Abstract
Aims/Introduction
Diabetic polyneuropathy is one of the most frequent diabetic complications, and impairs patients’ quality of life. We evaluated the efficacy and safety of ranirestat (40 mg/day) in patients with diabetic polyneuropathy.
Materials and Methods
This was a multicenter, placebo‐controlled, randomized double‐blind, parallel‐group, phase III study in which 557 patients were randomly assigned to either the ranirestat or placebo group and assessed for 52 weeks. The co‐primary end‐points were the changes in tibial motor nerve conduction velocity and total modified Toronto Clinical Neuropathy Score as a measure of clinical symptoms.
Results
There was a significant increase in tibial motor nerve conduction velocity in the ranirestat group compared with the placebo group. The difference between groups in the change at last observation was 0.52 m/s (P = 0.021). Increases in nerve conduction velocity in the ranirestat group were found not only in the tibial motor nerves, but also in the median motor nerves, proximal median sensory nerves and distal median sensory nerves. No significant differences in modified Toronto Clinical Neuropathy Score or safety parameters were found between the two groups.
Conclusions
Ranirestat (40 mg/day) was well tolerated and improved nerve conduction velocity. Regarding symptoms and signs, no detectable benefits over the placebo were observed in the ranirestat group during the 52 weeks of treatment.
Keywords: Diabetic polyneuropathy, Nerve conduction velocity, Ranirestat
Introduction
Diabetic polyneuropathy (DPN) is a complication that often leads to foot ulceration and amputation, and its associated symptoms significantly impair patient quality of life1. Although therapies are available for the relief of symptoms associated with DPN, at present there are few basic therapies for maintaining and improving nerve function.
The mechanisms involved in the development and progression of DPN are complex. Recent studies have shown that hyperglycemia and dyslipidemia are associated with DPN through an impaired cellular mechanism in sensory neurons and microvascular endothelial cells2. Furthermore, activation of the polyol pathway, by which glucose is converted to sorbitol, has also been proposed as one of the factors. Aldose reductase is a rate‐limiting enzyme that regulates the polyol pathway. This enzyme is activated under hyperglycemic conditions, suggesting that an increased flux of polyol pathway is pathogenic for DPN3. A clinical study of diabetes patients showed that intracellular sorbitol concentrations were inversely related to the density of myelinated nerves4.
A clinical study of the aldose reductase inhibitor, zenarestat, showed that sorbitol suppression was accompanied by an improvement in nerve conduction velocity (NCV) and by an increase in the density of sural sensory nerve myelinated fibers5.
Ranirestat is an aldose reductase inhibitor synthesized by Sumitomo Dainippon Pharma Co., Ltd. (Osaka, Japan), and treatment at a dose of 20 mg/day for 12 weeks has been shown to result in an 83.5% inhibition of sorbitol accumulation in the sural sensory nerves of patients6. To date, phase II study of ranirestat has been carried out in Japan to evaluate the efficacy and safety of ranirestat 20 mg in DPN compared with a placebo. Sensory NCV was found to be significantly improved by ranirestat treatment7. Some global studies have also shown improvement in NCV by ranirestat administration from an electrophysiological standpoint8, 9, 10. Based on these results, in the present study we assessed the efficacy and safety of 40 mg/day ranirestat in patients with DPN.
Methods
Study design
The present study was a multicenter (151 sites in Japan), placebo‐controlled, randomized, double‐blind, parallel‐group, phase III study in patients with DPN. An observation period (4 weeks), treatment period (52 weeks) and follow‐up period (4 weeks) were established, and a placebo was administered under single‐blind conditions in the observation phase. In the treatment period, either a placebo or 40 mg ranirestat was orally administered once daily under double‐blind conditions. The co‐primary end‐points were the change in tibial motor NCV (TMNCV) from baseline and the change in total modified Toronto Clinical Neuropathy Score (mTCNS). The secondary end‐points were changes in NCV, amplitude and minimum F wave latency of each nerve, change in each mTCNS item, and change in the coefficient of variation at rest of RR intervals at each assessment time‐point. A nerve conduction study (NCS) was carried out, and the mTCNS11, 12, 13 was assessed at screening, baseline, and weeks 12, 24, 36 and 52. The coefficient of variation at rest of RR intervals was assessed at baseline, and weeks 24 and 52.
As for safety, events that occurred in the period from the start of the investigational drug treatment (the observation period) until the last test in the follow‐up period were recorded as adverse events. The Hospital Anxiety and Depression Scale was used to assess the effect of ranirestat on depression and anxiety disorders at baseline, week 24 and week 52. Electrocardiograms were assessed at baseline, and weeks 12, 24, 36 and 52, and laboratory tests and vital signs were taken at all visits.
This study was registered with the Japan Pharmaceutical Information Center (JapicCTI‐111702), and was carried out in accordance with the Declaration of Helsinki, the Pharmaceutical Affairs Law and Good Clinical Practice. The study protocol was approved by each institutional review board. Written informed consent was obtained from all participants before the screening visit.
Patients
The following were established as inclusion criteria: men and women between the ages of 20 and 75 years; patients with type 1 or 2 diabetes receiving drug therapy; patients with stable glycemic control; and patients with glycated hemoglobin (HbA1c) between 6.9 and 10.5% (National Glycohemoglobin Standardization Program) at screening. Patients were diagnosed with DPN when two or more items of the modified San Antonio Criteria were met: (i) symptoms of DPN; (ii) signs of DPN; (iii) abnormal NCS results with at least two abnormal nerves (at least one nerve was a lower limb nerve); and (iv) abnormal vibration perception threshold (<10 s using a 128‐Hz tuning fork. Criterion (iii) was mandatory. Patients with severe neuropathy, as indicated by the following nerve conduction test results at screening, were excluded: sural sensory nerve amplitude <1.0 μV; distal tibial motor nerve amplitude <3.0 mV; distal median sensory nerve amplitude <2.0 μV; and distal median motor nerve amplitude: <0.5 mV. As DPN progresses symmetrically, patients with differences between left and right sural sensory nerve amplitude or conduction velocity (amplitude: ≥6.0 μV; NCV: ≥7.0 m/s) were excluded. Other exclusion criteria were non‐diabetic neuropathy, hospitalization for diabetic control or ketoacidosis within 3 months of screening, use of other investigational drugs and serious comorbidities. Patients with mTCNS changes of two or more grades in the same items during screening or observation were excluded to eliminate patients showing excessive responses to the placebo or patients with unstable clinical symptoms at baseline.
Electrophysiological measurements
Nerve conduction study were carried out on the non‐dominant median motor, dominant tibial motor and non‐dominant median sensory nerves, and bilateral sural sensory nerves. Sensory NCSs were carried out antidromically. All stimulation and recording was carried out using surface electrodes. Measurements of response latencies and amplitudes were carried out in a standard fashion using onset latencies and baseline‐to‐peak amplitudes. Measurements from the initial positive peak to negative peak were made for sensory responses. F waves were generated for all motor nerves with 16 supramaximal stimuli per nerve, and the minimal reproducible latency of at least three responses was measured. The condition of temperature and distance was referred to in the previous study7. As centralized assessment is important in the assessment of NCS14, a NCS assessment committee composed of six experts was established in the present study to blind review the eligibility of participants at screening and to accept or reject data at all assessment time‐points.
Clinical symptoms and signs
In this trial, symptoms and signs were assessed with the mTCNS, which is a reliable and valid clinical tool to capture symptoms and signs of DPN11, 12, 13. The mTCNS consists of a symptom domain and a sensory test domain. In the symptom domain, the course of development of ‘Pain, Numbness, Tingling, Weakness, and Ataxia in Foot and Upper limb symptoms’ is separated into four stages: 0, absent; 1, present, but does not interfere with sense of well‐being or activities of daily living; 2, present, interferes with sense of well‐being, but not with activities of daily living; and 3, present and interferes with both sense of well‐being and activities of daily living. In the sensory test domain, ‘Pinprick, Temperature, Light touch, Vibration, Position sense’ were assessed as 0, normal; 1, reduced at the toes only; 2, reduced to a level above the toes, but only up to the ankles; and 3, reduced to a level above the ankles and/or absent at the toes. The mTCNS scale varies from 0 (no signs or no symptoms of DPN) to 33 (all symptoms and signs of DPN present with a maximum score of 18 symptom points and 15 sensory test points).
Pharmacodynamics
As a surrogate of nerve sorbitol concentration, the concentration of sorbitol in erythrocytes, which is easier to measure, was determined at baseline, and at weeks 4, 12 and 52.
Statistical analysis
Based on past studies, the difference between groups in the change from baseline in TMNCV at last observation was assumed to be 1.0 m/s, and the standard deviation was assumed to be 3.4. The difference between groups in the change from baseline in total mTCNS at last observation was assumed to be −1.1, and the standard deviation was assumed to be 4.0, based on past studies. Based on these assumptions, the target sample size was calculated as 260 participants per group, a total of 520 participants, to ensure at least 80% power simultaneously for both co‐primary end‐points with a 5% significance level for two‐sided tests.
The full analysis set (FAS) was used as the analysis population. The FAS included all participants who were randomized and received the investigational drug at least once in the treatment period, and who had TMNCV or mTCNS data during this time. The week 52 data were used as the last observation data, but if these data were missing the last observation carried forward (LOCF) approach was used.
The primary end‐points in the FAS were compared between the ranirestat and placebo groups based on the analysis of covariance model using treatment group as a factor and baseline measurements as covariates. The difference between groups in the least squares (LS) means at LOCF and the 95% confidence interval as well as the P‐value were calculated.
Summary statistics were calculated for the measurements and change from baseline in the secondary end‐points at each assessment time‐point by treatment group in the FAS. Then, after unblinding, subgroup analysis of the change from baseline in TMNCV in participants who completed 52 weeks was carried out using the mean HbA1c at week 52 (<7.0%, ≥7.0%) and duration of DPN (<3 years, ≥3 years). These results were compared between the ranirestat and placebo groups using analysis of covariance with treatment group as a factor and baseline measurements as covariates. The difference between groups in the LS mean at each assessment time‐point and last observation, and the 95% confidence interval as well as the P‐value were calculated.
Results
Participant demographics, baseline data and exposure
Informed consent was obtained from a total of 1,185 participants, 557 participants were randomized and 555 participants (placebo group 278; ranirestat group 277) started treatment with the investigational drug in the treatment period. Of these, 492 participants (placebo 248; ranirestat 244) completed the treatment phase, and 63 participants (30 and 33, respectively) discontinued the study. The FAS consisted of 537 participants (269 and 268, respectively), because two participants with no efficacy data related to the primary end‐points, 15 participants who were ineligible for efficacy assessment and one participant who met both criteria were excluded (Figure 1). There were no significant differences in baseline demographics between the two groups (Table 1). The baseline data for NCS and for mTCNS are summarized in Table 2.
Figure 1.
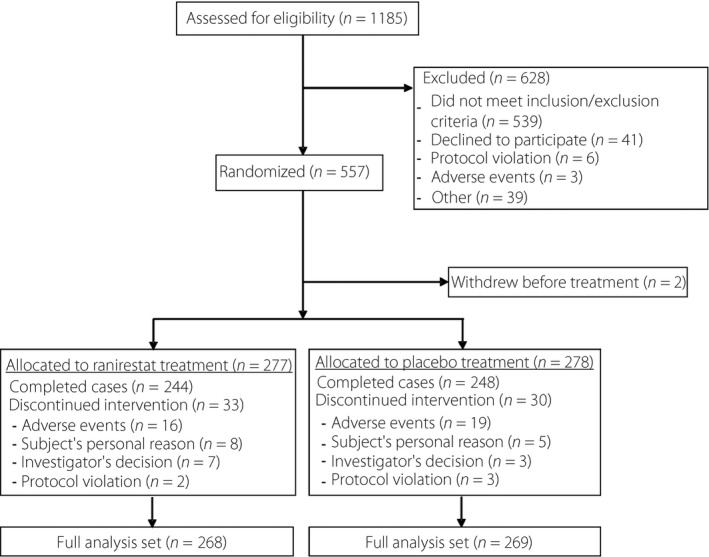
Study flow diagram. A total of 555 participants (placebo group 278; ranirestat group 277) started treatment with the investigational drug in the treatment period. The full analysis set consisted of 537 participants (269 and 268, respectively), because two participants with no efficacy data related to the primary end‐points, 15 participants who were ineligible for efficacy assessment and one participant who met both criteria were excluded.
Table 1.
Summary of baseline demographics
| Ranirestat (n = 268) | Placebo (n = 269) | |
|---|---|---|
| Age at informed consent (years) | 62.1 ± 9.1 | 60.9 ± 9.0 |
| Male sex | 185 (69.0) | 178 (66.2) |
| BMI (kg/m2) | 24.99 ± 3.96 | 25.55 ± 4.14 |
| Type 2 diabetes | 252 (94.0) | 253 (94.1) |
| Diabetes duration (years) | 15.0 ± 9.0 | 14.7 ± 8.3 |
| DPN duration (years) | 6.1 ± 4.9 | 5.8 ± 4.7 |
| HbA1c (%) | 7.46 ± 0.67 | 7.51 ± 0.77 |
Data are n (%) for sex and type 2 diabetes, and mean ± standard deviation for other parameters. BMI, body mass index; DPN, diabetic polyneuropathy; HbA1c, glycated hemoglobin.
Table 2.
Summary of the baseline data (nerve conduction study and mTCNS)
| Ranirestat (n = 268) | Placebo (n = 269) | |
|---|---|---|
| Tibial motor nerve | ||
| CV (m/s) | 41.09 ± 3.61 | 40.91 ± 3.88 |
| Distal amplitude (mV) | 9.23 ± 3.53 | 9.29 ± 3.65 |
| Proximal amplitude (mV) | 6.50 ± 2.76 | 6.56 ± 2.84 |
| F wave latency (ms) | 50.74 ± 4.54 | 51.14 ± 4.90 |
| Median motor nerve | ||
| CV (m/s) | 52.38 ± 4.24 | 51.82 ± 3.92 |
| Distal amplitude (mV) | 8.20 ± 2.48 | 8.18 ± 2.48 |
| Proximal amplitude (mV) | 7.67 ± 2.45 | 7.57 ± 2.43 |
| F‐wave latency (ms) | 27.77 ± 2.21 | 28.03 ± 2.45 |
| Sural sensory nerve | ||
| CV (m/s) | 46.98 ± 6.06 | 46.06 ± 5.56 |
| Amplitude (μV) | 5.45 ± 2.87 | 5.34 ± 3.08 |
| Median sensory nerve | ||
| Distal CV (m/s) | 47.91 ± 7.85 | 47.32 ± 7.94 |
| Proximal CV (m/s) | 58.86 ± 5.15 | 58.74 ± 5.00 |
| Distal amplitude (μV) | 15.62 ± 8.98 | 15.61 ± 9.03 |
| Proximal amplitude (μV) | 8.24 ± 4.81 | 8.32 ± 4.70 |
| mTCNS | ||
| Total score (0–33) | 9.76 ± 5.31 | 9.67 ± 5.51 |
| Symptom domain (0–18) | 4.25 ± 2.87 | 4.15 ± 3.02 |
| Sensory test domain (0–15) | 5.52 ± 3.61 | 5.52 ± 3.61 |
Data are means ± standard deviation for parameters of nerve conduction study and mean ± standard error for modified Toronto Clinical Neuropathy Score (mTCNS). The symptom domain score is the sum of individual symptom scores, and the sensory test domain score is the sum of individual sensory test scores. CV, conduction velocity.
The median duration of treatment in the treatment period was 364 days in both the placebo and ranirestat groups, showing no significant difference.
Primary end‐points
The change from baseline in TMNCV significantly improved at all assessment time‐points in the ranirestat group compared with the placebo group. The change at LOCF was −0.03 ± 0.16 m/s (LS mean ± standard error [SE]) in the placebo group, showing no change from baseline, but was 0.49 ± 0.16 m/s (LS mean ± SE) in the ranirestat group, indicating improvement. The difference in change between groups was 0.52 m/s, indicating a significant change between groups (P = 0.021; Figure 2, Figure S1).
Figure 2.
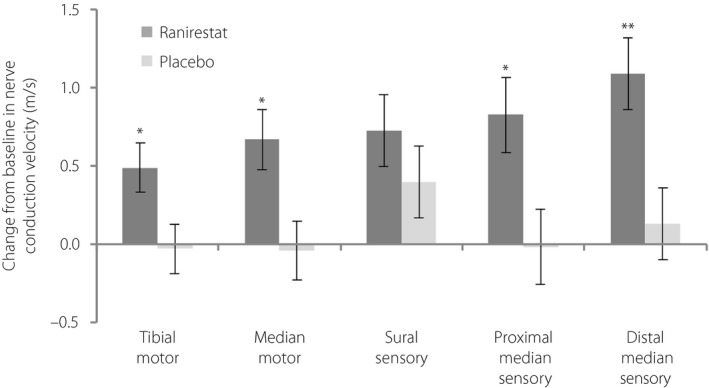
Change from baseline to last observation carried forward (LOCF) for each nerve conduction velocity (m/s; full analysis set). Nerve conduction velocities were increased in all investigated nerves in the ranirestat group. Data shown are least square mean ± standard error change from baseline. P‐values were obtained from an analysis of covariance model with change from baseline to LOCF and the baseline value as a covariate. *P < 0.05; **P < 0.01.
Conversely, the change in total mTCNS at LOCF was −2.31 ± 0.22 (LS mean ± SE) in the placebo group and −2.50 ± 0.22 (LS mean ± SE) in the ranirestat group, indicating improvement from baseline in both groups. The difference in change between groups was −0.19 (P = 0.528), showing no significant difference between the placebo and ranirestat groups (Figure 3). As for individual data of the symptom domain and sensory test domain, each score was improved in both groups; however, there was no significant difference between the placebo and ranirestat groups (Figures S2 and S3.
Figure 3.
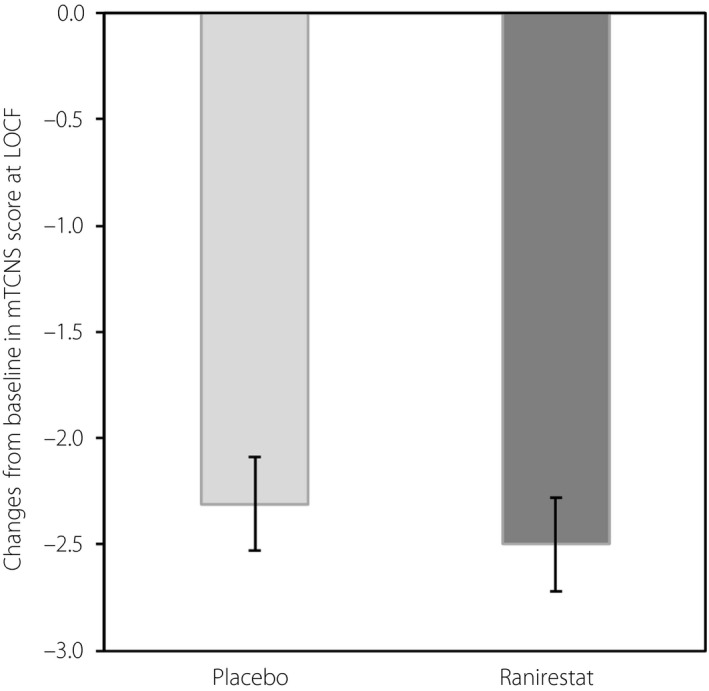
Change from baseline to (LOCF) in total modified Toronto Clinical Neuropathy Score (mTCNS; full analysis set). Ranirestat n = 268; placebo n = 269. There was no significant difference between the groups. Data shown are least square mean ± standard error change from baseline. P‐values were obtained from an analysis of covariance model with change from baseline to LOCF and the baseline value as a covariate.
HbA1c, a factor affecting efficacy, remained at a constant level of 7.49–7.59% (mean) in the placebo group and 7.45–7.62% (mean) in the ranirestat group throughout the treatment period (Figure S4).
Secondary end‐points
The change in NCV at LOCF in the median motor nerves, proximal median sensory nerves and distal median sensory nerves showed significant improvement in the ranirestat group compared with the placebo group. Although the difference in the sural NCV was not significant, there was a trend toward greater improvement in the ranirestat group compared with the placebo group (Figure 2).
The changes in amplitude showed a greater trend toward improvement in all nerves in the ranirestat group compared with the placebo group. The change in distal median motor nerve amplitude at LOCF showed significant improvement in the ranirestat group compared with the placebo group (P = 0.021; Figure 4).
Figure 4.
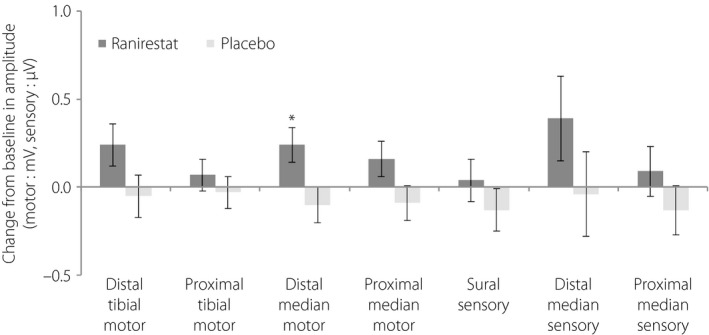
Change from baseline to last observation carried forward in each amplitude (motor nerve, mV; sensory nerve, μV; full analysis set). The compound muscle and sensory nerve action potential amplitudes were increased in all nerves investigated in the ranirestat group. Data shown are least square mean ± standard error change from baseline. P‐values were obtained from an analysis of covariance model with change from baseline to last observation carried forward and the baseline value as a covariate. *P < 0.05.
The change in minimum F wave latency in the median motor nerves at LOCF was 0.01 ± 0.07 (LS mean ± SE) in the placebo group and −0.27 ± 0.07 (LS mean ± SE) in the ranirestat group, indicating significant improvement in the ranirestat group (P = 0.003). The change in minimum F wave latency in the tibial motor nerves at LOCF was −0.08 ± 0.13 (LS mean ± SE) in the placebo group and −0.26 ± 0.13 (LS mean ± SE) in the ranirestat group, showing no significant difference between the two groups, although there was a trend toward greater improvement in the ranirestat group.
There was no significant difference between the ranirestat and placebo groups in the coefficient of variation at rest of RR intervals changes.
Safety
The incidence of adverse events during the treatment period was 88.5% in the placebo group and 87.7% in the ranirestat group. The incidence of adverse events for which a causal relationship to the investigational drug could not be ruled out (adverse drug reactions) was 14.0% in the placebo group and 16.2% in the ranirestat group, showing no significant difference in the incidence of adverse events or adverse drug reactions between groups. During the treatment period, one participant in the placebo group died (acute myocardial infarction and ventricle rupture) and one participant in the ranirestat group died (pancreatic carcinoma with metastases to the liver). There were no significant differences between groups in the incidence of serious adverse events during the treatment period or adverse events leading to discontinuation (Table S1).
There were no significant differences from the placebo group in the incidences of any of the most common adverse events, including these. Furthermore, there was no significant difference between the ranirestat and placebo groups in Hospital Anxiety and Depression Scale total score changes.
Pharmacodynamics
The erythrocyte sorbitol concentration was approximately 50 nmol/g Hb (mean) from baseline to week 52 in the placebo group, with a rate of change of −0.01% at week 52. In contrast, the concentration in the ranirestat group decreased from 53.19 ± 19.94 nmol/g Hb (mean ± standard deviation) at baseline to 12.20 ± 8.24 nmol/g Hb (mean ± standard deviation) at week 52, with a rate of change from baseline of −75.82% (Figure S5).
Discussion
Nerve conduction study have been reported to be useful for the diagnosis of DPN and assessment of its severity15, 16, so NCV was established as a primary end‐point in the present studies. Although the main pathophysiology of DPN is axonal degeneration, the NCV represents a reliable and reproducible parameter in the evaluation of DPN17. A previous report showed that motor NCV was an independent predictor for the development of new foot ulcers in patients with diabetes18. In another report, a relationship between motor nerve conduction deficit and muscle weakness was described19. NCV parameters, including TMNCV in NCS, have also been shown to correlate with the intra‐epidermal nerve fiber density of Aδ fibers and C fibers, as measured by skin biopsy20. In the present study, the conduction velocity in the tibial motor nerve was selected as the primary end‐point, because the peroneal motor nerves are often impaired as a result of the way people sit with their legs tucked underneath them in Japan21.
A significant difference in the change in TMNCV was found between the placebo and ranirestat groups beginning at week 12, with a difference between groups in the change at LOCF of 0.52 m/s. The progression of neuropathy in diabetes is slow, taking place over several years. A previous report of the natural course of diabetes has shown that TMNCV deteriorates at a rate of 0.5 m/s per year under long‐term (7.5 years) conditions of poor glycemic control22. Another study has shown that the rate of deterioration is approximately 0.3 m/s per year among patients with HbA1c ≤7.8% (mean 7.3%) over 18 years23. Therefore, the effect of ranirestat treatment (0.52 m/s increase in NCV in 1 year) might have a sufficient impact on the natural course of diabetic polyneuropathy.
Additionally, a close relationship has been reported between atrophy of the foot muscle, determined by ultrasonography, and nerve conduction parameters, not only as a decrease of amplitude, but also a decrease in conduction velocity in the peroneal motor and tibial motor nerves24. It has also been reported that TMNCV was decreased in diabetes patients with foot ulcers compared with diabetes patients without foot ulcers25. On the basis of these findings, the improvement in TMNCV observed in the present study can be considered clinically meaningful.
Furthermore, ranirestat was associated with a significant improvement in NCV not only in the tibial motor nerves, but also in the median motor nerves, proximal median sensory nerves and distal median sensory nerves compared with the placebo. A significant difference in sural NCV between the ranirestat and placebo groups was not found, likely because of the inherent technical difficulty and sensitivity to error arising from the short distance examined when measuring this parameter26.
With respect to amplitude, all measured amplitudes were decreased in the placebo group, but no deterioration was observed in the ranirestat group. A recent study reported that there was an approximately 1‐μV decrease in sural amplitude over 3 years in patients with well‐controlled diabetes27. In the present study, the change in sural amplitude over 1 year was too small to evaluate any therapeutic benefit, while the reproducibility of sural measurement is known to present challenges26. Based on these observations, sural amplitude might not have been an appropriate end‐point in the present study, whereas there was no decrease in ranirestat group.
A phase II/III study with a 2‐year treatment period also showed similar trends in peroneal motor nerves10, and clinical studies of ranirestat have shown consistent improvement in nerve conduction test parameters. These results show that ranirestat is effective at improving impaired nerve function associated with DPN.
The erythrocyte sorbitol concentration in the ranirestat group was also suppressed, confirming the pharmacological mechanism of the drug as an aldose reductase inhibitor. It has been reported that erythrocyte sorbitol concentration correlates with sorbitol concentration in nerves28, and the results of the present study are consistent with the results of a past study6.
In an additional analysis of the results of the present study in patients who had DPN for <3 years, a significant difference in the change in TMNCV was found between the placebo and ranirestat groups (difference 1.63 m/s; data not shown). These results suggest that the therapeutic effect of ranirestat might be greater in patients with early‐stage DPN.
In another analysis of the results of the present study in patients who had diabetes for <10 years, the difference between the placebo group and ranirestat group was similar to that of FAS (difference in subgroup 0.41 m/s; data not shown). The duration of diabetes might not be the factor to affect the efficacy of ranirestat.
It is known that the progress of DPN is affected by the status of blood glucose control29, so additional subgroup analysis was carried out according to whether or not patients achieved the target HbA1c level of <7.0%, as recommended for the prevention of complications30. In patients who completed the treatment period and had a mean HbA1c <7.0%, the TMNCV at week 52 had improved from baseline in both the placebo and ranirestat groups. The change in the ranirestat group was greater than that in the placebo group. In contrast, in the population with a mean HbA1c of ≥7.0%, the velocity was worse than at baseline in the placebo group, but significantly improved in the ranirestat group (difference between groups 0.55 ± 0.27 m/s [LS mean ± SE], P = 0.042), indicating improvement even in patients with poorly controlled blood glucose. These findings suggest that ranirestat has an effect in improving TMNCV regardless of blood glucose control status. These findings differ from those of a previous study showing that epalrestat was most effective in patients with good glycemic control31. This discrepancy between ranirestat and epalrestat might be attributable to the strength of the effect on sorbitol level. In vivo, ranirestat showed stronger suppression of sorbitol level in the sciatic nerves than eparlestat32, whereas a previous clinical study showed that epalrestat did not reduce erythrocytic sorbitol33. Therefore, ranirestat might exert different effects in patients with a mean HbA1c of ≥7.0%.
In the present study, we used the mTCNS to assess such symptoms, but no significant differences were found between the two groups. In contrast, the results of the NCS showed a clear improvement in nerve function, and it is possible that the effect on clinical symptoms might be revealed with longer treatment times. However, studies cannot reasonably follow patients for long enough to detect a pharmacological effect. Subjective symptoms also do not necessarily reflect the severity of diabetic neuropathy. In the case that fibers undergo active nerve fiber degeneration or impaired regeneration, they release impulse of positive symptoms. Once nerve fibers are lost, then the loss of sensation will take place34. Therefore, when evaluating the therapeutic effects of a drug that is intended to prevent the progression of DPN, a method of evaluation that sensitively captures the progress and improvement of disease is required.
In conclusion, ranirestat was well tolerated and significantly improved TMNCV vs the placebo. In regard to symptoms and signs, no detectable benefits over the placebo were observed in the ranirestat group. To evaluate effects on symptoms and signs, the longer assessment or a method of evaluation that sensitively captures the progress and improvement of disease would be required.
Disclosure
Nobuo Kohara, Yutaka Naito, Tomihiro Imai and Kenji Sekiguchi report receiving consulting fees from Sumitomo Dainippon Pharma Co., Ltd. Masayuki Baba and Tetsuo Komori, and Jo Satoh reports receiving grants from Sumitomo Dainippon Pharma Co., Ltd. Yasuyuki Yamaguchi and Tatsuto Hamatani are full‐time employees of Sumitomo Dainippon Pharma Co., Ltd.
Supporting information
Figure S1 | Change from baseline in tibial nerve conduction velocity (m/s) over time.
Figure S2 | Change from baseline in individual modified Toronto Clinical Neuropathy Score symptom domain at last observation carried forward (LOCF).
Figure S3 | Change from baseline in individual modified Toronto Clinical Neuropathy Score sensory test domain at last observation carried forward.
Figure S4 | Glycated hemoglobin control during treatment period.
Figure S5 | Sorbitol concentration in participants’ erythrocytes.
Table S1 | Summary of adverse events.
Acknowledgments
Jo Satoh served as the medical expert. Nobuo Kohara, Masayuki Baba, Tetsuo Komori, Yutaka Naito, Tomihiro Imai and Kenji Sekiguchi reviewed the data as the NCS assessment committee. This study was funded by Sumitomo Dainippon Pharma Co., Ltd.
J Diabetes Investig 2019; 10: 466–474
Clinical Trial Registry
Japan Pharmaceutical Information Center
JapicCTI‐111702
References
- 1. Vileikyte L, Rubin RR, Leventhal H. Psychological aspects of diabetic neuropathic foot complications: an overview. Diabetes Metab Res Rev 2004; 20: S13–S18. [DOI] [PubMed] [Google Scholar]
- 2. Vincent AM, Callaghan BC, Smith AL, et al Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. [DOI] [PubMed] [Google Scholar]
- 3. Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig 2011; 2: 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dyck PJ, Zimmerman BR, Vilen TH, et al Nerve glucose, fructose, sorbitol, myo‐inositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med 1988; 319: 542–548. [DOI] [PubMed] [Google Scholar]
- 5. Greene DA, Arezzo JC, Brown MB, et al Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Neurology 1999; 53: 580–591. [DOI] [PubMed] [Google Scholar]
- 6. Bril V, Buchanan RA, AS‐3201 Study Group . Aldose reductase inhibition by AS‐3201 in sural nerve from patients with diabetic sensorimotor polyneuropathy. Diabetes Care 2004; 27: 2369–2375. [DOI] [PubMed] [Google Scholar]
- 7. Satoh J, Kohara N, Sekiguchi K, et al Effect of ranirestat on sensory and motor nerve function in Japanese patients with diabetic polyneuropathy: a randomized double‐blind placebo‐controlled study. J Diabetes Res 2016; 2016: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bril V, Buchanan RA, Ranirestat Study Group . Long term effects of ranirestat (AS‐3201) on peripheral nerve function in patients with diabetic sensorimotor polyneuropathy. Diabetes Care 2006; 29: 68–72. [DOI] [PubMed] [Google Scholar]
- 9. Bril V, Hirose T, Tomioka S, et al Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care 2009; 32: 1256–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polydefkis M, Arezzo J, Nash M, et al Safety and efficacy of ranirestat in patients with mild‐to‐moderate diabetic sensorimotor polyneuropathy. J Peripher Nerv Syst 2015; 20: 363–371. [DOI] [PubMed] [Google Scholar]
- 11. Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care 2002; 25: 2048–2052. [DOI] [PubMed] [Google Scholar]
- 12. Bril V, Tomioka S, Buchanan RA, et al Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 2009; 26: 240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satoh J, Kohara N, Hamada C. Assessment of the reliability of a Japanese version of the Modified Toronto Clinical Neuropathy Score in Japanese patients with diabetic sensorimotor polyneuropathy. J Jpn Diabetes Soc 2013; 56: 932–937 (Japanese). [Google Scholar]
- 14. Bril V, Ellison R, Ngo M, et al Electrophysiological monitoring in clinical trials. Muscle Nerve 1998; 21: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 15. Tesfaye S, Boulton AJ, Dyck PJ, et al Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dyck PJ, Albers JW, Andersen H, et al Toronto Expert Panel on Diabetic Neuropathy. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev 2011; 27: 620–628. [DOI] [PubMed] [Google Scholar]
- 17. Shabeeb D, Najafi M, Hasanzadeh G, et al Electrophysiological measurements of diabetic peripheral neuropathy: a systematic review. Diabetes Metab Syndr 2018; 12: 591–600. [DOI] [PubMed] [Google Scholar]
- 18. Carrington AL, Shaw JE, Van Schie CH, et al Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6‐year outcome period? Diabetes Care 2002; 25: 2010–2015. [DOI] [PubMed] [Google Scholar]
- 19. van Schie CH, Vermigli C, Carrington AL, et al Muscle weakness and foot deformities in diabetes: relationship to neuropathy and foot ulceration in Caucasian diabetic men. Diabetes Care 2004; 27: 1668–1673. [DOI] [PubMed] [Google Scholar]
- 20. Quattrini C, Tavakoli M, Jeziorska M, et al Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007; 56: 2148–2154. [DOI] [PubMed] [Google Scholar]
- 21. Baba M, Jin C, Shen Z. [Peroneal A‐waves in healthy Japanese subjects]. Clin Electroencephalogr 2007; 49: 369–372 (Japanese). [Google Scholar]
- 22. Reichard P, Nilsson BY, Rosenqvist U. The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 1993; 329: 304–309. [DOI] [PubMed] [Google Scholar]
- 23. Larsen JR, Sjoholm H, Hanssen KF, et al Optimal blood glucose control during 18 years preserves peripheral nerve function in patients with 30 years’ duration of type 1 diabetes. Diabetes Care 2003; 26: 2400–2404. [DOI] [PubMed] [Google Scholar]
- 24. Severinsen K, Andersen H. Evaluation of atrophy of foot muscles in diabetic neuropathy ‐ a comparative study of nerve conduction studies and ultrasonography. Clin Neurophysiol 2007; 118: 2172–2175. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki E, Kashiwagi A, Hidaka H, et al 1H‐ and 31P‐magnetic resonance spectroscopy and imaging as a new diagnostic tool to evaluate neuropathic foot ulcers in Type II diabetic patients. Diabetologia 2000; 43: 165–172. [DOI] [PubMed] [Google Scholar]
- 26. Kohara N, Kimura J, Kaji R, et al F‐wave latency serves as the most reproducible measure in nerve conduction studies of diabetic polyneuropathy: multicentre analysis in healthy subjects and patients with diabetic polyneuropathy. Diabetologia 2000; 43: 915–921. [DOI] [PubMed] [Google Scholar]
- 27. Gibbons CH, Freeman R, Tecilazich F, et al The evolving natural history of neurophysiologic function in patients with well‐controlled diabetes. J Peripher Nerv Syst 2013; 18: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kikkawa R, Hatanaka I, Yasuda H, et al Effect of a new aldose reductase inhibitor, (E)‐3‐carboxymethyl‐5‐[(2E)‐methyl‐3‐phenylpropenylidene] rhodanine (ONO‐2235) on peripheral nerve disorders in streptozotocin‐diabetic rats. Diabetologia 1983; 24: 290–292. [DOI] [PubMed] [Google Scholar]
- 29. DCCT Research Group . Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). Diabetes 1988; 37: 476–481. [PubMed] [Google Scholar]
- 30. The Japan Diabetes Society . The Japanese evidence‐based practice guideline for the treatment for diabetes. Nankodo 2013.
- 31. Hotta N, Akanuma Y, Kawamori R, et al Long‐term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3‐year, multicenter, comparative Aldose Reductase Inhibitor‐Diabetes Complications Trial. Diabetes Care 2006; 29: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 32. Ota A, Kakehashi A, Toyoda F, et al Effects of long‐term treatment with ranirestat, a potent aldose reductase inhibitor, on diabetic cataract and neuropathy in spontaneously diabetic torii rats. J Diabetes Res 2013; 2013: 175901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asano T, Saito Y, Kawakami M, et al Erythrocytic sorbitol contents in diabetic patients correlate with blood aldose reductase protein contents and plasma glucose levels, and are normalized by the potent aldose reductase inhibitor fidarestat (SNK‐860). J Diabetes Complications 2004; 18: 336–342. [DOI] [PubMed] [Google Scholar]
- 34. Yagihashi S, Yamagishi S, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Pract 2007; 77: S184–S189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Change from baseline in tibial nerve conduction velocity (m/s) over time.
Figure S2 | Change from baseline in individual modified Toronto Clinical Neuropathy Score symptom domain at last observation carried forward (LOCF).
Figure S3 | Change from baseline in individual modified Toronto Clinical Neuropathy Score sensory test domain at last observation carried forward.
Figure S4 | Glycated hemoglobin control during treatment period.
Figure S5 | Sorbitol concentration in participants’ erythrocytes.
Table S1 | Summary of adverse events.


