motor neuropathy, which are important reasons for the occurrence of foot ulcerations. Microvascular alterations, such as impaired vasodilation and increased3 vasoconstriction, could also be detected in smokers, leading to tissue hypoxia and interfering with the healing process. Smoking also has negative impacts on wound healing in almost all phases through very complex mechanisms. The effects are not separate, but interact with each other. For instance, axonal degeneration and demyelination of the nerve fibers could be partly due to ischemia of the microvasculature20 caused by smoking, and in turn, autonomous nerve dysfunctions regarding several nerve reflexes could also regulate skin blood flow through opening arteriovenous shunts and blunting vasodilation, leading to hypoxia of the skin tissue53, forming a vicious cycle. All the effects of cigarette smoking together further jeopardize the already impaired capacity of diabetes patients to maintain skin integrity and repair the wound. One of the most important mechanisms underlying these effects is the cellular oxidative stress, as cigarette smoking is known to be a source of oxidants and increases the production of ROS inside cells from almost all tissues. Although there might still be many unknown mechanisms, quitting smoking has been confirmed to be effective in reducing mortality and increasing amputation‐free survival. The current effective therapies for smoking cessation include behavioral interventions and pharmacological options, such as NRT, bupropion, varenicline or the combination use of them. In order to achieve long‐term smoking abstinence success, the therapy should be individualized according to the will of the smokers, and behavioral support should always be recommended if possible. Successful smoking cessation would make the situation of diabetic foot ulcers better and lead to a better prognosis.
Figure 3.
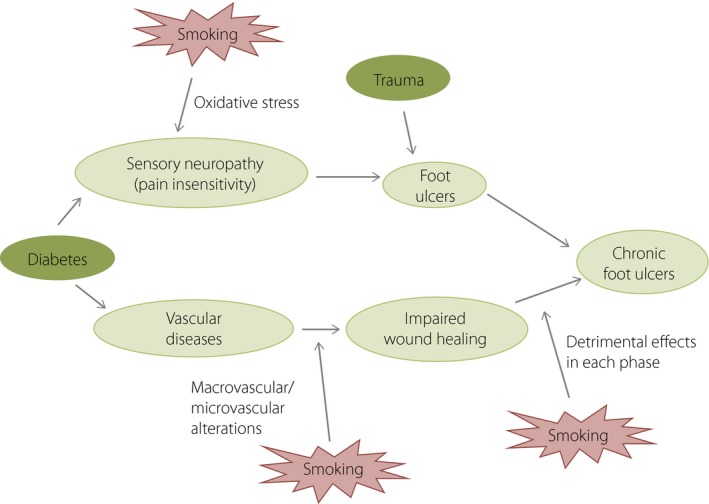
Effects of cigarette smoking on formation of diabetic foot ulcers. The effects of smoking on the onset of chronic diabetic foot ulcers have been shown, from worsening neuropathy and vascular alterations to slowing wound healing.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant number: 81770810).
J Diabetes Investig 2019; 10: 202–215
References
- 1. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293: 217–228. [DOI] [PubMed] [Google Scholar]
- 2. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, et al The global burden of diabetic foot disease. Lancet 2005; 366: 1719–1724. [DOI] [PubMed] [Google Scholar]
- 3. Zhang P, Lu J, Jing Y, et al Global epidemiology of diabetic foot ulceration: a systematic review and meta‐analysis (dagger). Ann Med 2017; 49: 106–116. [DOI] [PubMed] [Google Scholar]
- 4. Boyko EJ, Ahroni JH, Stensel V, et al A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999; 22: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 5. Ng M, Freeman MK, Fleming TD, et al Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA 2014; 311: 183–192. [DOI] [PubMed] [Google Scholar]
- 6. Jha P, Ranson MK, Nguyen SN, et al Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health 2002; 92: 1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loeken MR. Passive smoking as an independent risk factor for gestational diabetes that synergizes with prepregnancy obesity in urban Chinese women. Diabetes Metab Res Rev 2017; 33: e2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bucheli JR, Manshad A, Ehrhart MD, et al Association of passive and active smoking with pre‐diabetes risk in a predominantly Hispanic population. J Investig Med 2017; 65: 328–332. [DOI] [PubMed] [Google Scholar]
- 9. Sattar N, Sorensen T, Taylor AE, et al Smoking and diabetes risk: building a causal case with clinical implications. Lancet Diabetes Endocrinol 2015; 3: 918–920. [DOI] [PubMed] [Google Scholar]
- 10. Pan A, Wang Y, Talaei M, et al Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta‐analysis and systematic review. Circulation 2015; 132: 1795–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yokomichi H, Nagai A, Hirata M, et al Survival of macrovascular disease, chronic kidney disease, chronic respiratory disease, cancer and smoking in patients with type 2 diabetes: bioBank Japan cohort. J Epidemiol 2017; 27: S98–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walsh JW, Hoffstad OJ, Sullivan MO, et al Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med 2016; 33: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 13. Liedberg E, Persson BM. Age, diabetes and smoking in lower limb amputation for arterial occlusive disease. Acta Orthop Scand 1983; 54: 383–388. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong EJ, Wu J, Singh GD, et al Smoking cessation is associated with decreased mortality and improved amputation‐free survival among patients with symptomatic peripheral artery disease. J Vasc Surg 2014; 60: 1565–1571. [DOI] [PubMed] [Google Scholar]
- 15. Pan A, Wang Y, Talaei M, et al Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tesfaye S, Stevens LK, Stephenson JM, et al Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996; 39: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 17. Young MJ, Boulton AJ, MacLeod AF, et al A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993; 36: 150–154. [DOI] [PubMed] [Google Scholar]
- 18. Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int J Low Extr Wound 2005; 4: 154–159. [DOI] [PubMed] [Google Scholar]
- 19. Callaghan BC, Cheng HT, Stables CL, et al Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2012; 11: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Said G. Diabetic neuropathy–a review. Nat Clin Pract Neurol 2007; 3: 331–340. [DOI] [PubMed] [Google Scholar]
- 21. Vincent AM, Russell JW, Low P, et al Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 2004; 25: 612–628. [DOI] [PubMed] [Google Scholar]
- 22. Nathan C, Cunningham‐Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol 2013; 13: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 1999; 9: 69–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincent AM, Callaghan BC, Smith AL, et al Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. [DOI] [PubMed] [Google Scholar]
- 25. Tesfaye S, Chaturvedi N, Eaton SE, et al Vascular risk factors and diabetic neuropathy. N Engl J Med 2005; 352: 341–350. [DOI] [PubMed] [Google Scholar]
- 26. Morrow JD, Frei B, Longmire AW, et al Increase in circulating products of lipid peroxidation (F2‐isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med 1995; 332: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 27. Zhou JF, Yan XF, Guo FZ, et al Effects of cigarette smoking and smoking cessation on plasma constituents and enzyme activities related to oxidative stress. Biomed Environ Sci 2000; 13: 44–55. [PubMed] [Google Scholar]
- 28. Prasad C, Imrhan V, Marotta F, et al Lifestyle and advanced glycation end products (AGEs) burden: its relevance to healthy aging. Aging Dis 2014; 5: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogawa K, Tanaka T, Nagoshi T, et al Increase in the oxidised low‐density lipoprotein level by smoking and the possible inhibitory effect of statin therapy in patients with cardiovascular disease: a retrospective study. BMJ Open 2015; 5: e005455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cerami C, Founds H, Nicholl I, et al Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 1997; 94: 13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. G SB, Choi S, Krishnan J, et al Cigarette smoke and related risk factors in neurological disorders: an update. Biomed Pharmacother 2017; 85: 79–86. [DOI] [PubMed] [Google Scholar]
- 32. Knight‐Lozano CA, Young CG, Burow DL, et al Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 2002; 105: 849–854. [DOI] [PubMed] [Google Scholar]
- 33. Masayesva BG, Mambo E, Taylor RJ, et al Mitochondrial DNA content increase in response to cigarette smoking. Cancer Epidemiol Biomarkers Prev 2006; 15: 19–24. [DOI] [PubMed] [Google Scholar]
- 34. Facchini FS, Hollenbeck CB, Jeppesen J, et al Insulin resistance and cigarette smoking. Lancet 1992; 339: 1128–1130. [DOI] [PubMed] [Google Scholar]
- 35. Bergman BC, Perreault L, Hunerdosse D, et al Novel and reversible mechanisms of smoking‐induced insulin resistance in humans. Diabetes 2012; 61: 3156–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu Y, Song P, Zhang W, et al Activation of AMPKα2 in adipocytes is essential for nicotine‐induced insulin resistance in vivo . Nat Med 2015; 21: 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab 2012; 23: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garbin U, Fratta Pasini A, Stranieri C, et al Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS One 2009; 4: e8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naha N, Gandhi D, Gautam A, et al Involvement of neurotransmitter and Nrf2 in nicotine‐ and cigarette smoke‐induced testicular toxicity in adult rats. Reprod Biol Insight 2016; 5: 5–17. [Google Scholar]
- 40. Lee JM, Li J, Johnson DA, et al Nrf2, a multi‐organ protector? FASEB J 2005; 19: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 41. Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res 2008; 79: 360–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ross R. The pathogenesis of atherosclerosis–an update. N Engl J Med 1986; 314: 488–500. [DOI] [PubMed] [Google Scholar]
- 43. Wissler RW. Update on the pathogenesis of atherosclerosis. Am J Med 1991; 91: 3S–9S. [DOI] [PubMed] [Google Scholar]
- 44. Haverich A. A surgeon's view on the pathogenesis of atherosclerosis. Circulation 2017; 135: 205–207. [DOI] [PubMed] [Google Scholar]
- 45. Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003; 361: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 46. Iribarren C, Tekawa IS, Sidney S, et al Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med 1999; 340: 1773–1780. [DOI] [PubMed] [Google Scholar]
- 47. Sytkowski PA, Kannel WB, D'Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med 1990; 322: 1635–1641. [DOI] [PubMed] [Google Scholar]
- 48. Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol 2013; 10: 219–230. [DOI] [PubMed] [Google Scholar]
- 49. LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes. N Engl J Med 1984; 311: 1615–1619. [DOI] [PubMed] [Google Scholar]
- 50. Williamson JR, Kilo C. Basement‐membrane thickening and diabetic microangiopathy. Diabetes 1976; 25(2 Suppl.): 925–927. [PubMed] [Google Scholar]
- 51. Rayman G, Malik RA, Sharma AK, et al Microvascular response to tissue injury and capillary ultrastructure in the foot skin of type I diabetic patients. Clin Sci (Lond) 1995; 89: 467–474. [DOI] [PubMed] [Google Scholar]
- 52. Fiordaliso F, Clerici G, Maggioni S, et al Prospective study on microangiopathy in type 2 diabetic foot ulcer. Diabetologia 2016; 59: 1542–1548. [DOI] [PubMed] [Google Scholar]
- 53. Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev 2009; 25: 604–614. [DOI] [PubMed] [Google Scholar]
- 54. Pellaton C, Kubli S, Feihl F, et al Blunted vasodilatory responses in the cutaneous microcirculation of cigarette smokers. Am Heart J 2002; 144: 269–274. [DOI] [PubMed] [Google Scholar]
- 55. Avery MR, Voegeli D, Byrne CD, et al Age and cigarette smoking are independently associated with the cutaneous vascular response to local warming. Microcirculation 2009; 16: 725–734. [DOI] [PubMed] [Google Scholar]
- 56. Rossi M, Pistelli F, Pesce M, et al Impact of long‐term exposure to cigarette smoking on skin microvascular function. Microvasc Res 2014; 93: 46–51. [DOI] [PubMed] [Google Scholar]
- 57. Fujii N, Reinke MC, Brunt VE, et al Impaired acetylcholine‐induced cutaneous vasodilation in young smokers: roles of nitric oxide and prostanoids. Am J Physiol Heart Circ Physiol 2013; 304: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Talukder MA, Johnson WM, Varadharaj S, et al Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol 2011; 300: H388–H396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lehr HA. Microcirculatory dysfunction induced by cigarette smoking. Microcirculation 2000; 7(6 Pt. 1): 367. [PubMed] [Google Scholar]
- 60. Jensen JA, Goodson WH, Hopf HW, et al Cigarette smoking decreases tissue oxygen. Arch Surg 1991; 126: 1131–1134. [DOI] [PubMed] [Google Scholar]
- 61. van Adrichem LN, Hovius SE, van Strik R, et al Acute effects of cigarette smoking on microcirculation of the thumb. Br J Plast Surg 1992; 45: 9–11. [DOI] [PubMed] [Google Scholar]
- 62. Sorensen LT, Jorgensen S, Petersen LJ, et al Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res 2009; 152: 224–230. [DOI] [PubMed] [Google Scholar]
- 63. Acevedo A, Schnell A. Effect of cigarette smoking upon the finger circulation in normal and diabetic subjects. Basic Res Cardiol 1975; 70: 350–353. [DOI] [PubMed] [Google Scholar]
- 64. Henriksson P, Lu Q, Diczfalusy U, et al Immediate effect of passive smoking on microcirculatory flow. Microcirculation 2014; 21: 587–592. [DOI] [PubMed] [Google Scholar]
- 65. Heitzer T, Just H, Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 1996; 94: 6. [DOI] [PubMed] [Google Scholar]
- 66. Enoch S, Leaper DJ. Basic science of wound healing. Surgery (Oxford) 2008; 26: 31–37. [Google Scholar]
- 67. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005; 366: 1736–1743. [DOI] [PubMed] [Google Scholar]
- 68. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012; 2012: 918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roy S, Khanna S, Nallu K, et al Dermal wound healing is subject to redox control. Mol Ther 2006; 13: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010; 89: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Turan A, Mascha EJ, Roberman D, et al Smoking and perioperative outcomes. Anesthesiology 2011; 114: 837–846. [DOI] [PubMed] [Google Scholar]
- 72. Kuri M, Nakagawa M, Tanaka H, et al Determination of the duration of preoperative smoking cessation to improve wound healing after head and neck surgery. Anesthesiology 2005; 102: 892–896. [DOI] [PubMed] [Google Scholar]
- 73. Moller AM, Villebro N, Pedersen T, et al Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002; 359: 114–117. [DOI] [PubMed] [Google Scholar]
- 74. Kean J. The effects of smoking on the wound healing process. J Wound Care 2010; 19: 5–8. [DOI] [PubMed] [Google Scholar]
- 75. Sorensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg 2012; 255: 1069–1079. [DOI] [PubMed] [Google Scholar]
- 76. Barua RS, Sy F, Srikanth S, et al Effects of cigarette smoke exposure on clot dynamics and fibrin structure: an ex vivo investigation. Arterioscler Thromb Vasc Biol 2010; 30: 75–79. [DOI] [PubMed] [Google Scholar]
- 77. Sorensen LT, Toft B, Rygaard J, et al Smoking attenuates wound inflammation and proliferation while smoking cessation restores inflammation but not proliferation. Wound Repair Regen 2010; 18: 186–192. [DOI] [PubMed] [Google Scholar]
- 78. Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol 2009; 9: 377. [DOI] [PubMed] [Google Scholar]
- 79. Sorensen LT, Nielsen HB, Kharazmi A, et al Effect of smoking and abstention on oxidative burst and reactivity of neutrophils and monocytes. Surgery 2004; 136: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 80. Lauhio A, Farkkila E, Pietilainen KH, et al Association of MMP‐8 with obesity, smoking and insulin resistance. Eur J Clin Invest 2016; 46: 757–765. [DOI] [PubMed] [Google Scholar]
- 81. Snitker S, Xie K, Ryan KA, et al Correlation of circulating MMP‐9 with white blood cell count in humans: effect of smoking. PLoS One 2013; 8: e66277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Riber U, Espersen F, Skinhoj P, et al Induction of oxidative burst response in human neutrophils by adherent staphylococci. Comparison between Staphylococcus epidermidis and Staphylococcus aureus . APMIS 1993; 101: 55–60. [DOI] [PubMed] [Google Scholar]
- 83. Bryan N, Ahswin H, Smart N, et al Reactive oxygen species (ROS)–a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cells Mater 2012; 24: 249–265. [DOI] [PubMed] [Google Scholar]
- 84. Stringer KA, Tobias M, O'Neill HC, et al Cigarette smoke extract‐induced suppression of caspase‐3‐like activity impairs human neutrophil phagocytosis. Am J Physiol Lung Cell Mol Physiol 2007; 292: L1572–L1579. [DOI] [PubMed] [Google Scholar]
- 85. Zappacosta B, Martorana GE, Papini S, et al Morpho‐functional modifications of human neutrophils induced by aqueous cigarette smoke extract: comparison with chemiluminescence activity. Luminescence 2011; 26: 331–335. [DOI] [PubMed] [Google Scholar]
- 86. van Eeden SF, Hogg JC. The response of human bone marrow to chronic cigarette smoking. Eur Respir J 2000; 15: 915–921. [DOI] [PubMed] [Google Scholar]
- 87. Yanbaeva DG, Dentener MA, Creutzberg EC, et al Systemic effects of smoking. Chest 2007; 131: 1557–1566. [DOI] [PubMed] [Google Scholar]
- 88. Wong LS, Martins‐Green M. Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing. Wound Repair Regen 2004; 12: 471–484. [DOI] [PubMed] [Google Scholar]
- 89. Yin L, Morita A, Tsuji T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res 2000; 292: 188–194. [DOI] [PubMed] [Google Scholar]
- 90. Knuutinen A, Kokkonen N, Risteli J, et al Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol 2002; 146: 588–594. [DOI] [PubMed] [Google Scholar]
- 91. Lahmann C, Bergemann J, Harrison G, et al Matrix metalloproteinase‐1 and skin ageing in smokers. Lancet 2001; 357: 935–936. [DOI] [PubMed] [Google Scholar]
- 92. Dinh T, Tecilazich F, Kafanas A, et al Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012; 61: 2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Heeschen C, Jang JJ, Weis M, et al Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 2001; 7: 833–839. [DOI] [PubMed] [Google Scholar]
- 94. Morimoto N, Takemoto S, Kawazoe T, et al Nicotine at a low concentration promotes wound healing. J Surg Res 2008; 145: 199–204. [DOI] [PubMed] [Google Scholar]
- 95. Michaud SE, Dussault S, Groleau J, et al Cigarette smoke exposure impairs VEGF‐induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol 2006; 41: 275–284. [DOI] [PubMed] [Google Scholar]
- 96. Michaud SE, Menard C, Guy LG, et al Inhibition of hypoxia‐induced angiogenesis by cigarette smoke exposure: impairment of the HIF‐1alpha/VEGF pathway. FASEB J 2003; 17: 1150–1152. [DOI] [PubMed] [Google Scholar]
- 97. Asahara T, Murohara T, Sullivan A, et al Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–966. [DOI] [PubMed] [Google Scholar]
- 98. Loomans CJ, de Koning EJ, Staal FJ, et al Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004; 53: 195–199. [DOI] [PubMed] [Google Scholar]
- 99. Chen MC, Sheu JJ, Wang PW, et al Complications impaired endothelial progenitor cell function in Type 2 diabetic patients with or without critical leg ischaemia: implication for impaired neovascularization in diabetes. Diabet Med 2009; 26: 134–141. [DOI] [PubMed] [Google Scholar]
- 100. Marrotte EJ, Chen DD, Hakim JS, et al Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 2010; 120: 4207–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Michaud SE, Dussault S, Haddad P, et al Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis 2006; 187: 423–432. [DOI] [PubMed] [Google Scholar]
- 102. Kondo T, Hayashi M, Takeshita K, et al Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol 2004; 24: 1442–1447. [DOI] [PubMed] [Google Scholar]
- 103. Silverstein P. Smoking and wound healing. Am J Med 1992; 93: 22S–24S. [DOI] [PubMed] [Google Scholar]
- 104. Ahn C, Mulligan P, Salcido RS. Smoking‐the bane of wound healing: biomedical interventions and social influences. Adv Skin Wound Care 2008; 21: 227–236. quiz 237–228. [DOI] [PubMed] [Google Scholar]
- 105. Siddiqui A, Galiano RD, Connors D, et al Differential effects of oxygen on human dermal fibroblasts: acute versus chronic hypoxia. Wound Repair Regen 1996; 4: 211–218. [DOI] [PubMed] [Google Scholar]
- 106. Eisenbud DE. Oxygen in wound healing: nutrient, antibiotic, signaling molecule, and therapeutic agent. Clin Plast Surg 2012; 39: 293–310. [DOI] [PubMed] [Google Scholar]
- 107. Hohn DC, MacKay RD, Halliday B, et al Effect of O2 tension on microbicidal function of leukocytes in wounds and in vitro . Surg Forum 1976; 27: 18–20. [PubMed] [Google Scholar]
- 108. Schreml S, Szeimies RM, Prantl L, et al Oxygen in acute and chronic wound healing. Br J Dermatol 2010; 163: 257–268. [DOI] [PubMed] [Google Scholar]
- 109. Liu M, Zhang W, Yan Z, et al Smoking increases the risk of diabetic foot amputation: a meta‐analysis. Exp Ther Med 2018; 15: 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lindstrom D, Sadr Azodi O, Wladis A, et al Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 2008; 248: 739–745. [DOI] [PubMed] [Google Scholar]
- 111. Li R, Zhang P, Barker LE, et al Cost‐effectiveness of interventions to prevent and control diabetes mellitus: a systematic review. Diabetes Care 2010; 33: 1872–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hobbs SD, Bradbury AW. Smoking cessation strategies in patients with peripheral arterial disease: an evidence‐based approach. Eur J Vasc Endovasc Surg 2003; 26: 341–347. [DOI] [PubMed] [Google Scholar]
- 113. Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017; 3: CD001292. [DOI] [PubMed] [Google Scholar]
- 114. Wu L, He Y, Jiang B, et al Effectiveness of additional follow‐up telephone counseling in a smoking cessation clinic in Beijing and predictors of quitting among Chinese male smokers. BMC Public Health 2016; 16: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rogers ES, Smelson DA, Gillespie CC, et al Telephone smoking‐cessation counseling for smokers in mental health clinics: a patient‐randomized controlled trial. Am J Prev Med 2016; 50: 518–527. [DOI] [PubMed] [Google Scholar]
- 116. Cunningham P. Patient engagement during medical visits and smoking cessation counseling. JAMA Intern Med 2014; 174: 1291–1298. [DOI] [PubMed] [Google Scholar]
- 117. Olaiya O, Sharma AJ, Tong VT, et al Impact of the 5As brief counseling on smoking cessation among pregnant clients of Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) clinics in Ohio. Prev Med 2015; 81: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Patnode CD, Henderson JT, Thompson JH, et al Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S. preventive services task force. Ann Intern Med 2015; 163: 608–621. [DOI] [PubMed] [Google Scholar]
- 119. Tindle HA, Greevy RA. Smoking cessation pharmacotherapy, even without counseling, remains a cornerstone of treatment. J Natl Cancer Inst 2018; 110: 545–546. [DOI] [PubMed] [Google Scholar]
- 120. Voci SC, Zawertailo LA, Hussain S, et al Association between adherence to free nicotine replacement therapy and successful quitting. Addict Behav 2016; 61: 25–31. [DOI] [PubMed] [Google Scholar]
- 121. Moore D, Aveyard P, Connock M, et al Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta‐analysis. BMJ 2009; 338: b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Flowers L. Nicotine replacement therapy. Am J Psychiatry Resid J 2016; 11: 4–7. [Google Scholar]
- 123. Stead LF, Perera R, Bullen C, et al Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008(1):CD000146. [DOI] [PubMed] [Google Scholar]
- 124. Jorenby DE, Smith SS, Fiore MC, et al Varying nicotine patch dose and type of smoking cessation counseling. JAMA 1995; 274: 1347–1352. [PubMed] [Google Scholar]
- 125. Heydari G, Marashian M, Ebn Ahmady A, et al Which form of nicotine replacement therapy is more effective for quitting smoking? A study in Tehran, Islamic Republic of Iran. East Mediterr Health J 2012; 18: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 126. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol 1997; 29: 1422–1431. [DOI] [PubMed] [Google Scholar]
- 127. Dollerup J, Vestbo J, Murray‐Thomas T, et al Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol 2017; 9: 231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kowalski M, Udy AA, McRobbie HJ, et al Nicotine replacement therapy for agitation and delirium management in the intensive care unit: a systematic review of the literature. J Intensive Care 2016; 4: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Klemperer EM, Hughes JR. After precessation nicotine replacement therapy, abrupt cessation increases abstinence more than gradual cessation in smokers ready to quit. Evid Based Med 2016; 21: 174. [DOI] [PubMed] [Google Scholar]
- 130. Jorenby DE, Leischow SJ, Nides MA, et al A controlled trial of sustained‐release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999; 340: 685–691. [DOI] [PubMed] [Google Scholar]
- 131. Hurt RD, Sachs DP, Glover ED, et al A comparison of sustained‐release bupropion and placebo for smoking cessation. N Engl J Med 1997; 337: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 132. Glassman AH, Covey LS, Stetner F, et al Smoking cessation and the course of major depression: a follow‐up study. Lancet 2001; 357: 1929–1932. [DOI] [PubMed] [Google Scholar]
- 133. Stapleton J, West R, Hajek P, et al Randomized trial of nicotine replacement therapy (NRT), bupropion and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction 2013; 108: 2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bullen C, Howe C, Laugesen M, et al Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382: 1629–1637. [DOI] [PubMed] [Google Scholar]
- 135. Grana R, Benowitz N, Glantz SA. E‐Cigarettes: a scientific review. Circulation 2014; 129: 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among US adolescents. JAMA Pediatr 2014; 168: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Coe JW, Brooks PR, Vetelino MG, et al Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 2005; 48: 3474–3477. [DOI] [PubMed] [Google Scholar]
- 138. Cahill K, Stevens S, Perera R, et al Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database Syst Rev. 2013. (5): CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mills EJ, Wu P, Lockhart I, et al Comparisons of high‐dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta‐analysis. Ann Med 2012; 44: 588–597. [DOI] [PubMed] [Google Scholar]
- 140. Taylor GMJ, Taylor AE, Thomas KH, et al The effectiveness of varenicline versus nicotine replacement therapy on long‐term smoking cessation in primary care: a prospective cohort study of electronic medical records. Int J Epidemiol 2017; 46: 1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Baker TB, Piper ME, Stein JH, et al Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: a randomized clinical trial. JAMA 2016; 315: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tulloch HE, Pipe AL, Els C, et al Flexible, dual‐form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med 2016; 14: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nides M, Oncken C, Gonzales D, et al Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7‐week, randomized, placebo‐ and bupropion‐controlled trial with 1‐year follow‐up. Arch Intern Med 2006; 166: 1561–1568. [DOI] [PubMed] [Google Scholar]
- 144. Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2012; 10: CD008286. [DOI] [PubMed] [Google Scholar]
- 145. Severson HH, Danaher BG, Ebbert JO, et al Randomized trial of nicotine lozenges and phone counseling for smokeless tobacco cessation. Nicotine Tob Res 2015; 17: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Baker A, Richmond R, Haile M, et al A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. Am J Psychiatry 2006; 163: 1934–1942. [DOI] [PubMed] [Google Scholar]
- 147. Pollak KI, Oncken CA, Lipkus IM, et al Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med 2007; 33: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sweeney CT, Fant RV, Fagerstrom KO, et al Combination nicotine replacement therapy for smoking cessation. CNS Drugs 2001; 15: 453–467. [DOI] [PubMed] [Google Scholar]
- 149. Kornitzer M, Boutsen M, Dramaix M, et al Combined use of nicotine patch and gum in smoking cessation: a placebo‐controlled clinical trial. Prev Med 1995; 24: 41–47. [DOI] [PubMed] [Google Scholar]
- 150. Bohadana A, Nilsson F, Rasmussen T, et al Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized, double‐blind, placebo‐controlled trial. Arch Intern Med 2000; 160: 3128–3134. [DOI] [PubMed] [Google Scholar]
- 151. Ebbert JO, Hatsukami DK, Croghan IT, et al Combination varenicline and bupropion sr for tobacco‐dependence treatment in cigarette smokers. JAMA 2014; 311: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ebbert JO, Croghan IT, Sood A, et al Varenicline and bupropion sustained‐release combination therapy for smoking cessation. Nicotine Tob Res 2009; 11: 234–239. [DOI] [PubMed] [Google Scholar]
- 153. George TP, Vessicchio JC, Sacco KA, et al A placebo‐controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry 2008; 63: 1092–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]


