Abstract
Aims/Introduction
To investigate the prevalence of sarcopenia, its related factors and indicators of physical evaluation in elderly diabetes patients.
Materials and Methods
This was a cross‐sectional observation study. A total of 267 diabetes patients (159 men, 108 women) aged >65 years were recruited in the present study. Skeletal muscle mass index, grip strength and usual gait speed were measured to diagnose sarcopenia according to the Asian Working Group for Sarcopenia. Body composition was measured using bioelectrical impedance analysis. Body mass index (BMI) and body fat percentage were evaluated in quartiles to investigate the relationship with sarcopenia. A multiple logistic regression analysis examined sarcopenia‐related factors.
Results
The prevalence of sarcopenia in all participants was 18.7% and increased with age. Sarcopenia decreased as BMI increased (P < 0.01, Cochran–Armitage test). In contrast, the third quartile body fat percentage group showed the lowest prevalence of sarcopenia. A strong positive correlation was observed between body mass and skeletal muscle mass indices (R = 0.702–0.682). Multiple logistic regression analysis showed that sarcopenia was associated with lower BMI, non‐use of metformin and lower bone mineral content in men (P < 0.05), and lower bone mineral content, lower serum levels of albumin and older age in women (P < 0.05).
Conclusions
The present study suggests that diabetes patients with a high body fat percentage in addition to low BMI might develop sarcopenia. It is suggested that physical management in elderly diabetes patients should be carried out based on the evaluation of BMI and body fat percentage to prevent sarcopenia.
Keywords: Body fat percentage, Elderly diabetes, Sarcopenia
Introduction
Sarcopenia is defined by the European Working Group on Sarcopenia in Older People as a loss of skeletal muscle mass, decrease in muscle strength or decline in physical ability that occurs with advancing age1. Some studies have shown that sarcopenia is associated with falls, increased fracture risk, movement disorders and reduced activities of daily living2, 3, 4, 5, 6, 7, leading to a worsened life prognosis8, 9. Limb skeletal muscles from older men and women are 25–35% smaller than limb muscles from younger individuals10.
Sarcopenia results from a collapse of the balance between protein synthesis and degradation. The insufficient action of insulin, a protein assimilation‐related hormone, leads to a decrease in skeletal muscle mass. In diabetes patients, muscle mass, muscular strength and physical ability decrease, leading to sarcopenia11, 12, 13. In a previous report, the lean body of mass of the extremities of diabetes patients tended to decrease compared with their non‐diabetic counterparts14, especially in patients with uncontrolled diabetes13. Taking insulin sensitizers reportedly attenuates lean body mass loss15. The degree of sarcopenia of elderly diabetes patients becomes more prominent as the diabetes duration increases, particularly when glycemic control is poor12. On the contrary, a decrease in muscle mass increases insulin resistance, which further deteriorates glycemic control. Thus, sarcopenia and diabetes are intertwined.
The definition of sarcopenia was that proposed in the European Working Group on Sarcopenia in Older People criteria in 2010. In addition, the Asian Working Group for Sarcopenia (AWGS) presented standards for Asians in 201416. These definitions were not fully supported by evidence, and investigators in the USA published a series of studies attempting to establish an evidence‐based data‐driven definition17. The present study examined the presence or absence of sarcopenia according to the AWGS standards. The prevalence of sarcopenia per AWGS standards is reportedly 16.5% in men and 19.9% in women aged >65 years in Japan18, and 14.8% in type 2 diabetes patients aged >60 years in China19. Diabetes patients were reportedly at a threefold higher risk of sarcopenia than healthy individuals after the adjustment for age, sex and body mass index (BMI)20.
In elderly diabetes patients, sarcopenia has been considered a preliminary stage to the need for long‐term care. It is important to maintain a patient's activities of daily living through appropriate evaluation and intervention, but few studies to date have evaluated sarcopenia in diabetes patients, particularly those aged >75 years. The purposes of the present study were to investigate the prevalence of sarcopenia in elderly diabetes patients, investigate its related factors and examine the indicators of physical evaluation that consider the prevention or progression of sarcopenia using a body composition analysis.
Methods
Patients
The present cross‐sectional observational study investigated the prevalence and related factors of sarcopenia by examining 267 patients (159 men, 108 women) aged >65 years. All patients provided written informed consent before participation. Participants included in the present study were all outpatients aged >65 years who visited the Diabetes and Endocrinology/Geriatric Medicine Unit of Akita University Hospital between February and July 2015. A total of 317 patients underwent evaluation of body composition, usual gait speed and grip strength. Excluding 40 non‐diabetes patients (diabetes mellitus was defined according to The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus)21, seven dialysis patients and three who withdrew informed consent, a total of 267 patients were included. The study's design was approved by the Health Research Ethics Committee at Akita University Graduate School of Medicine. The Akita prefecture, which has a population of 1 million, where Akita University Hospital is located, has an aged proportion (people aged ≥65 years) of 35.5%, an unprecedented statistic.
Parameters
Here, we measured the limb skeletal muscle mass, grip strength and usual gait speed as indicators of physical ability, and judged the presence or absence of sarcopenia using AWGS standards. Body composition was measured using the bioelectrical impedance analyzer (InBody770; InBody Japan Inc., Tokyo, Japan). Skeletal muscle mass index (SMI) was calculated by dividing the limb skeletal muscle mass (kg) by the square of the height (m2), and low muscle mass was defined as SMI <7.0 kg/m2 in men and <5.7 kg/m2 in women. Grip strength was measured with a Smedley‐type (mechanical) handgrip dynamometer (Smedley; Matsumiya Ika Seiki Seisakujo, Tokyo, Japan). Patients were tested twice on each side in a standing position with the elbow at full extension, and the maximum value was taken as the analysis value. The cut‐off value for reduced grip strength was set at 26 kg for men and 18 kg for women. Usual gait speed was measured at the 6‐m mark of a 12‐m walking test, and the cut‐off value of usual gait speed reduction was set at ≤0.8 m/s. Sarcopenia was diagnosed as a condition in which skeletal muscle mass was reduced, and grip strength and/or usual gait speed was decreased. In addition, the condition in which one's skeletal muscle mass was reduced but physical ability was maintained was classified as “pre‐sarcopenia,” whereas the condition in which both grip strength and usual gait speed in addition to skeletal muscle mass were decreased was classified as “severe sarcopenia.” Bodyweight, body fat percentage and bone mineral content were calculated in a body composition analysis. The prevalence of sarcopenia was investigated by sex and age class. To investigate the relationship with sarcopenia, BMI and body fat percentage were evaluated by quartile.
We studied diabetes‐related factors, including glycated hemoglobin (HbA1c), disease duration, degree of diabetic retinopathy progression, urinary albumin:creatinine ratio, insulin use (unit per bodyweight) and use or non‐use of oral antidiabetes drugs (sulfonylurea, dipeptidyl peptidase 4 inhibitor, biguanide, glinide, α‐glucosidase inhibitor, thiazolidine, sodium–glucose cotransporter 2 inhibitor) and glucagon‐like peptide‐1 receptor agonists.
As blood and biochemical parameters, hemoglobin, serum levels of total protein, albumin, alanine aminotransferase, aspartate aminotransferase, gamma‐glutamyl transpeptidase, creatinine, urea nitrogen, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglyceride, uric acid, sodium, potassium, calcium, 25‐hydroxyvitamin D (25‐OHD) and estimated glomerular filtration rate were measured. Serum 25‐OHD quantification was carried out using chemiluminescence immunoassay, and the sampling period was restricted from September to October to avoid seasonal fluctuations. Vitamin D deficiency was defined as a 25‐OHD of <10 ng/mL, and insufficiency was defined as a 25‐OHD of 10.0–19.9 ng/mL22. The use of angiotensin receptor blocker, calcium channel blocker and statin, and history of cerebral infarction or cardiovascular disease were investigated from the patients’ medical records.
Statistical analysis
A statistical analysis was carried out of the sarcopenia and non‐sarcopenia groups. Data are presented as mean ± standard deviation or number (%). For the body composition analysis value, sarcopenia‐related factors, diabetes‐related factors and vascular complications, the Mann–Whitney U‐test and χ2‐test were used for continuous and categorical variables, respectively. To determine the relationship between sarcopenia and age, sensitivity and specificity were analyzed using a receiver operating characteristic curve. For the trend analysis between sarcopenia and BMI or body fat percentage, a Cochran–Armitage test was used. In the analysis of factors related to sarcopenia, a multiple logistic regression analysis was carried out, using potential factors including significant variables in univariate analysis. All statistical analysis was carried out using Statflex version 6.0 (Artech Co., Ltd., Osaka, Japan) and JMP Pro version 12 (SAS Institute Inc., Cary, NC, USA), and the significance level was <5%.
Results
The clinical characteristics of the study population are summarized in Table 1. The mean age of the study participants was 73.7 ± 6.3 years; mean BMI was 24.0 ± 3.3 kg/m2 in men and 24.3 ± 4.7 kg/m2 in women; and mean diabetes duration was 14.3 ± 9.5 years. Sarcopenia was present in 18.7% (50/267) of all patients. Pre‐sarcopenia was present in 13.9%, whereas severe sarcopenia was present in 4.9% (Figure 1a). When the prevalence of sarcopenia was evaluated by age group, it significantly increased with age (P < 0.0001, Cochran–Armitage test; Figure 1b). Regarding the relationship between age and sarcopenia, in the receiver operating characteristic curve of sarcopenia prevalence, sensitivity and specificity were the highest at a cut‐off value of 75 years (sensitivity 0.660, specificity 0.682). The prevalence of sarcopenia was examined by BMI quartiles. In men, it was 45.0% in the first quartile group and 2.5% in the fourth quartile group. In women, it was 37.0% in the first quartile group and 7.4% in the fourth quartile group. The prevalence of sarcopenia showed a decreasing trend as BMI increased in both sexes (men P < 0.0001, women P = 0.0030; Figure 1c). The prevalence of sarcopenia by body fat percentage quartile is shown in Figure 1d. In men, the first quartile group had a significantly higher prevalence of sarcopenia than the third quartile group (30.0% vs 5.0%, P = 0.0018; univariate logistic analysis). In women, the third quartile group also had the lowest prevalence of sarcopenia ratio of 11.1%, but no significant difference was observed between the groups.
Table 1.
Characteristics of patients
| Parameter | All patients | Non‐sarcopenia | Sarcopenia |
|---|---|---|---|
| n | 267 | 217 | 50 |
| Women, n (%) | 108 (40.4) | 84 (38.7) | 24 (48.0) |
| Age (years) | 73.7 ± 6.3 | 72.9 ± 5.8 | 77.2 ± 7.0** |
| Diabetes duration (years) | 14.3 ± 9.5 | 13.6 ± 9.4 | 17.1 ± 9.7** |
| Height (m) | |||
| Men | 1.65 ± 0.06 | 1.66 ± 0.06 | 1.63 ± 0.06* |
| Women | 1.53 ± 0.06 | 1.53 ± 0.06 | 1.50 ± 0.05* |
| Bodyweight (kg) | |||
| Men | 65.7 ± 9.7 | 67.9 ± 8.9 | 54.6 ± 4.8** |
| Women | 56.4 ± 10.9 | 58.6 ± 10.5 | 48.6 ± 8.3** |
| BMI (kg/m2) | |||
| Men | 24.0 ± 3.3 | 24.7 ± 3.1 | 20.6 ± 2.3** |
| Women | 24.3 ± 4.7 | 25.0 ± 4.7 | 21.8 ± 4.3** |
| Body fat percentage (%) | |||
| Men | 25.5 ± 6.9 | 26.1 ± 6.8 | 22.5 ± 6.7** |
| Women | 32.5 ± 9.8 | 33.0 ± 9.2 | 31.0 ± 11.5 |
| Bone mineral content (kg) | |||
| Men | 2.69 ± 0.34 | 2.74 ± 0.32 | 2.40 ± 0.23** |
| Women | 2.18 ± 0.29 | 2.23 ± 0.29 | 2.01 ± 0.17** |
| Hypertension | 161 (60.2) | 134 (61.8) | 27 (54.0) |
| Statin treatment | 129 (48.3) | 107 (49.3) | 22 (44.0) |
| Coronary heart disease | 46 (17.2) | 37 (17.1) | 9 (18.0) |
| Stroke | 58 (21.7) | 45 (20.7) | 13 (26.0) |
| Retinopathy | |||
| Non | 174 (65.2) | 142 (65.4) | 32 (64.0) |
| Simple | 66 (24.7) | 55 (25.3) | 11 (22.0) |
| Proliferative | 27 (10.1) | 20 (9.2) | 7 (14.0) |
| Nephropathy | |||
| Normoalbuminuria | 136 (50.9) | 110 (50.7) | 26 (52.0) |
| Microalbuminuria | 90 (33.7) | 74 (34.1) | 16 (32.0) |
| Macroalbuminuria | 41 (15.4) | 33 (15.2) | 8 (16.0) |
| Diabetes treatment contents | |||
| Insulin | 87 (32.6) | 75 (34.6) | 12 (24.0) |
| GLP‐1RA | 4 (1.5) | 3 (1.4) | 1 (2.0) |
| Sulfonylurea | 69 (25.9) | 59 (27.1) | 10 (20.0) |
| DPP4 inhibitor | 132 (49.7) | 106 (48.8) | 26 (52.0) |
| Biguanide | 56 (20.9) | 53 (24.4) | 3 (6.0)** |
| Glinide | 11 (4.1) | 8 (3.7) | 3 (6.0) |
| Thiazolidine | 19 (7.1) | 16 (7.3) | 3 (6.0) |
| α‐Glucosidase inhibitor | 58 (21.7) | 48 (22.1) | 10 (20.0) |
| SGLT2 inhibitor | 2 (0.7) | 2 (0.9) | 0 (0.0) |
Data are presented as mean ± standard deviation or number (percentage). Comparison of non‐sarcopenia and sarcopenia. Continuous variables: Mann–Whitney U‐test. Categorical variables: χ2‐test. *P < 0.05, **P < 0.01.DPP4, dipeptidyl peptidase 4; GLP‐1RA, glucagon‐like peptide‐1 receptor agonists; SGLT2, sodium–glucose cotransporter 2.
Figure 1.
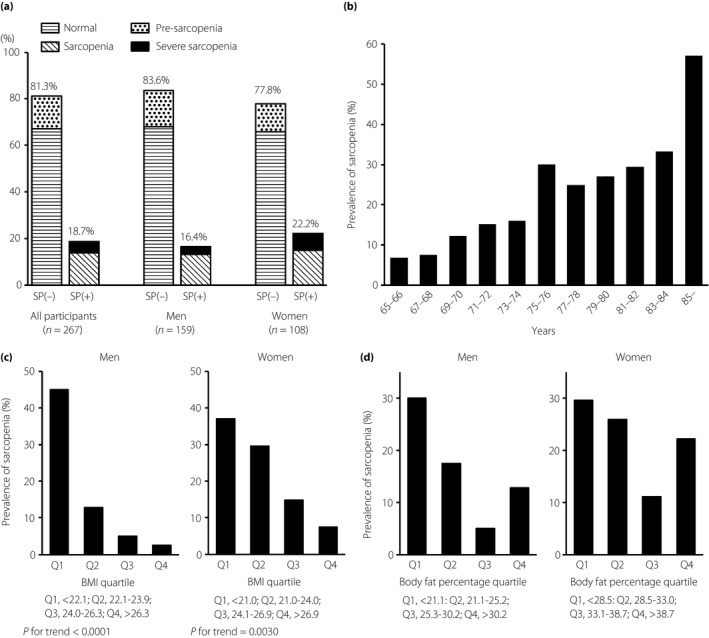
Prevalence of sarcopenia. (a) All patients. (b) Evaluation of sarcopenia by age. (c) Evaluation of sarcopenia by body mass index quartile. In men: Q1, first quartile group, <22.1 kg/m2; Q2, second quartile group, 22.1–23.9 kg/m2; Q3, third quartile group, 24.0–26.3 kg/m2; Q4, fourth quartile group, >26.3 kg/m2. In women: Q1, <21.0 kg/m2; Q2, 21.0–24.0 kg/m2; Q3, 24.1–26.9 kg/m2; Q4, >26.9 kg/m2. (d) Evaluation of sarcopenia by body fat percentage quartile. In men: Q1, <21.1%; Q2, 22.1–25.2%; Q3, 25.3–30.2%; Q4, >30.2%. In women: Q1, <28.5%; Q2, 28.5–33.0%; Q3, 33.1–38.7%; Q4, >38.7%. SP, diagnosis of sarcopenia.
Table 1 compares the sarcopenia and non‐sarcopenia groups. In the sarcopenia group compared with the non‐sarcopenia group, the mean age was higher (P < 0.01); the mean diabetes duration was longer (P < 0.01); height, bodyweight and BMI were significantly lower (P < 0.01); body fat percentage was higher in men (P < 0.01); and bone mineral content was lower in both sexes (P < 0.01). Metformin use was significantly lower in the sarcopenia group (P < 0.01), but the usage rates of other oral antidiabetes drugs and insulin did not differ significantly. There were no significant differences in the prevalence of diabetic retinopathy, nephropathy, cerebral infarction or cardiovascular disease.
Table 2 shows the patients’ blood and biochemical parameters. HbA1c did not differ significantly between groups. Serum levels of alanine aminotransferase, gamma‐glutamyl transpeptidase, albumin, creatinine and uric acid were significantly lower in the sarcopenia group. Serum levels of 25‐OHD were not significantly different between groups.
Table 2.
Blood and biochemical parameters
| Parameter | All patients | Non‐sarcopenia | Sarcopenia |
|---|---|---|---|
| HbA1c (%) | 7.04 ± 1.02 | 7.04 ± 1.03 | 7.02 ± 1.00 |
| AST (IU/L) | 25.2 ± 10.2 | 25.5 ± 17.6 | 24.2 ± 8.5 |
| ALT (IU/L) | 22.1 ± 11.8 | 22.7 ± 12.0 | 19.4 ± 10.7* |
| γ‐GTP (IU/L) | 33.9 ± 29.1 | 35.3 ± 29.5 | 29.2 ± 27.1** |
| BUN (mg/dL) | 18.9 ± 8.0 | 18.9 ± 8.0 | 18.7 ± 8.0 |
| Creatinine (mg/dL) | 0.96 ± 0.50 | 0.96 ± 0.39 | 0.94 ± 0.85* |
| eGFR (mL/min/1.73 m2) | 60.3 ± 18.6 | 59.6 ± 18.3 | 63.7 ± 19.5 |
| ACR (mg/g creatinine) | 177 ± 436 | 190 ± 472 | 118 ± 206 |
| Hemoglobin (g/dL) | 12.9 ± 1.5 | 12.9 ± 1.6 | 12.7 ± 1.5 |
| TP (g/dL) | 6.96 ± 0.50 | 6.99 ± 0.50 | 6.86 ± 0.50 |
| Alb (g/dL) | 4.03 ± 0.39 | 4.07 ± 0.38 | 3.88 ± 0.41** |
| TG (mg/dL) | 122 ± 69 | 124 ± 71 | 109 ± 59 |
| HDL‐C (mg/dL) | 55.4 ± 15.7 | 55.1 ± 15.7 | 56.8 ± 15.6 |
| LDL‐C (mg/dL) | 95.3 ± 24.7 | 95.3 ± 24.0 | 95.4 ± 27.5 |
| UA (mg/dL) | 5.32 ± 1.25 | 5.44 ± 1.20 | 4.76 ± 1.32** |
| Sodium (mEq/L) | 140 ± 2 | 140 ± 2 | 140 ± 2 |
| Potassium (mEq/L) | 4.40 ± 0.40 | 4.39 ± 0.40 | 4.41 ± 0.42 |
| Corrected calcium (mg/dL) | 9.19 ± 0.51 | 9.16 ± 0.53 | 9.31 ± 0.38* |
| 25‐OHD (ng/mL) | |||
| Men | 24.0 ± 7.8 | 24.6 ± 6.7 | 23.9 ± 7.9 |
| Women | 19.9 ± 7.2 | 20.8 ± 9.7 | 19.6 ± 6.5 |
Data are presented as mean ± standard deviation. *P < 0.05, **P < 0.01. Comparison of non‐sarcopenia and sarcopenia. 25‐OHD, 25‐hydroxyvitamin D; ACR, urinary albumin:creatinine ratio; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; γ‐GTP, gamma‐glutamyl transpeptidase; HbA1c, glycated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TP, total protein; TG, triglyceride; UA, uric acid.
Table 3 shows factors for the diagnosis of sarcopenia. SMI, grip strength and usual gait speed were lower in the sarcopenia group than in the non‐sarcopenia group (P < 0.01), except for usual gait speed in men (not significantly different).
Table 3.
Factors for diagnosis of sarcopenia
| Parameter | All patients | Non‐sarcopenia | Sarcopenia |
|---|---|---|---|
| Grip strength (kg) | |||
| Men | 25.5 ± 9.3 | 32.1 ± 7.2 | 22.3 ± 4.3* |
| Women | 17.9 ± 4.3 | 19.2 ± 5.1 | 13.3 ± 3.5* |
| Usual gait speed (m/s) | |||
| Men | 1.16 ± 0.25 | 1.19 ± 0.21 | 1.11 ± 0.29 |
| Women | 1.13 ± 0.29 | 1.17 ± 0.29 | 0.97 ± 0.21* |
| SMI (kg/m2) | |||
| Men | 7.37 ± 0.82 | 7.57 ± 0.72 | 6.35 ± 0.44* |
| Women | 6.21 ± 0.86 | 6.47 ± 0.78 | 5.32 ± 0.39* |
Data are presented as mean ± standard deviation. *P < 0.01. Comparison of non‐sarcopenia and sarcopenia. SMI, skeletal muscle mass index.
Figure 2 shows the correlation between SMI and BMI (Figure 2a) and age (Figure 2b). SMI showed a strong positive correlation with BMI (men R = 0.70, P < 0.001; women R = 0.68, P < 0.001). SMI decreased with age (men R = −0.22, P < 0.01; women R = −0.26, P < 0.01).
Figure 2.
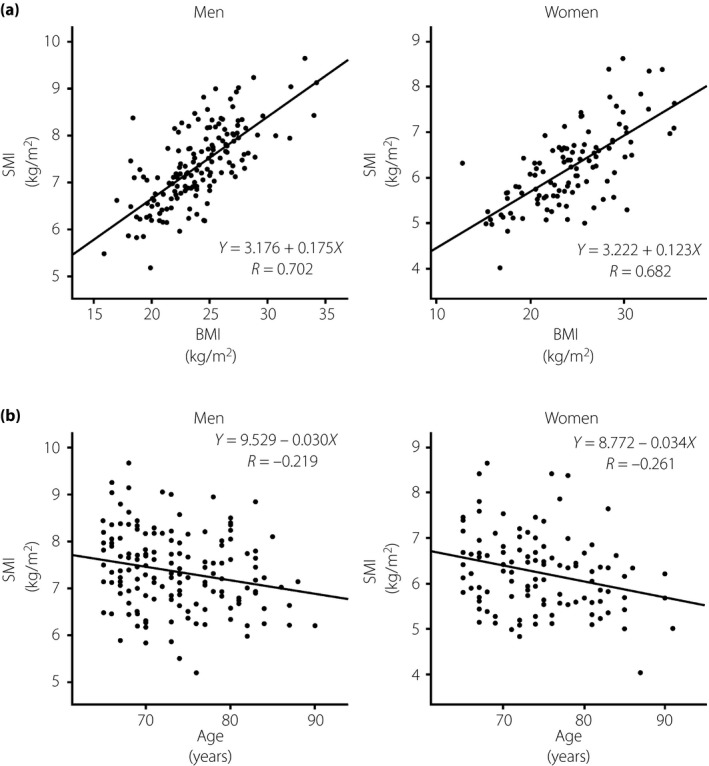
Correlation of skeletal muscle mass index (SMI) and body mass index (BMI) and age. (a) The correlation of SMI and BMI. (b) The correlation of SMI and age.
Figure 3 shows the patients divided into four groups by high and low SMI and body fat percentage. We defined group A as “appropriate physical,” group B as “obesity,” group C as “sarcopenia” and group D as “sarcopenic obesity.” The meaning divided in this way is described in the Discussion.
Figure 3.
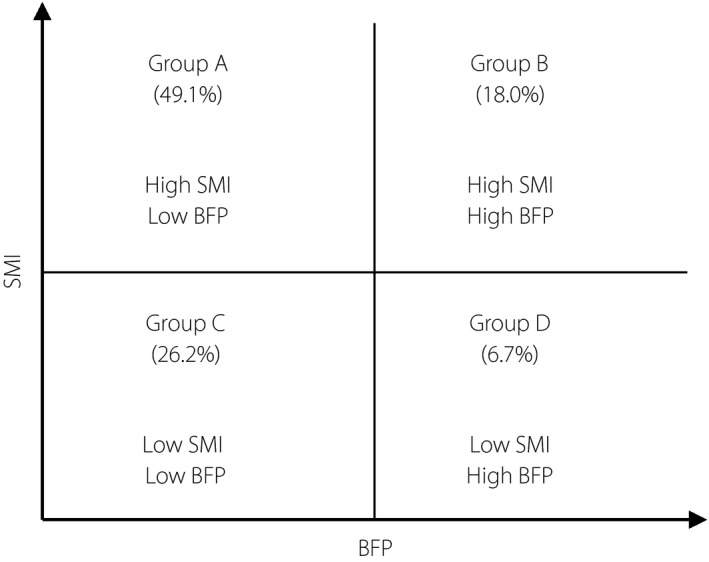
Patients divided by high and low skeletal muscle mass index (SMI) and body fat percentage (BFP). The SMI cut‐off was determined according to the Asia Working Group for Sarcopenia standards (<7.0 kg/m2 in men, <5.7 kg/m2 in women), whereas that of BFP is the fourth quartile level in the present study (>30.2% in men, >38.7% in women). The numbers show the proportion of each group.
Finally, we carried out multiple logistic regression analysis for sarcopenia in men and women. In univariate analysis, age, BMI, body fat percentage, bone mineral content, metformin use, diabetes duration and uric acid levels were significant factors in men, and age, BMI, bone mineral content, and serum levels of total protein and albumin were significant factors in women. In addition to these significant variables in univariate analysis, HbA1c was used in multiple logistic regression analysis (Table 4). In men, the prevalence of sarcopenia was correlated with lower BMI, non‐use of metformin and lower bone mineral content (P < 0.05). In women, the prevalence of sarcopenia was correlated with lower bone mineral content, lower serum levels of albumin and higher age (P < 0.05).
Table 4.
Multiple logistic regression with objective variables of sarcopenia
| Variables | Odds ratio | (95% CI) | P |
|---|---|---|---|
| Men | |||
| BMI (kg/m2) | 0.499 | (0.322–0.777) | 0.0006 |
| Metformin usage | 0.307 | (0.098–0.959) | 0.0129 |
| 10 × Bone mineral content (kg) | 0.719 | (0.545–0.950) | 0.0159 |
| Uric acid (mg/dL) | 0.665 | (0.387–1.142) | 0.1287 |
| Diabetes duration (years) | 1.045 | (0.977–1.117) | 0.1876 |
| Body fat percentage (%) | 1.102 | (0.947–1.281) | 0.1970 |
| Age (years) | 1.053 | (0.952–1.165) | 0.3150 |
| HbA1c (%) | 0.685 | (0.977–1.117) | 0.3635 |
| Women | |||
| 10 × Bone mineral content (kg) | 0.628 | (0.476–0.828) | 0.0001 |
| Albumin (g/dL) | 0.076 | (0.013–0.432) | 0.0031 |
| Age (years) | 1.150 | (1.038–1.275) | 0.0037 |
| BMI (kg/m2) | 0.899 | (0.791–1.022) | 0.0890 |
| HbA1c (%) | 0.879 | (0.539–1.433) | 0.5984 |
| Total protein (g/dL) | 0.862 | (0.212–3.502) | 0.8356 |
BMI, body mass index; CI, confidence interval; HbA1c, glycated hemoglobin.
Discussion
In the present study, the prevalence of sarcopenia among all patients was 18.7%, similar to that of a previous report after the European Working Group on Sarcopenia in Older People definition was announced. Evaluated by age, the prevalence of sarcopenia has been increasing over the past 75 years. Compared with the report that the prevalence of sarcopenia in the average 75‐year‐old Asian non‐diabetes patient was 18.8%13, the prevalence in patients aged >75 years in the present study was higher. The reduction in skeletal muscle mass with aging is expected to be greater in diabetes patients than in non‐diabetes patients, suggesting that it is a population at a high risk of sarcopenia. There have been few reports extracting diabetes for individuals aged >75 years, and future follow‐up investigations are necessary.
A BMI of ≥25 indicates obesity in Japan; especially in diabetes patients, dietary guidance is often provided to decrease bodyweight. However, there is concern that providing guidance to lose weight without considering the patient's characteristics leads to lower muscle mass and increases the patient's risk of developing sarcopenia. Based on the results of the body composition analysis, the prevalence of sarcopenia was lower as BMI levels increased. However, an evaluation of body fat percentage showed that the third quartile group (men 25.3–30.2%, women 33.1–38.7%) had the lowest prevalence of sarcopenia, whereas the fourth quartile group tended to have a higher prevalence of sarcopenia than the third group. This finding suggests that diabetes patients with a high body fat percentage and low BMI are at an increased risk of developing sarcopenia. Therefore, an evaluation of obesity in elderly diabetes patients should not be judged by BMI alone; rather, it should be considered in combination with body fat percentage. In past studies of the relationship between weight and all‐cause mortality, a too low or too high BMI and high body fat percentage were associated with increased mortality23. In the present study, BMI and SMI were strongly correlated (Figure 2a). Thus, in elderly diabetes patients, BMI might reflect lean mass. A patient with a low BMI and high body fat percentage is likely to develop sarcopenia, which can negatively influence life prognosis. When we manage the physicals of elderly diabetes patients, we can divide them into four groups by body fat percentage and SMI (Figure 3). It is important to achieve the “appropriate physical” body composition whenever possible. Patients in group B must reduce their body fat percentage while maintaining their skeletal muscle mass. Group C must increase their skeletal muscle mass. Group D must reduce their body fat percentage while also increasing their skeletal muscle mass. Nutritional intake is the most important assimilatory stimulus for skeletal muscle protein synthesis. To maintain skeletal muscle mass, a high protein diet and vitamin D intake are recommended24, 25. Furthermore, combining resistance training is considered effective for maintaining and increasing muscle mass. To reduce body fat, energy intake restriction, fat restriction and aerobic exercise are important. However, it is important not to limit protein intake to prevent skeletal muscle loss due to energy intake restriction. For elderly diabetes patients, the dietary and exercise therapy should be chosen after consideration of background factors (such as presence in group B to D) to improve patient life prognosis. Patients with sarcopenic obesity are reportedly more susceptible to death than those with sarcopenia or obesity alone8, with a high risk of insulin resistance and metabolic syndrome26. Patients in group D likely have the poorest prognosis.
The skeletal muscle mass decrease rate with aging is reportedly higher in the lower limbs than in the upper limbs27. The lower limb skeletal muscle mass declines predominantly when activity decreases due to aging. Because the lower limbs have load‐bearing joints, obese patients should theoretically maintain their lower‐limb muscle strength. Sarcopenic obesity is considered evidence that the opportunities for loading are decreasing; that is, one has an inactive lifestyle. Thus, sarcopenic obesity requires more aggressive lifestyle interventions.
The therapeutic or management approach of sarcopenia and diabetes in older adults, in particular the very old, also depends on other considerations, including the presence of multimorbidity and life expectancy. In the present study, HbA1c, an indicator of glycemic control, was not related to the prevalence of sarcopenia. There was no significant difference between the prevalence of sarcopenia and microangiopathy of diabetic retinopathy and nephropathy. Major vascular disorders, such as cerebral infarction and myocardial infarction, were similar between the two groups (Table 1). There was no significant difference in the number of oral medications and or rate of insulin use between the two groups. It is not clear why glycemic control was not poor in the sarcopenia group, but this result is important in considering the relationship between sarcopenia and diabetes mellitus. In elderly diabetes patients, interventions for hyperglycemia alone cannot prevent the development of sarcopenia associated with aging.
Multiple logistic regressions showed that the prevalence of sarcopenia was correlated with lower bone mineral content in both sexes. In sarcopenia, one's bone mineral content is often low. Thus, it is better to measure bone density in patients with sarcopenia. By doing so, it is important to manage and treat the bone mineral content to prevent reduced mobility caused by fracture and maintain the patient's daily activity. The present study measured serum 25‐OHD, which is considered important for maintaining muscle mass and strength. We found no significant intergroup differences, but women in both groups showed vitamin D insufficiency. The Akita prefecture is the region with the fewest daylight hours in Japan, and the serum 25‐OHD level measured in the present study might be the minimum value in Japanese elderly diabetes patients. Diabetes patients reportedly have decreased serum 25‐OHD levels28, 29, 30, but no difference from controls was reported in another study31; therefore, inconsistent findings have been reported. A study investigating serum 25‐OHD levels in postmenopausal women with type 2 diabetes mellitus in Japan reported that levels were <20 ng/mL in 50.0% and 20–29 ng/mL in 41.8%30. These values were similar to the mean values of the women in the present study, whereas the mean value in men was approximately 4.0 ng/mL higher than that of women regardless of sarcopenia status.
The present study had several limitations. It is undeniable that the sample number of this study was small (n = 267). The study was carried out in a single facility, and largely reflects the characteristics of the targeted group. This cross‐sectional study also had the limitation of temporal causality. Thus, future prospective studies that examine conditions such as exercise habits and meal contents are required. Patients who were unable to maintain a standing position for 1 min could not undergo a body composition analysis and could not participate in this study. Therefore, the actual prevalence of sarcopenia might be higher. The present study was cross‐sectional, and the judgment about whether metformin contributes to preventing sarcopenia requires a prospective investigation of users and non‐users. We used a height2‐adjusted skeletal muscle mass model for analysis in this study, because this model was used as an AWGS diagnostic criterion. However, a critical limitation of the height2‐adjusted model is that it results in a significant positive correlation with BMI, so that the prevalence of sarcopenia could be low in individuals with higher BMI32. Whether a BMI‐adjusted model or a weight‐adjusted model is appropriate is a subject for further study33, 34, 35.
In conclusion, the present findings suggest that diabetes patients had the greatest age‐related decreasing rate of skeletal muscle mass and were at a higher risk of sarcopenia than non‐diabetes patients. The body composition analysis results highlight the importance of evaluating the balance between SMI and body fat percentage rather than evaluating BMI alone to manage the physical of elderly diabetes patients. A low BMI and high body fat percentage tend to increase one's risk of developing sarcopenia. The bone mineral density of patients with sarcopenia is often low. Thus, is important to measure bone mineral content in elderly diabetes patients with sarcopenia, and its management might be useful for preventing mobility reductions induced by fracture.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We appreciate nurse M Nishida and K Kitabayashi for measuring body composition. This work was in part supported by funds for Research and Development Grants for Dementia from the Japan Agency for Medical Research and Development, and The Research Fund for Longevity Sciences from the National Center for Geriatrics and Gerontology.
J Diabetes Investig 2019; 10: 322–330
References
- 1. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregg EW, Mangione CM, Cauley JA, et al Diabetes and incidence of functional disability in older women. Diabetes Care 2002; 25: 61–67. [DOI] [PubMed] [Google Scholar]
- 3. Araki A, Nakano T, Oba K, et al Low well‐being, cognitive impairment and visual impairment associated with functional disabilities in elderly Japanese patients with diabetes mellitus. Geriatr Gerontol Intern 2004; 4: 15–24. [Google Scholar]
- 4. Park SW, Goodpaster BH, Strotmeyer ES, et al Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007; 30: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz AV, Hillier TA, Sellmeyer DE, et al Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002; 25: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 6. Strotmeyer ES, Cauley JA, Schwartz AV, et al Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med 2005; 165: 1612–1617. [DOI] [PubMed] [Google Scholar]
- 7. Baumgartner RN, Koehler KM, Gallagher D, et al Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 8. Atkins JL, Whincup PH, Morris RW, et al Sarcopenic obesity and risk of cardiovascular disease and mortality: a population‐based cohort study of older men. J Am Geriatr Soc 2014; 62: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanimoto Y, Watanabe M, Sun W, et al Sarcopenia and falls in community‐dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European Working Group on Sarcopenia in Older People. Arch Gerontol Geriatr 2014; 59: 295–299. [DOI] [PubMed] [Google Scholar]
- 10. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci 1995; 50: 11–16. [DOI] [PubMed] [Google Scholar]
- 11. Solini A, Penno G, Bonora E, et al Age, renal dysfunction, cardiovascular disease, and antihyperglycemic treatment in type 2 diabetes mellitus: findings from the Renal Insufficiency and Cardiovascular Events Italian Multicenter Study. J Am Geriatr Soc 2013; 61: 1253–1261. [DOI] [PubMed] [Google Scholar]
- 12. Park SW, Goodpaster BH, Strotmeyer ES, et al Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006; 55: 1813–1818. [DOI] [PubMed] [Google Scholar]
- 13. Wang CP, Hazuda HP. Better glycemic control is associated with maintenance of lower‐extremity function over time in Mexican American and European American older adults with diabetes. Diabetes Care 2011; 34: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong E, Backholer K, Gearon E, et al Diabetes and risk of physical disability in adults: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2013; 1: 106–114. [DOI] [PubMed] [Google Scholar]
- 15. Lee CG, Boyko EJ, Barrett‐Connor E, et al Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011; 34: 2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen LK, Liu LK, Woo J, et al Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 17. Correa‐de‐Araujo R, Hadley E. Skeletal muscle function deficit: a new terminology to embrace the evolving concepts of sarcopenia and age‐related muscle dysfunction. J Gerontol A Biol Sci Med Sci 2014; 69: 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamada M, Nishiguchi S, Fukutani N, et al Prevalence of sarcopenia in community‐dwelling Japanese older adults. J Am Med Dir Assoc 2013; 14: 911–915. [DOI] [PubMed] [Google Scholar]
- 19. Wang T, Feng X, Zhou J, et al Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre‐sarcopenia in Chinese elderly. Sci Rep 2016; 6: 38937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TN, Park MS, Yang SJ, et al Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010; 33: 1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 19: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Institute of Medicine . Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press, Washington D.C., 2011. [PubMed] [Google Scholar]
- 23. Padwal R, Leslie WD, Lix LM, et al Relationship among body fat percentage, body mass index, and all‐cause mortality: a cohort study. Ann Intern Med 2016; 164: 532–541. [DOI] [PubMed] [Google Scholar]
- 24. Mikanae Y, Fujita S. Role of exercise and nutrition in the prevention of sarcopenia. J Nutr Sci Vitaminol 2015; 61: S125–S127. [DOI] [PubMed] [Google Scholar]
- 25. Viljakainen HT, Palssa A, Kärkkäinen M, et al How much vitamin D3 do the elderly need? J Am Coll Nutr 2006; 25: 429–439. [DOI] [PubMed] [Google Scholar]
- 26. Kohara K. Sarcopenic obesity in aging population: current status and future directions for research. Endocrine 2014; 45: 15–25. [DOI] [PubMed] [Google Scholar]
- 27. Janssen I, Heymsfield SB, Wang ZM, et al Skeletal muscle mass and distribution in 468 men and women aged 18‐88 yr. J Appl Physiol 2000; 89: 81–88. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki A, Kotake M, Ono Y, et al Hypovitaminosis D in type 2 diabetes mellitus: association with microvascular complications and type of treatment. Endocr J 2006; 53: 503–510. [DOI] [PubMed] [Google Scholar]
- 29. Kostoglou‐Athanassiou I, Athanassiou P, Gkountouvas A, et al Vitamin D and glycemic control in diabetes mellitus type 2. Ther Adv Endocrinol Metab 2013; 4: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mori H, Okada Y, Tanaka Y. Incidence of vitamin D deficiency and its relevance to bone metabolism in Japanese postmenopausal women with type 2 diabetes mellitus. Intern Med 2015; 54: 1599–1604. [DOI] [PubMed] [Google Scholar]
- 31. Ishida H, Seino Y, Matsukura S, et al Diabetic osteopenia and circulating levels of vitamin D metabolites in type 2 (noninsulin‐dependent) diabetes. Metabolism 1985; 34: 797–801. [DOI] [PubMed] [Google Scholar]
- 32. Newman AB, Kupelian V, Visser M, et al Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003; 51: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 33. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height‐, weight‐, and body mass index‐adjusted models in assessing sarcopenia. Korean J Intern Med 2016; 31: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cawthon PM, Peters KW, Shardell MD, et al Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014; 69: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002; 50: 889–896. [DOI] [PubMed] [Google Scholar]


