Abstract
Aims/Introduction
Glinides are antidiabetic drugs that enhance the early phase of insulin secretion, but have been considered to be less effective at lowering blood glucose than sulfonylureas. However, glinides show a lower risk of hypoglycemia and a greater effect on postprandial hyperglycemia, and are particularly recommended for use in elderly patients with type 2 diabetes. We investigated the efficacy and safety of repaglinide compared with sulfonylurea for the treatment of elderly patients.
Materials and Methods
In the present multicenter, prospective, randomized, open‐label, controlled trial, 57 elderly lean patients with type 2 diabetes who were being treated with sulfonylureas were studied. They were either switched to repaglinide (Repa group) or continued a sulfonylurea (SU group) for 12 weeks. The primary outcome comprised the change in glycemic control, and among the secondary outcomes was the presence of hypoglycemia and drug compliance.
Results
Although glycated hemoglobin (HbA1c) was not significantly different between the two groups (SU +0.02% vs Repa −0.07%), greater improvements in the glycated albumin (GA) and GA to HbA1c ratio (GA/HbA1c) were observed in the Repa group (ΔGA, SU +0.12% vs Repa −1.15%; ΔGA/HbA1c, SU +0.01 vs Repa −0.13; each P < 0.01) without increasing hypoglycemia. When the Repa group was subdivided according to whether GA improved, the SU dose before switching to repaglinide was significantly smaller and the homeostatic model assessment of β‐cell function was significantly higher in the GA improvement subgroup.
Conclusions
Switching from SU to Repa improved GA and GA/HbA1c, and had favorable effects on glucose fluctuation in elderly patients with type 2 diabetes.
Keywords: Elderly, Glucose variability, Repaglinide
Introduction
The mechanism of action of repaglinide involves the promotion of insulin release from the pancreas, like that of sulfonylureas (SUs), and its principal side‐effect includes hypoglycemia. However, the risk of hypoglycemia while using repaglinide is considered to be potentially lower than SUs1. During the treatment of type 2 diabetes, the importance of managing glycated hemoglobin (HbA1c) has been shown in numerous large‐scale clinical studies2, 3, 4. However, as shown in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial5, it is also important to avoid severe hypoglycemia, but its occurrence is not usually reflected in the HbA1c value. An association between postprandial hyperglycemia and macrovascular disease has been shown in several previous studies, and the suppression of postprandial blood glucose can prevent atherosclerosis6. Daily glycemic fluctuations and high postprandial blood glucose potentially contribute to diabetic complications, such as atherosclerosis, through more glycation or oxidative stress7. Some studies have also shown an association between cognitive impairment and postprandial hyperglycemia or daily acute glucose fluctuations8, 9. Therefore, the avoidance of hypoglycemia and a reduction in postprandial hyperglycemia, with lower glycemic fluctuation, is especially beneficial for elderly patients with type 2 diabetes.
Analysis of continuous blood glucose monitoring (CGM) systems to evaluate the glycemic control achieved using SUs has shown that asymptomatic hypoglycemia is common10, 11. In addition, SUs are often insufficient to manage postprandial hyperglycemia. It is well known that insulin secretion is already attenuated at the stage of impaired glucose tolerance in the Asian population, relative to the Western population12, and that the ability to secrete insulin gradually decreases during the progression of diabetes, resulting in lower insulin secretion by elderly patients. In addition, elderly patients have lower muscle mass, thus having less capacity for glucose disposal. Accordingly, postprandial hyperglycemia is more common in elderly patients than younger patients13.
Glinides could therefore be indicated specifically for elderly patients with type 2 diabetes because of the lower risk of hypoglycemia associated with their use, and their greater effect on postprandial hyperglycemia. However, they have been believed to be less efficacious at lowering blood glucose than SUs. In contrast, our pilot study comparing the effects of repaglinide and SU on blood glucose revealed the superiority of repaglinide for glycemic control in lean elderly patients who had not reached their glycemic goal14. Here, we aimed to assess the use of repaglinide for glycemic control in lean elderly patients with type 2 diabetes in a multicenter, prospective, randomized, parallel‐group comparison study design, which was based on our pilot study.
Methods
Study population
We enrolled 57 patients with type 2 diabetes from seven medical service units located in Hokkaido, Japan (Hokkaido University Hospital, Kuriyama Red Cross Hospital, Hokkaido Spinal Cord Injury Center, Manda Memorial Hospital, Oki Medical Clinic, Kurihara Clinic and Aoki Clinic). The participants were recruited to this trial between July 2016 and February 2017. All participants provided written informed consent before study enrollment. The inclusion criteria were as follows: patients with type 2 diabetes, aged 60–90 years, with HbA1c 7.0–8.9%, body mass index <25 kg/m2 and who had been taking SUs for ≥12 weeks before enrollment. In calculating the SU dose, we defined that 1 mg glimepiride was equivalent to 40 mg gliclazide and 1.25 mg glibenclamide, as previously reported15, 16. We excluded patients with serious liver dysfunction and those taking high doses of SU (>3 mg glimepiride).
Protocol
This was a multicenter, prospective randomized, open‐label, parallel‐group comparison trial. Patients were randomly assigned to continue taking a SU once daily or to switch from SUs to three daily doses of 0.5 mg repaglinide (1.5 mg/day), according to HbA1c, body mass index and SU dose using computer software. All patients were encouraged to continue diet and exercise therapy during the study. Treatment was supervised at the appropriate medical care center for 12 weeks.
The primary outcome was the difference in glycemic control between the two groups. The sample size was calculated using the assumption that switching to glinides from SUs would improve HbA1c by 0.41%, based on data from our retrospective pilot study. Average HbA1c was improved from 7.68% (standard deviation [SD] 0.50%) to 7.27% (SD 0.51%) by switching from SUs to repaglinide. It was determined that 25 patients (28 patients including a 10% dropout estimate) were required in each group to detect a significant difference, with 80% power and at a statistical significance level of 5%. The secondary outcomes were changes in metabolic parameters and surrogate markers of β‐cell function in blood samples, frequency of hypoglycemia and drug compliance. We defined hypoglycemia as the appearance of hypoglycemic symptoms as reported in the questionnaire used in the present study.
Drug compliance was checked using a questionnaire containing a five‐stage evaluation, before and after the trial.
Biochemical analysis
Fasting serum biomarkers were measured at baseline and at the end of the study. Of these, insulin was not measured in patients taking exogenous insulin. The homeostatic model assessment of insulin resistance (HOMA‐IR) and the homeostatic model assessment of β‐cell function (HOMA‐β) were calculated to assess insulin resistance and β‐cell function, respectively. HOMA‐IR and HOMA‐β were assessed using the following formulas: HOMA‐IR = fasting plasma glucose (mg/dL) × fasting insulin (μU/mL)/405, HOMA‐β = fasting insulin (μU/mL) × 360/(fasting plasma glucose [mg/dL] − 63).
Statistical analysis
The results are expressed as means or medians and range. Differences in baseline characteristics between groups were assessed using unpaired Student's t‐tests for continuous variables, and the χ2‐test for categorical variables. Differences in time series baseline characteristics in the same group were assessed using paired Student's t‐tests for continuous variables. The Kolmogorov–Smirnov test for normality was used to determine the appropriate statistical test for the continuous variables. The primary outcome was analyzed using the full analysis set. Single correlation analyses were undertaken using Spearman's rank correlation. HOMA‐IR was analyzed using the Wilcoxon signed‐rank test, because normality was rejected for this variable. For the secondary end‐points, the mean changes between baseline and the end of the treatment period were analyzed descriptively for both groups in the complete case population. We also used the paired t‐test or Wilcoxon signed test without an adjustment for multiple analyses, because these secondary analyses were exploratory in nature. P < 0.05 was considered to represent statistical significance. Data were analyzed using JMP pro version 12 (SAS Institute Inc., Cary, North Carolina, USA) and BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Ethics statement
The study was registered at the University Hospital Medical Information Network Center under the identifier UMIN000022531. The protocol for this research was approved by the institutional review board of Hokkaido University Hospital Clinical Research and Medical Innovation Center, and conformed to the provisions of the Declaration of Helsinki (as revised in Fortaleza, Brazil, in October 2013). Signed informed consent was obtained from the all the participants.
Results
Baseline characteristics
A total of 57 patients were initially enrolled in the trial. Each patient was randomly assigned to either the SU group or the Repa group, and 52 patients completed the study (26 patients per group). One patient in the SU group and two patients in the Repa group withdrew their consent, and one patient in the Repa group withdrew because of hypoglycemia and the requirement for surgery to treat an unrelated disease (Figure 1). The participants consisted of 38 men and 19 women, with a mean age of 72.3 ± 6.7 years and mean HbA1c levels of 7.4 ± 0.5%. Other baseline characteristics of each group are shown in Table 1. There were no differences between the two groups in body mass index, blood biochemistry, diabetic complications or the proportions taking oral antihyperglycemic agents. SUs and repaglinide were well tolerated throughout the study.
Figure 1.
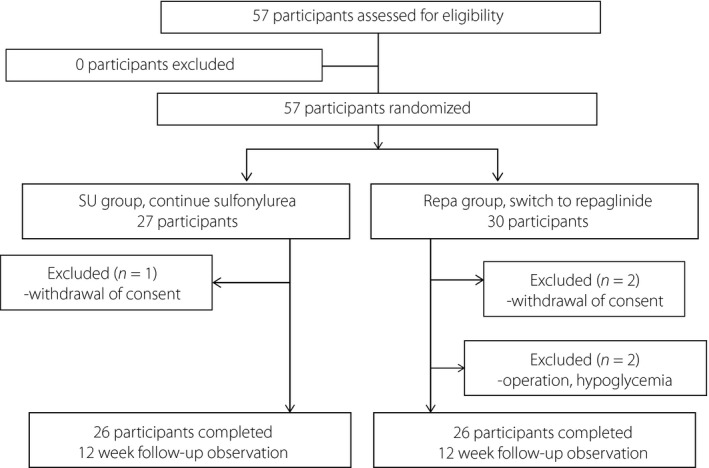
Flow diagram. Repa group, switched from sulfonylurea to repaglinide; SU group, continued a sulfonylurea.
Table 1.
Clinical characteristics of the study population
| Variables | SU (n = 27) | Repaglinide (n = 30) | P‐value |
|---|---|---|---|
| Age (years) | 73.9 ± 6.6 | 72.3 ± 6.7 | 0.37 |
| Male sex (%) | 66.7 | 66.7 | 1.00 |
| Body mass index (kg/m2) | 21.4 ± 2.21 | 21.7 ± 1.93 | 0.54 |
| FPG (mmol/L) | 8.38 ± 1.54 | 8.2 ± 2.57 | 0.74 |
| Glycated hemoglobin (%) | 7.52 ± 0.43 | 7.44 ± 0.47 | 0.50 |
| GA (%)† | 21.0 ± 2.4 | 21.0 ± 3.0 | 0.97 |
| GA/HbA1c† | 2.79 ± 0.28 | 2.85 ± 0.35 | 0.55 |
| IRI (μU/mL) ‡ | 4.41 ± 3.30 | 4.50 ± 3.50 | 0.93 |
| HOMA‐IR¶ , ‡ | 1.43 (0.88–1.92) | 1.36 (0.85–1.81) | 0.90 |
| HOMA‐β‡ | 20.0 ± 16.5 | 20.6 ± 14.5 | 0.89 |
| SU (equivalent to glimepiride mg) | 1.20 ± 0.67 | 0.97 ± 0.49 | 0.13 |
| Serum creatinine (mg/dL) | 0.81 ± 0.21 | 0.85 ± 0.23 | 0.52 |
| eGFR (mL/min/1.73 m2) | 68.6 ± 18.8 | 65.8 ± 16.9 | 0.56 |
| ALT (IU/mL) | 21.8 ± 8.34 | 21.3 ± 14.2 | 0.87 |
| γ‐GT (IU/mL) | 32.0 ± 23.3 | 26.7 ± 18.7 | 0.35 |
| TG (mg/dL) | 99.3 ± 35.9 | 107.3 ± 55.7 | 0.52 |
| Total cholesterol (mg/dL) | 171 ± 21.0 | 175 ± 27.7 | 0.56 |
| HDL cholesterol (mg/dL) | 59.5 ± 14.8 | 62.2 ± 17.5 | 0.53 |
| LogUACR (mg/gCre) | 1.52 ± 0.69 | 1.47 ± 0.81 | 0.82 |
| Hypertension (%) | 66.7 | 66.7 | 1.00 |
| Dyslipidemia (%) | 81.5 | 76.7 | 0.66 |
| Metformin (%) | 81.5 | 70.0 | 0.37 |
| DPP‐4 inhibitors (%) | 88.9 | 83.3 | 0.71 |
| SGLT2 inhibitors (%) | 7.4 | 7.0 | 1.00 |
| Thiazolidine (%) | 3.7 | 16.7 | 0.20 |
| α‐GI (%) | 22.2 | 17.6 | 1.00 |
| GLP‐1RA (%) | 3.7 | 0 | 1.00 |
| Insulin (%) | 11.1 | 10.0 | 1.00 |
| Diabetic retinopathy (%) | 18.5 | 33.3 | 0.18 |
| Diabetic nephropathy (%) | 44.4 | 36.7 | 0.60 |
| §Hypoglycemic episodes (%) | 11.5 | 14.3 | 1.00 |
| Duration of diabetes >10 years (%) | 80.8 | 82.8 | 1.00 |
| History of taking SU >10 years (%) | 77.8 | 75.9 | 0.87 |
Values are mean ± standard deviation or median (range). P‐value of sulfonylurea (SU)‐ vs repaglinide‐treated groups. †Data were obtained in 53 patients (SU n = 26, repaglinide n = 27). ‡Data were obtained in 47 patients (SU n = 23, repaglinide n = 24). §Data were obtained in 54 patients (SU n = 26, repaglinide n = 28). ¶The Mann–Whitney U‐test was applied to homeostatic model assessment of insulin resistance (HOMA‐IR). α‐GI, alpha glucosidase inhibitor; γ‐GT, γ‐glutamyltransferase; ALT, alanine aminotransferase; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GA, glycated albumin; GA/HbA1c, glycated albumin to glycated hemoglobin ratio; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HDL, high‐density lipoprotein; HOMA‐β, homeostatic model assessment of β‐cell function; IRI, immunoreactive insulin; SGLT2, sodium–glucose cotransporter 2; TG, triacylglycerol; UACR, urinary albumin creatinine ratio.
Outcomes
After 12 weeks of SU or repaglinide treatment, HbA1c levels were changed in neither group. No difference was found in the HbA1c change (SU, 7.53% before and 7.55% after; Repa, 7.41% before and 7.31% after, P = 0.37; Figure 2a). However, glycated albumin (GA) and the GA to HbA1c ratio (GA/HbA1c) significantly improved in the Repa group during the study, and this improvement was greater than that observed in the SU group (ΔGA +0.12 vs −1.15, P < 0.01; ΔGA/HbA1c +0.01 vs −0.13, P < 0.01; Figure S1; Figure 2b).
Figure 2.
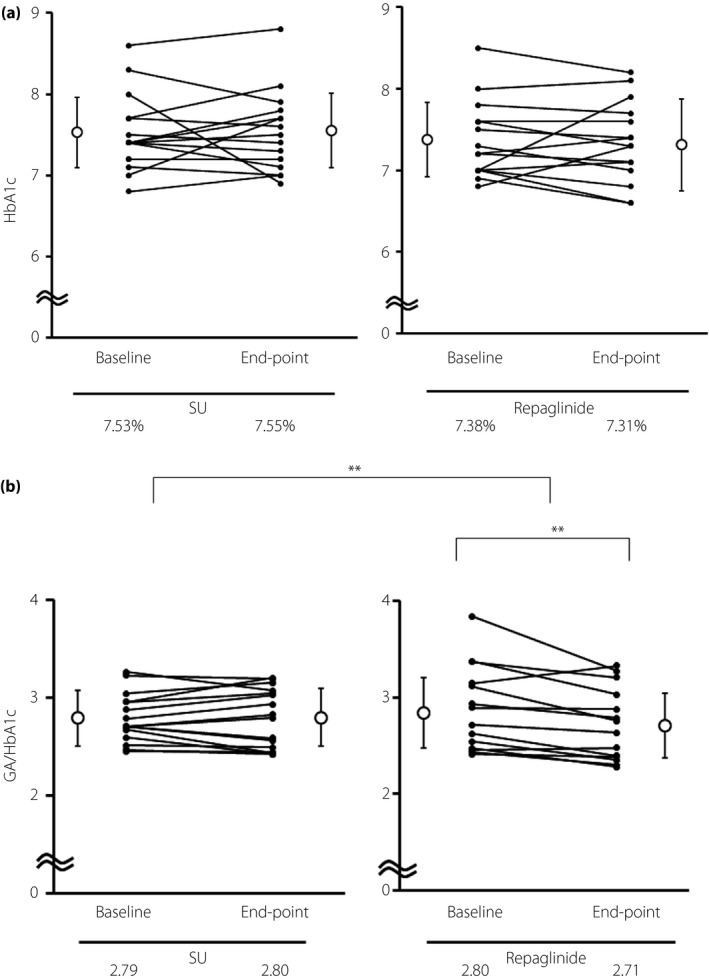
(a) Comparison of changes in glycated hemoglobin (HbA1c) before and after treatment in each group. (b) Comparison of changes in the glycated albumin (GA) to HbA1c (GA/HbA1c) ratio before and after treatment in each group. Values are means, and P‐values were generated using paired or unpaired Student's t‐tests, with **P < 0.01 representing statistical significance. Repaglinide, the group that switched from sulfonylurea to repaglinide from the start of the trial period; SU, the group that continued sulfonylurea during the trial period.
There were no differences in body masses, blood pressure, or liver or renal function between baseline and 12 weeks, or between the two groups (Table S1). Similarly, there were no significant differences in the number of hypoglycemic episodes or drug compliance between the groups. The protocol of the present study did not allow the addition or switching of all medications except for SU and insulin, which could be reduced in patients at risk of hypoglycemia. However, no participant changed or added any medications during the trial.
Subsequently, we divided the Repa group into subgroups according to whether GA did or did not improve during the study (Table 2). Although the number of insulin users before enrollment was small in the Repa group (n = 3), none showed an improvement in GA after switching their medication. The GA improvement subgroup had been taking a lower dose of SU (0.8 ± 0.4 mg vs 1.4 ± 0.5 mg, P < 0.01) and had higher HOMA‐β (25 ± 13% vs 9.1 ± 3.6%, P = 0.03; Table 2).
Table 2.
Comparison of the characteristics and measurements in subgroups of repaglinide‐treated patients that showed an improvement or no improvement in glycated albumin
| Variables | Improved (n = 18) | Not improved (n = 7) | P‐value |
|---|---|---|---|
| Age (years) | 71.7 ± 6.79 | 72.9 ± 4.84 | 0.69 |
| Male sex (%) | 61.1 | 85.7 | 0.36 |
| Body mass index (kg/m2) | 21.8 ± 2.24 | 21.5 ± 1.60 | 0.78 |
| FPG (mmol/L) | 8.18 ± 2.20 | 8.50 ± 1.32 | 0.73 |
| Glycated hemoglobin (%) | 7.35 ± 0.45 | 7.41 ± 0.53 | 0.76 |
| GA (%) | 20.8 ± 3.12 | 21.3 ± 3.43 | 0.74 |
| GA/HbA1c | 2.83 ± 0.36 | 2.87 ± 0.40 | 0.81 |
| IRI (μU/mL)† | 5.37 ± 3.83 | 2.29 ± 1.28 | 0.13 |
| HOMA‐IR† , § | 1.51 (1.19–2.03) | 0.73 (0.51–1.11) | 0.07 |
| HOMA‐β† | 24.7 ± 13.7 | 9.14 ± 3.57 | 0.04* |
| SU (equivalent to glimepiride mg) | 0.78 ± 0.39 | 1.36 ± 0.48 | <0.01** |
| Serum creatinine (mg/dL) | 0.85 ± 0.28 | 0.85 ± 0.18 | 0.51 |
| eGFR (mL/min/1.73 m2) | 66.5 ± 19.5 | 67.7 ± 15.1 | 0.89 |
| ALT (IU/mL) | 19.9 ± 13.8 | 23.7 ± 17.9 | 0.58 |
| γ‐GT (IU/mL) | 22.0 ± 11.7 | 36.6 ± 27.3 | 0.21 |
| TG (mg/dL) | 101.5 ± 46.3 | 103.1 ± 40.2 | 0.94 |
| Total cholesterol (mg/dL) | 173.2 ± 30.4 | 185.4 ± 25.3 | 0.35 |
| HDL cholesterol (mg/dL) | 59.9 ± 19.1 | 70.0 ± 16.5 | 0.23 |
| LogUACR (mg/gCre) | 1.44 ± 0.60 | 1.55 ± 1.01 | 0.72 |
| Hypertension (%) | 66.7 | 57.1 | 0.67 |
| Dyslipidemia (%) | 77.8 | 71.4 | 1.00 |
| Metformin (%) | 66.7 | 57.1 | 0.67 |
| DPP‐4 inhibitors (%) | 77.8 | 100 | 0.29 |
| Insulin (%) | 0 | 43 | 0.02* |
| Diabetic retinopathy (%) | 22.2 | 57.1 | 0.16 |
| Diabetic nephropathy (%) | 38.9 | 28.6 | 1.00 |
| Duration of diabetes >10 years (%) | 83.3 | 71.4 | 0.59 |
| History of taking SU >10 years (%) | 72.2 | 71.4 | 1.00 |
†Data were obtained in 20 patients. ‡Values are mean ± standard deviation. §Values were analyzed using the Wilcoxon signed‐rank test, because normality was rejected for these variables. P‐value for the difference between glycated albumin (GA) improved and not improved population in the repaglinide group, with *P < 0.05 and **P < 0.01 representing statistical significance. α‐GI, alpha glucosidase inhibitor; γ‐GT, γ‐glutamyltransferase; ALT, alanine aminotransferase; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; GA, glycated albumin; GA/HbA1c, glycated albumin to glycated hemoglobin ratio; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HDL, high‐density lipoprotein; HOMA‐β, homeostatic model assessment of β‐cell function; IRI, immunoreactive insulin; SGLT2, sodium–glucose cotransporter 2; SU, sulfonylurea; TG, triacylglycerol; UACR, urinary albumin creatinine ratio.
In addition, we divided the Repa group into three categories according to their original SU dose (0.5, 1.0 and ≥1.5 mg; Table S2). Improvement in GA was significantly associated with the SU dose, although the number of patients being treated with ≥1.5 mg of SU dose was small, and 100% (n = 10) of those taking 0.5 mg SU at baseline showed an improvement in GA. However, no contributing factors, including SU dose or HOMA‐β, were found in single correlation analyses with respect to improvements in GA or GA/HbA1c (Table S3).
Discussion
This is the first randomized controlled trial to compare the effect of SU and a glinide in lean elderly patients with type 2 diabetes, in whom postprandial blood glucose is usually high. Glinides have not been widely used to date, because they are considered to be less effective at lowering blood glucose, despite costing more and requiring more frequent administration than other antidiabetic drugs. However, several studies have reported that dipeptidyl peptidase (DPP‐4) inhibitors augment glinide‐induced early‐phase insulin secretion and significantly enhance their postprandial blood glucose‐lowering effect17, 18. Recently, in accordance with the increase of DPP‐4 inhibitors users and the growing population of elderly patients in Japan, the number of glinide users has become larger.
DPP‐4 inhibitors amplify the pro‐insulin secretory effects of SUs and enhance their blood glucose‐lowering effect, but hypoglycemia can develop more frequently on combination therapy. Indeed, it has been reported that the addition of DPP‐4 inhibitors to SU therapy is associated with a 50% increase in the risk of hypoglycemia19. This is partially because of the enhancement of insulin secretion due to the interaction between exchange proteins directly activated by cyclic adenosine monophosphate 2 and SUs20. More than 80% of participants had been taking DPP‐4 inhibitors before starting the present study, and this high rate of DPP‐4 inhibitor use could have been at least in part responsible for the comparable or better glycemic control in the Repa group in this trial.
Although a superior effect of Repa on HbA1c was not observed in this trial, significant improvement in GA and GA/HbA1c were observed in the Repa group. One possible reason for the discrepancy between the HbA1c and GA data is the disparity in the timescale required to reveal an effect on glycemic control: 12 weeks could be too short a period to detect a change in HbA1c. In addition, there is also a difference in how these measurements reflect blood glucose fluctuations. Albumin is 10‐fold more rapidly glycated than hemoglobin, and that postprandial glucose levels are reflected more readily in the glycosylation of albumin21. For this reason, GA and the GA/HbA1c ratio would reflect more acute increases in plasma glucose, whereas HbA1c is a marker of chronic hyperglycemia. Indeed, a previous study showed that the GA/HbA1c ratio, but not HbA1c, correlated with the glycemic fluctuations assessed using CGM22, 23. In clinical research, glycemic SD in CGM has been strongly associated with the GA/HbA1c ratio. Previous studies have shown a remarkable difference in glycemic SD values between patients with and without a GA/HbA1c ratio ≥2.8, and a ratio of 2.8 has therefore been proposed as a cut‐off value for discriminating between patients with smaller and larger SD values. In addition, other Japanese groups have shown that GA or GA/HbA1c correlates with SD and MAGE in CGM23, 24. Although CGM is relatively convenient in our clinics, it is not practical or cost‐efficient to use CGM for every patient. Thus, the GA/HbA1c ratio might represent a useful alternative means of predicting blood glucose fluctuations in daily medical practice.
A previous clinical study showed a negative correlation between glycemic fluctuation (mean amplitude of glycemic excursions) and cognitive performance (Mini‐Mental State Examination Score) in elderly patients with type 2 diabetes8, whereas another study found that the incidence of Alzheimer's disease was significantly higher when the GA/HbA1c ratio was high25. The other study showed a significant relationship of large white matter hyperintensity volume on brain magnetic resonance imaging, which could lead to the decline of cognition, with a high GA/HbA1c, but not with HbA1c level26. GA/HbA1c was also negatively associated with both the Mini‐Mental State Examination Score and instrumental activities of daily living in elderly patients with type 2 diabetes. The authors suggested that increased glucose variability might cause oxidative stress, leading to cognitive impairment. Thus, it is important to manage GA/HbA1c, especially in the elderly.
When switching from SU to repaglinide, patients were expected to have a lower rate of hypoglycemia and poorer compliance. However, the results of the present study showed that hypoglycemic episodes, as analyzed using the questionnaire, were infrequent before switching from SU to repaglinide, and this might explain why a difference was not detected after switching.
Although we did not identify independent characteristic markers with which to predict an improvement in GA after switching, a smaller dose of SU and higher HOMA‐β could be used. Higher HOMA‐β is indicative of a greater capacity for insulin secretion, and these data are consistent with the present finding that none of the insulin users in the Repa group showed an improvement in GA. In addition, the beneficial effect of switching to Repa is likely to be limited when the corrected glimepiride dose is ≥1.5 mg. Although we did not identify independent characteristic markers that could predict an improvement in GA after switching, a smaller dose of SU and higher HOMA‐β might represent indicative factors of successful switching.
The main limitations of the present study were the small sample size and the short duration of treatment. A further limitation was the lack of double blinding, which was because of the ambulatory care setting. To circumvent these potential issues, our findings need to be replicated by a larger‐scale, long‐term, diet‐controlled, double‐blind trial. Although it was reported that the half dose of repaglinaide (0.25 mg × 3/day) could also be useful16 in Japanese patients, the administration dose of repaglinide was set at 1.5 mg (0.5 mg × 3/day) in this trial. Further study is required to elucidate the clinical efficacy of lower‐dose repaglinide.
In conclusion, repaglinide lowered GA or GA/HbA1c, and might be more effective at improving glycemic fluctuations than SUs in lean elderly patients with type 2 diabetes. Switching from SUs to repaglinide acceptably is safe and might be associated with a lower risk of subsequent atherosclerosis or cognitive impairment.
Disclosure
AN has received research funding from Mitsubishi Tanabe Pharma Co. and Ono Pharmaceutical Co., Ltd. NM has received honoraria for lectures from Ono Pharmaceutical Co., Ltd. YK has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Mitsubishi Tanabe Pharma Co., Ltd., MSD, Ono Pharmaceutical Co., Ltd., Sanofi, Shionogi & Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. TA has received honoraria for lectures from Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Pfizer Inc., AbbVie Inc., Eisai Co., Ltd., Daiichi Sankyo Co., Ltd., Bristol‐Myers Squibb Co., UCB Japan Co., Ltd. and Eli Lilly Japan K.K., and has received research funding from Astellas Pharma Inc., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Inc. and Alexion Inc. HM has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Dainippon Pharma Co., Eli Lilly, Kissei, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma, Takeda Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Sanofi, and has received research funding from Astellas Pharma Inc., AstraZeneca, Daiichi Sankyo, Dainippon Pharma Co., Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novo Nordisk Pharma, Sanofi, Takeda Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd. The other authors declare no conflict of interest.
Supporting information
Figure S1 Comparison of changes in glycated albumin (GA) before and after treatment in each group. Values indicate the mean, and P‐values were generated using paired or unpaired Student's t‐tests, with P < 0.01 (**) being considered to represent statistical significance. Repaglinide, the group that switched from a sulfonylurea to repaglinide at the start of the trial period; SU, the group that continued sulfonylurea during the trial period.
Table S1 Laboratory data for the study population at baseline and 12 weeks.
Table S2 Association between sulfonylurea dose and rate of improvement after switching from sulfonylurea to repaglinide.
Table S3 Analysis of the contributing factors to the change in glycated albumin or the glycated albumin to glycated hemoglobin ratio in the repaglinide group.
Acknowledgments
The authors thank Ms N Fujimori and Ms M Watanabe for technical assistance. We thank the Translational Research and Clinical Trial Centre in Hokkaido University Hospital for data analysis and useful discussion. We thank Mark Cleasby, PhD, from Edanz Group for editing a draft of this manuscript.
J Diabetes Investig 2019; 10: 367–374
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000022531
References
- 1. Papa G, Fedele V, Rizzo MR, et al Safety of type 2 diabetes treatment with repaglinide compared with glibenclamide in elderly people: a randomized, open‐label, two‐period, cross‐over trial. Diabetes Care 2006; 29: 1918–1920. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Genuth S, Lachin J, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 3. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–853. [PubMed] [Google Scholar]
- 4. Shichiri M, Kishikawa H, Ohkubo Y, et al Long‐term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(Suppl 2): B21–B29. [PubMed] [Google Scholar]
- 5. Gerstein HC, Miller ME, Byington RP, et al Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Esposito K, Giugliano D, Nappo F, et al Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004; 110: 214–219. [DOI] [PubMed] [Google Scholar]
- 7. Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci 2014; 15: 18381–18406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizzo MR, Marfella R, Barbieri M, et al Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care 2010; 33: 2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohara T, Doi Y, Ninomiya T, et al Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology 2011; 77: 1126–1134. [DOI] [PubMed] [Google Scholar]
- 10. Hay LC, Wilmshurst EG, Fulcher G. Unrecognized hypo‐ and hyperglycemia in well‐controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring. Diabetes Technol Therap 2003; 5: 19–26. [DOI] [PubMed] [Google Scholar]
- 11. Gehlaut RR, Dogbey GY, Schwartz FL, et al Hypoglycemia in type 2 diabetes–more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol 2015; 9: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukushima M, Usami M, Ikeda M, et al Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross‐sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835. [DOI] [PubMed] [Google Scholar]
- 13. Qiao Q, Nakagami T, Tuomilehto J, et al Comparison of the fasting and the 2‐h glucose criteria for diabetes in different Asian cohorts. Diabetologia 2000; 43: 1470–1475. [DOI] [PubMed] [Google Scholar]
- 14. Nomoto H, Sekizaki T, Jyoudo S. The effect of switching from sulfonlylureas to repaglinide ‐observational trial. Diabetes Front 2015; 26: 613–617 (Japanese). [Google Scholar]
- 15. Basit A, Riaz M, Fawwad A. Glimepiride: evidence‐based facts, trends, and observations (GIFTS) [corrected]. Vasc Health Risk Manag 2012; 8: 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamiyama H, Aoki K, Nakajima S, et al Effect of switching from sulphonylurea to repaglinide twice or three times daily for 4 months on glycemic control in japanese patients with type 2 diabetes. Int Med 2016; 55: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 17. Kudo‐Fujimaki K, Hirose T, Yoshihara T, et al Efficacy and safety of nateglinide plus vildagliptin combination therapy compared with switching to vildagliptin in type 2 diabetes patients inadequately controlled with nateglinide. J Diabetes Investig 2014; 5: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanimoto M, Kanazawa A, Hirose T, et al Comparison of sitagliptin with nateglinide on postprandial glucose and related hormones in drug‐naive Japanese patients with type 2 diabetes mellitus: a pilot study. J Diabetes Investig 2015; 6: 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salvo F, Moore N, Arnaud M, et al Addition of dipeptidyl peptidase‐4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta‐analysis. BMJ 2016; 353: i2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang CL, Katoh M, Shibasaki T, et al The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009; 325: 607–610. [DOI] [PubMed] [Google Scholar]
- 21. Day JF, Ingebretsen CG, Ingebretsen WR Jr, et al Nonenzymatic glucosylation of serum proteins and hemoglobin: response to changes in blood glucose levels in diabetic rats. Diabetes 1980; 29: 524–527. [DOI] [PubMed] [Google Scholar]
- 22. Ogawa A, Hayashi A, Kishihara E, et al New indices for predicting glycaemic variability. PLoS One 2012; 7: e46517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koga M, Murai J, Saito H, et al Glycated albumin and glycated hemoglobin are influenced differently by endogenous insulin secretion in patients with type 2 diabetes. Diabetes Care 2010; 33: 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka C, Saisho Y, Tanaka K, et al Factors associated with glycemic variability in Japanese patients with diabetes. Diabetol Int 2014; 5: 36–42. [Google Scholar]
- 25. Mukai N, Ohara T, Hata J, et al Alternative measures of hyperglycemia and risk of Alzheimer's disease in the community: the Hisayama Study. J Clin Endocrinol Metab 2017; 102: 3002–3010. [DOI] [PubMed] [Google Scholar]
- 26. Tamura Y, Kimbara Y, Yamaoka T, et al White matter hyperintensity in elderly patients with diabetes mellitus is associated with cognitive impairment, functional disability, and a high glycoalbumin/glycohemoglobin ratio. Front Aging Neurosci 2017; 9: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of changes in glycated albumin (GA) before and after treatment in each group. Values indicate the mean, and P‐values were generated using paired or unpaired Student's t‐tests, with P < 0.01 (**) being considered to represent statistical significance. Repaglinide, the group that switched from a sulfonylurea to repaglinide at the start of the trial period; SU, the group that continued sulfonylurea during the trial period.
Table S1 Laboratory data for the study population at baseline and 12 weeks.
Table S2 Association between sulfonylurea dose and rate of improvement after switching from sulfonylurea to repaglinide.
Table S3 Analysis of the contributing factors to the change in glycated albumin or the glycated albumin to glycated hemoglobin ratio in the repaglinide group.


