Abstract
Over two billion people worldwide lack access to an improved sanitation facility that adequately retains or treats feces. This results in the potential for fecal material containing enteric pathogens to contaminate the environment, including household floors. This study aimed to assess how floor type and sanitation practices impacted the concentration of fecal contamination on household floors. We sampled 189 floor surfaces within 63 households in a peri-urban community in Iquitos, Peru. All samples were analyzed for colony forming units (CFUs) of E. coli and households were evaluated for their water, sanitation and hygiene characteristics. Results of multivariate linear regression indicated that households with improved sanitation and cement floors in the kitchen area had reduced fecal contamination to those with unimproved sanitation and dirt floors (Beta: −1.18 log10 E. coli CFU/900 cm2; 95% confidence interval [CI]: −1.77, −0.60). Households that did not versus did share their sanitation facility also had less contaminated kitchen floors (Beta: −0.65 log10 E. coli CFU/900 cm2; 95% CI: −1.15, −0.16). These findings suggest that the sanitation facilities of a home may impact the microbial load found on floors, contributing to the potential for household floors to serve as an indirect route of fecal pathogen transmission to children.
Graphical Abstract

INTRODUCTION
Diarrheal diseases are a leading cause of malnutrition and death in children under five years old, accounting for 10 percent of all deaths (approximately 760,000 children annually).1 Children living in low-income countries disproportionately suffer from malnutrition, which has been shown to increase mortality risk, affect cognitive development, increase infection risk, limit physical capacity and childbearing, and reduce adult economic productivity.2 Fecal contamination in the environment due to a lack of sanitation leads to high rates of diarrhea and is hypothesized to impact malnutrition through environmental enteropathy (EE), a condition in the gut caused by exposure to enteric pathogens that lead to alterations in intestinal structure, function, and local and systemic immune activation.3 EE is also considered to negatively impact growth. A growing body of evidence supports the contribution of environmental factors related to poor water, sanitation and hygiene conditions to stunting in children.4-6
There are many fecal-oral transmission pathways, which account for important routes of exposure for the pathogens that cause enteric infection. These pathways can broadly be categorized by the F-diagram, which depicts the concept that human-derived enteric pathogens are transmitted through food, flies, floors, fingers, and fluids.7 A lack of access to clean water is often implicated as the primary fecal-oral transmission route; however, a number of randomized, controlled trials investigating the effect of drinking water on gastrointestinal health have shown no additional benefit from point-of-use interventions.8-11 This lack of benefit from clean water is hypothesized to stem from the additive contributions of poor sanitation and hygiene, which allow for exposures through alternative fecal- oral transmission pathways and negate any potential benefit observed from improved water quality alone. In addition, a recent review of epidemiological studies on the effect of water and sanitation interventions on self-reported diarrhea episodes revealed no difference in point-of-use water interventions when blinding was taken into account.12 These studies emphasize the importance of investigating other transmission routes to understand which fecal-oral pathways pose the greatest risk for ingestion of pathogens.
One of the pathways that has not been well characterized in communities with significant fecal contamination are household floors. This transmission pathway is especially important for infants (7-12 months) who are more likely to remain indoors and spend more time playing on the floor than older children.13, 14 Younger children are also more likely to engage in object-to-mouth and hand-to-mouth activity than older children.15, 16 These behaviors, combined with a lack of immunity, render the youngest children most vulnerable to enteric infections.Despite its importance, limited research has focused on floors as a critical pathway for pathogen transmission. The few studies conducted have highlighted the importance of quantifying fecal indicator bacteria on household floors and surfaces to understand the distribution of fecal matter.17-19 One limitation of these studies is that no duplicate samples were processed at the sample collection level to understand if the fecal contamination is significantly associated with location within a household. Repeat samples are also necessary to characterize between sample variability and understand if the fecal contamination within a household is consistent or varies over time and displays a “patchiness” as has been demonstrated in quantifying bacteria in beach sands.20
Our study reports on the Escherichia coli bacteria levels of the main floor surfaces in the homes of children near Iquitos, Peru enrolled in the Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) cohort study. The use of E. coli concentration as a fecal indicator bacteria within the household has been shown to be effective in a number of studies17-19, 21 as well as at this study site in Iquitos Peru.22 The aim of this study was to compare concentrations of E. coli recovered from household floors according to characteristics of household sanitation. A secondary aim was to characterize the variability of recovery of E. coli within households.
METHODS
Study Setting and Population
This study was nested within the MAL-ED cohort in three peri-urban communities of Iquitos, Peru: Santa Clara de Nanay, Santo Tomas, and La Union (3°47’S, 73°20’W). In order to be eligible for the floor sampling study, a household had to have a child less than 48 months of age who was still enrolled in the MAL-ED study at the time of sampling. Households were enrolled in the MAL-ED study if they were located within the catchment area and had a healthy infant born during the two years of enrollment.23
Prior work has shown that these communities lag behind the rest of the Peru in terms of development indicators.24 Only 20.2 percent of the population had access to an improved sanitation facility while 58.4 percent of the overall Peru population had access. Similarly, 46.7 percent of households in the study communities had access to clean water versus 77.1 percent in all of Peru. Child growth also lagged behind with 46.3 percent of children under 5 years old being stunted versus 19.5 percent in Peru. Children under 5 years old who were reported to have diarrhea in the past week was 35.4 percent versus 13.9 percent in Peru.24 The households were low-income with the mean monthly per-capita income at $28 US dollars.25 The temperature ranges between 21.9 and 32.4 degrees Celsius with an average of 25.8 degrees Celsius.24 Rainfall is frequent and occurs throughout the year on about half of all days with the heaviest rainfall in January.24
The communities are located proximal to the Nanay River, which is a major branch of the Amazon river system. The river levels rise until March and, at the time of initiation of the study, the Nanay River was receding and no flooding was apparent within any of the households visited. There is no centralized sewerage infrastructure in the community and hence open ditches are used to drain storm and gray water away from the home. The frequent flooding in this riverine community also leads to fecal matter from latrines being released into the environment.
Classification of Floors and Sanitation Practices
During each household visit, a household questionnaire was administered in Spanish prior to floor sampling. The questionnaire was based on the Demographic and Health Surveys3 and was a shortened version of the standardized questionnaire. In addition, study staff conducted a standardized visual inspection of floor type by room within households and noted the materials used as either dirt, wood, cement or tile.
The questionnaire assessed the primary exposure variable of the type of sanitation facility used by household members and whether or not this facility was shared. The options for type of sanitation facility were: i) no facility/open field; ii) pit latrine; iii) pour flush toilet to a septic; iv) flush to somewhere else; or v) ventilated improved pit latrine. Responses to water and hygiene questions provided covariate data on the household’s primary water source, mode of water treatment, time it takes to fetch water, hygiene behavior and crowding. Information was also collected on socio-economic factors such as housing construction materials, length of tenancy, electricity access, maternal education, and monthly income. Given the propensity for households to keep free-ranging or corralled chickens in this community, participants also were interviewed regarding the presence of chickens in the home to evaluate the influence of chicken feces on the bacterial contamination of household floors.
Floor Sampling
From August to September 2015 household floors were sampled for E. coli bacteria using a modified dry electrostatic cloth method based on one designed for household settings.26 Samples were collected from highly trafficked areas, namely the household entrance and the kitchen, which has been shown to have higher levels of fecal bacteria than the bathroom areas.18, 27 These areas were also selected for high likelihood of fecal pathogen exposure for children under five years of age who spend large amounts of time in play near the entrance and near the primary caregiver engaged in cooking activities. The first area sampled was the entryway floor, typically located at the front of the house and closest to the open drains that conveyed untreated wastewater and had a tendency to overflow during periods of rainfall. The second area sampled was the kitchen floor area, typically located at the back of the house (See Figure S1, Supporting Information). If there was a latrine or toilet, it was most commonly in the back of the house, closest to the kitchen area. The kitchen area was also observed to be the area of the house where most water use and storage activities took place, creating a potentially favorable environment for bacteria. Homes were visually inspected for presence of animals inside the home at the time of sampling. Duplicate samples were taken side by side at the entryway location to investigate the heterogeneity of fecal contamination across floors. To assess the potential influence of different floor material types (e.g. dirt, wood, cement) on fecal contamination, we recorded information about the floor material types at the household entrance and in the kitchen area at the time of sampling.
Prior to field collection, sterile packets of dry electrostatic cloths (Swiffer™; Proctor & Gamble, Cincinnati, OH) were separated, quartered, and individually wrapped in autoclave paper (Fisher Scientific™, Pittsburgh, PA). Wrapped packets were then sterilized by autoclaving. For each collection an adapted protocol from Davis et al. (2012) was used where a prepared cloth was passed over a 30 cm by 30 cm floor surface with medium pressure to maximize the amount of pick-up from the surface.26 The cloth was then placed into a sterile 700 mL Whirlpak bag (Nasco, Fork Atkinson, WI) and 5 mL of sterilized Milli-Q ultra-pure water to guard against microbial desiccation during transport. Samples were stored in a cooler on ice at 4°C during field collection and transported to the fully equipped microbiologic, immunologic, and PCR based diagnostic laboratory of the Asociación Benéfica PRISMA, approximately 15km from the study site in the city center of Iquitos. Samples were processed within six hours of collection.
Microbiological evaluation
For elution, 100 ml of sterile 0.1% Peptone buffer was added into the Whirlpak bag containing the cloth and vigorously shaken for one minute. The cloth was aseptically removed and E. coli in the buffer were enumerated following USEPA Method 160428 using m-coliblue24 commercial media (HACH, Loveland, U.S.A.). Positive E. coli were identified as blue colonies. Pre, intermittent and post blanks were run to confirm the absence of cross contamination of samples. To obtain a countable number of colonies (i.e. 20–200), undilute, 10-fold, 100-fold and 1000-fold dilutions of eluate for samples collected on dirt floors and undilute, 10-fold and 100-fold for samples collected on wood and cement floors were processed, enabling a detection range of 0 to 200,000 colony forming units (CFU) per 900 cm2of floor area to be enumerated.
Data Analysis
The primary independent variable of sanitation facility was categorized into “improved” and “unimproved” sanitation facilities as defined by the Joint Monitoring Program (JMP) for Water Supply and Sanitation.29 The JMP classifies improved facilities as those that ensure hygienic separation of human excreta from human contact and include facilities that flush or pour flush to a piped sewer system, septic tank or pit latrine. Unimproved facilities on the other hand, do not ensure hygienic separation of human excreta from human contact and include pit latrines without a slab. For the purposes of this study, those households that did not have a toilet facility were also categorized as “unimproved”.
Water source, water treatment and floor type covariates were analyzed as categorical variables. A hygiene index variable score was calculated as a cumulative score from the following four questions: i) Do you wash your hands after helping your child defecate? ii) Do you wash your hands before preparing food? iii) Do you wash your hands after going to the bathroom? and iv) Do you use toilet paper?. The hygiene index score had three levels with good indicating the interviewee answered all questions as always practicing the hygienic behaviors; average indicated that for one of the four questions the interviewee only sometimes practiced the hygienic behavior; and poor indicated that for two or more questions the interviewee only sometimes practiced the hygienic behavior. E. coli concentrations were log10-transformed and reported as log10 E. coli CFU/900 cm2.
Two-sample t-tests with equal variances and Pearson Chi-squared analysis were used to compare household characteristics across improved and unimproved sanitation facility types. Unadjusted linear regression analyses were conducted to evaluate associations between water, sanitation, hygiene (WASH) and household characteristics with log10 E. coli CFU/900 cm2 in the entrance and kitchen areas. Using generalized linear models we conducted a stratified analysis by sanitation type (improved versus unimproved). For this analysis of the relation between floor types and the levels of log10 E. coli CFU in strata of household sanitation type (unimproved and improved), observations in the entrance and kitchen of each house were combined. We adjusted for potential confounding covariates in linear regression models using a backward elimination approach. A final parsimonious covariate adjustment set was selected based on considerations of sample size and the minimization of the Akaike Information Criterion (AIC).30 Interaction terms between sanitation type and floor type were included to determine if the association between sanitation and log10 E. coli CFU/900 cm2 was modified by floor type. Beta coefficients and 95% confidence intervals were estimated and represent the log10-unit change in E. coli CFU/900 cm2 per unit of each of the independent variables (household sanitation type, floor type, etc). Pearson correlation coefficients and 95% confidence intervals were calculated to estimate the variability between duplicate floor samples within the same household.
The data processing and visualization were performed in R 3.0.331 using the ggplot2 package32 and subsequent statistical analyses were conducted using Stata version 12.1 (College Station, TX).
Ethics
The study protocol and questionnaires were approved by the institutional review boards from Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) and Asociación Benéfica Proyectos de Informática, Salud, Medicina, y Agricultura (A.B. PRISMA), Iquitos, Peru. All participants gave written consent prior to household sampling.
RESULTS
Table 1 illustrates that, among 63 household visits during the study period, 189 samples were collected, representing 63 entrance floor samples, 63 additional samples (duplicates) from adjoining areas to the primary entrance floor samples, and 63 samples from the kitchen floor. There were a total of 31 households that were classified as having unimproved sanitation and 32 households with improved sanitation facilities. In the entrance area there were 36 homes with dirt floors, 3 with wood floors and 24 with cement floors. In the kitchen area there were 46 homes with dirt floors, 4 with wood floors and 13 with cement floors. One household in each category for sanitation type had ceramic tile in either the entrance and kitchen area. These households were categorized as having a cement floor for analysis due to the common composition and construction characteristics between the local tile and cement. Chickens were the dominant species typically observed in homes and all other species, (i.e. dogs and cats) were observed infrequently. There were no missing data for the log10 E. coli CFU/900 cm2 outcome variable and less than ten percent of data were missing when all variables were considered in the full model.
Table 1.
Household characteristics by sanitation type (Pearson Chi-squared tests and two-sample t-tests with equal variances performed)
| Unimproved Sanitation Facility (N=31) |
Improved Sanitation Facility (N=32) |
|
|---|---|---|
| Sanitation facility is shared (n=58) | 38.5% | 21.9% |
| Entrance floor type**: | ||
| Dirt (n=36) | 77.4% | 37.5% |
| Wood (n=3) | 3.2% | 6.3% |
| Cement (n=24) | 19.4% | 56.3% |
| Kitchen floor type*: | ||
| Dirt (n=46) | 87.1% | 59.4% |
| Wood (n=4) | 3.2% | 9.4% |
| Cement (n=13) | 9.7% | 31.3% |
| Drinking water source: | ||
| Faucet in house (n=2) | 3.3% | 3.1% |
| Public tap (n=8) | 9.7% | 15.6% |
| Community hand pump (n=44) | 71.0% | 68.8% |
| Open well (without top) (n=1) | 3.2% | 0.0% |
| Surface water (n=2) | 0.0% | 6.3% |
| Other (n=6) | 12.9% | 6.3% |
| Time to fetch water in minutes (n=62) | 8.6 (6.2, 11.1) | 5.9 (4.0, 7.7) |
| Household uses chlorine to treat water (n=63) | 25.8% | 25.0% |
| Presence of chickens in HH** (n=63) | 45.2% | 12.5% |
| Crowding (Number of people sleeping in HH/ Number of rooms) (n=62) |
1.9 (1.5, 2.4) | 1.7 (1.2, 2.2) |
| Hygiene Score: | ||
| Good (n=41) | 64.5% | 65.6% |
| Average (n=11) | 12.9% | 21.9% |
| Poor (n=11) | 22.6% | 12.5% |
| Monthly income per capita (in USD) (n=61) | 26.1 (19.8, 32.3) | 27.7 (20.2, 35.3) |
| Maternal Education (years) (n=62) | 6.6 (5.5, 7.6) | 8.1 (7.0, 9.2) |
| Electricity connection (n=62) | 77.4% | 93.5% |
| Wall type: | ||
| Wood (n=48) | 83.9% | 68.8% |
| Concrete (n=14) | 12.9% | 31.3% |
| Other (n=1) | 3.2% | 0.0% |
| Roof type: | ||
| Tin (n=60) | 93.6% | 96.9% |
| Palm (n=2) | 3.3% | 3.1% |
| Other (n=1) | 3.3% | 0.0% |
| Tenancy in household: | ||
| Less than a year (n=13) | 32.3% | 9.4% |
| Between one and five years (n=19) | 22.6% | 37.5% |
| Between five and ten years (n=14) | 25.8% | 18.8% |
| Between ten and twenty years (n=9) | 12.9% | 15.6% |
| More than twenty years (n=8) | 6.5% | 18.8% |
Significant difference at the p<0.05 level, Pearson Chi-squared
Significant difference at the p<0.01 level, Pearson Chi-squared
Figure 1 depicts the log10 E. coli CFU/900 cm2 in both the entrance and kitchen areas of the home by floor type. The entrance area of homes had an average of 3.40 log10 E. coli CFU (standard deviation=1.00) per 30 by 30 cm sample and the kitchen areas had significantly higher levels of log10 E. coli with 3.91 log10 E. coli CFU (sd=1.00) (p-value = 0.005). Within the entrance areas, dirt floors had statistically significantly higher levels of log10 E. coli CFU than cement floors (3.75 vs 2.86, p-value<0.001) and within the kitchen areas, dirt floors also had statistically significantly higher levels of log10 E. coli CFU than cement floors (4.27 vs 2.96, p-value=0.002) and wood floors (4.27 vs 2.89, p-value=0.023). Lastly, when comparing dirt floors between the entrance and kitchen areas within a household, the levels of log10 E. coli CFU in the kitchen area were statistically significantly higher than in the entrance (4.27 vs 3.75, p-value=0.013).
Figure 1.
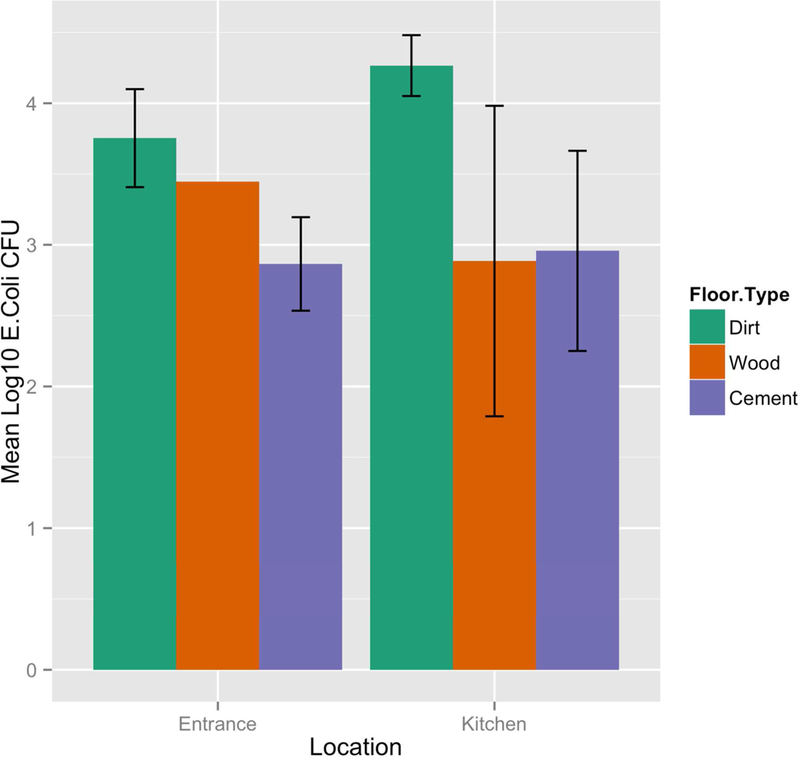
Concentrations of E.coli in entrance and kitchen by floor type (mean logi10 E. coli CFU per 900 cm2 with error bars representing 95% confidence intervals)
Household characteristic differences by sanitation type
For households with unimproved versus improved sanitation facilities, there were significant differences in household characteristics (Table 1). Households with unimproved versus improved sanitation had a higher percentage of dirt floors in both the entrance (77.4 vs 37.5, p<0.01) and kitchen (87.1 vs 59.4, p<0.05 level) and a more frequent reporting of chickens in the home (45.2 vs 12.5, p<0.01). Households with improved versus unimproved sanitation had a higher percentage of cement floors in both the entrance (56.3 vs 19.4, p<0.01) and kitchen (31.3 vs 9.7, p<0.05). There were no significant differences across sanitation type for other household WASH characteristics such as sharing sanitation facilities, type water connection, time to fetch water, household chlorine use to treat drinking water, crowding, income, education, electricity connection, wall and roof type and tenancy in the house.
Unadjusted analysis of household WASH characteristics and E. coli levels on floors
Linear regression models comparing individual household WASH characteristics and the levels of log10 E. coli CFU demonstrated significant associations in both the entrance and kitchen areas (Table 2). Households with improved sanitation had lower levels of log10 E. coli CFU/900 cm2 on floors when compared to homes with unimproved sanitation in both the entrance and kitchen (Beta: −0.63 (95% CI: −1.12, −0.15); and Beta: −0.80 (95% CI: −1.27, −0.33) respectively). For shared sanitation, households that reported not sharing their sanitation facility versus those did share had lower levels of log10 E. coli CFU/900 cm2 (Beta: −0.70; 95% CI: −1.27, −0.13) in the kitchen area. Household entrance and kitchen areas with cement floors also had lower levels of log10 E. coli CFU/900 cm2 when compared to household entrance and kitchen areas with dirt floors (Beta: −0.89 (95% CI: −1.38, −0.40); and Beta: −1.31 (95% CI: −1.83, −0.79) respectively). For every additional minute that interviewees reported needing to fetch water, corresponding increases in the concentrations of log10 E. coli CFU/900 cm2 on entrance floors (Beta: 0.06; 95% CI: 0.02, 0.10) and kitchen floors (Beta: 0.05; 95% CI: 0.01, 0.09) were observed. Table 2 illustrates that wall type, crowding, electricity access, maternal education and housing tenancy were independently associated with increases in log10 E. coli CFU/900 cm2.
Table 2.
Relation of household characteristics with log10 E. coli CFU per 900 cm2 in entrance and kitchen areas
| Entrance Log10 E. coli CFU/900cm2 Beta1 (95% CI) |
Kitchen Log10 E. coli CFU/900cm2 Beta (95% CI) |
|
|---|---|---|
| Sanitation Type: | ||
| Unimproved (n=31) | REF | REF |
| Improved (n=32) | −0.63 (−1.12, −0.15)** | −0.80 (−1.27, −0.33)† |
| Shared Sanitation Facility: | ||
| Shared (n=17) | REF | REF |
| Unshared (n=41) | −0.53 (−1.09, 0.03) | −0.70 (−1.27, −0.13)* |
| Floor Type (Entrance, Kitchen): | ||
| Dirt (n=36, n=46) | REF | REF |
| Wood (n=3, n=4) | −0.31 (−1.42, 0.81) | −1.38 (−2.27, −0.51)** |
| Cement (n=24, n=13) | −0.89 (−1.38, −0.40)† | −1.31 (−1.83, −0.79)†† |
| Drinking water source: | ||
| Community hand pump (n=44) | REF | REF |
| Faucet in house (n=2) | −0.04 (−1.54, 1.46) | 1.10 (−0.36, 2.56) |
| Public tap (n=3) | 0.33 (−0.47, 1.13) | 0.003 (−0.77, 0.78) |
| Open well (without bottom) (n=1) | −0.43 (−2.53, 1.67 | −1.05 (−3.09, 1.00) |
| Surface water (n=2) | 0.38 (−1.12, 1.89) | −0.46 (−1.92, 1.00) |
| Other (n=6) | 0.22 (−0.68, 1.13) | 0.48 (−0.40, 1.36) |
| Time to fetch water in minutes (n=62) | 0.06 (0.02, 0.10)** | 0.05 (0.01, 0.09)* |
| Household uses chlorine to treat water: | ||
| No (n=47) | REF | REF |
| Yes (n=16) | 0.08 (−0.51, 0.66) | −0.004 (−0.59, 0.59) |
| Presence of chickens in HH: | ||
| Yes (n=18) | REF | REF |
| No (n=45) | −0.38 (−0.93, 0.18) | −0.53 (−1.08, 0.02) |
| Crowding (Number of people sleeping in HH/ Number of rooms) (n=62) |
0.22 (0.02, 0.42)* | 0.16 (−0.04, 0.36) |
| Hygiene Score: | ||
| Good (n=41) | REF | REF |
| Average (n=11) | 0.26 (−0.43,0.95) | 0.10 (−0.59, 0.79) |
| Poor (n=11) | 0.18 (−0.51, 0.87) | 0.39 (−0.30, 1.08) |
| Monthly income per capita (in USD) (n=61) | 0.002 (−0.01, 0.02) | −0.0004 (−0.01, 0.01) |
| Maternal Education (years) (n=62) | −0.09 (−0.17, −0.01)* | −0.04 (−0.13, 0.04) |
| Electricity connection: | ||
| Yes (n=53) | REF | REF |
| No (n=9) | 0.78 (0.07, 1.49)* | 0.67 (−0.05, 1.39) |
| Wall type: | ||
| Wood (n=48) | REF | REF |
| Concrete (n=14) | −0.88 (−1.45, −0.31)** | −1.05 (−1.61, −0.52)†† |
| Roof type: | ||
| Tin (n=60) | REF | REF |
| Palm (n=2) | 1.16 (−0.26, 2.58) | 0.41 (−1.04, 1.85) |
| Tenancy in household: | ||
| Less than a year (n=13) | REF | REF |
| Between one and five years (n=19) | −0.72 (−1.44, −0.01)* | −0.40 (−1.13, 0.32) |
| Between five and ten years (n=14) | −0.78 (−1.54, −0.02)* | −0.76 (−1.53, 0.02) |
| Between ten and twenty years (n=9) | −0.49 (−1.35, 0.37) | −0.33 (−1.21, 0.54) |
| More than twenty years (n=8) | −0.86 (−1.74, 0.03) | −0.61 (−1.52, 0.30) |
Significance at the p<0.05 level
Significance at the p<0.01 level
Significant difference at the p<0.001 level
Significant difference at the p<0.0001 level
The beta coefficient represents the log10-unit change in E. coli CFU/900 cm2 between the exposed and unexposed (REF) categories. For the continuous independent variables the beta coefficient represents the log10-unit change in E. coli per increase in a unit change of the variable.
To further understand the relationship between floor type and sanitation type, the stratified data by sanitation type (improved versus unimproved) are shown in Figure 2. The lowest log10 E. coli CFU/900 cm2 were found in the homes with both improved sanitation and improved floor types (defined by their ability to be disinfected such that wood and cement floors are combined into the improved category and dirt as unimproved). The reduction in log10 E. coli CFU/900 cm2 among households with unimproved sanitation was −0.60 (95% CI: −1.03, −0.17) when comparing wood or cement (improved) floors to dirt floors (unimproved). An even greater reduction of −1.17 log10 E. coli CFU/900 cm2 (95% CI: −1.68, - 0.66) was observed among households with improved sanitation when comparing wood or cement floors to dirt floors (Table 3).
Figure 2.
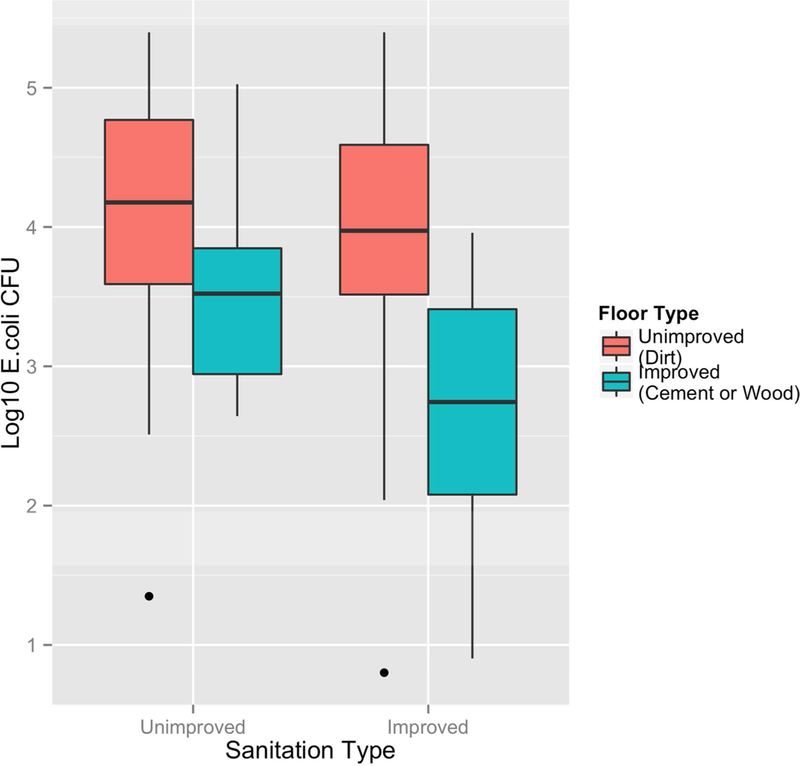
Log10 E. coli CFU per 900 cm2 by sanitation and floor type
Table 3.
Relation between floor type and log10 E. coli CFU per 900 cm2 by sanitation type
| Improved Sanitation Type2 (n=64) |
Unimproved Sanitation Type3 (n=62) |
|
|---|---|---|
| Floor Type1: | ||
| Unimproved | REF (n=31) | REF (n=51) |
| Improved | −1.17 (−1.68, −0.66)†† (n=32) | −0.60 (−1.03, −0.17)** (n=11) |
Improved floor type is classified as either cement or wood and unimproved as dirt.
Among homes with improved sanitation, Beta 0 for dirt floors = 3.90 log10 E. coli CFU/900 cm2 versus 2.74 log10 E. coli CFU/900 cm2 for cement or wood floors
Among homes with unimproved sanitation, Beta 0 for dirt floors = 4.12 log10 E. coli CFU/900 cm2 versus 3.52 log10 E. coli CFU/900 cm2 for cement or wood floors
Significance at the p<0.01 level
Significant difference at the p<0.0001 level
Adjusted analysis of household WASH characteristics and E. coli levels on floors
Two multivariate linear regression models were run for the entrance and kitchen floor areas with predictor variables that included both the sanitation type (improved or unimproved) as an interaction term with floor type and the variable for whether the sanitation facility was shared (Table 4). The models adjusted for time to fetch water, presence of chickens in the household, crowding, maternal education and wall type. For the entrance floor area, households with improved sanitation and cement floors had lower log10 E. coli CFU/900 cm2 on their floors when compared to households with unimproved sanitation and dirt floors (Beta: - 0.43; 95% CI: −1.08, 0.21). For the kitchen floor area, households with unimproved sanitation and wood floors and households with improved sanitation and cement floors both had statistically significantly lower log10 E. coli CFU/900 cm2 on their floors when compared to households with unimproved sanitation and dirt floors (Beta: −2.36 (95% CI: −3.86, −0.86) and (Beta: −1.18 (95% CI: −1.77, −0.60) respectively). Households that did not share their sanitation facility also had significantly reduced log10 E. coli CFU/900 cm2 on their kitchen floors (Beta: - 0.65; 95% CI: −1.15, −0.16) when compared to kitchen floors in households that did share their sanitation facility. The significant covariates in the adjusted model for the kitchen area included lack of chickens in the household (Beta: −0.63; 95% CI: - 1.12, −0.15; indicating lower log10 E. coli CFU/900 cm2 for those without versus with a presence of chickens) and maternal education (Beta: −0.08; 95% CI: (−0.15, - 0.004; indicating lower log10 E. coli CFU/900 cm2 in homes for every year increase in of education). The significant covariates in the adjusted model for the entrance area, were time to fetch water (Beta: 0.05; 95% CI: 0.003, 0.09; indicating higher log10 E. coli CFU/900 cm2 for every minute increase in time to fetch water) and maternal education (Beta: −0.10; 95% CI: −0.19, 0.00; indicating lower log10 E. coli CFU/900 cm2 for every year increase in of education).
Table 4.
Adjusted regression model of household characteristics with log10 E. coli CFU per 900 cm2 in entrance and kitchen areas (models adjust for time to fetch water, presence of chickens in the household, crowding, maternal education and wall type)
| Entrance Log10 E. coli CFU |
Kitchen Log10 E. coli CFU |
|||||
|---|---|---|---|---|---|---|
| N | 56 | 56 | ||||
| R-Squared (Adjusted R-squared) | 0.392 (0.241) | 0.651 (0.564) | ||||
| β (SE) | 95% CI | p-value | β (SE) | 95% CI | p-value | |
| Primary independent variables: | ||||||
| Sanitation Type with Floor Type: | ||||||
| Unimproved with Dirt | REF | REF | REF | REF | REF | REF |
| Unimproved with Wood | −1.13 (0.92) | (−2.99, 0.74) | 0.230 | −2.36 (0.75) | (−3.86, −0.86) | 0.003 |
| Unimproved with Cement | −0.51 (0.57) | (−1.66, 0.64) | 0.372 | 0.40 (0.52) | (−0.65, 1.45) | 0.444 |
| Improved with Dirt | 0.45 (0.36) | (−0.28, 1.18) | 0.271 | 0.32 (0.26) | (−0.20, 0.83) | 0.222 |
| Improved with Wood | 0.25 (0.69) | (−1.14, 1.64) | 0.721 | −0.74 (0.47) | (−1.68, 0.20) | 0.119 |
| Improved with Cement | −0.43 (0.32) | (−1.08, 0.21) | 0.183 | −1.18 (0.29) | (−1.77, −0.60) | <0.001 |
| Shared Sanitation Facility: | ||||||
| Shared | REF | REF | REF | REF | REF | REF |
| Unshared | −0.40 (0.31) | (−1.02, 0.22) | 0.203 | −0.65 (0.25) | (−1.15, −0.16) | 0.011 |
| Adjustment covariates: | ||||||
| Time to fetch water in minutes | 0.05 (0.02) | (.003, 0.09) | 0.038 | 0.03 (0.02) | (−0.002, 0.07) | 0.063 |
| Presence of chickens in HH: | ||||||
| Yes | REF | REF | REF | REF | −− | −− |
| No | −0.63 (0.24) | (−1.12, −0.15) | 0.185 | −0.63 (0.24) | (−1.12, −0.15) | 0.012 |
| Crowding | −0.06 (0.13) | (−0.32, 0.19) | 0.622 | −0.17 (0.10) | (−0.37, 0.02) | 0.084 |
| Maternal education (years) | −0.10 (0.05) | (−0.19, 0.00) | 0.048 | −0.08 (0.04) | (−0.15,−0.004) | 0.040 |
| Wall type: | ||||||
| Wood | REF | REF | REF | REF | REF | REF |
| Concrete | −0.11 (0.35) | (−0.81, 0.60) | 0.766 | −0.33 (0.25) | (−0.84, 0.18) | 0.198 |
Variability of recovery of E. coli within households
For the entrance area where side-by-side samples were collected to understand the distribution of E. coli bacteria across floor surfaces, the Pearson correlation coefficient between the initial and duplicate samples was 0.83 with a p-value < 0.001 (n=63) (See Figure S2, Supporting Information). The 95% confidence interval for the Pearson correlation coefficient ranged from 0.73 to 0.89 indicating a homogenous spread of bacteria across the sampling area.
DISCUSSION
This study found evidence that household floors carried differential loads of fecal contamination depending on the type of sanitation facility and whether or not that sanitation facility was shared. The kitchen area had a higher level of E. coli than the entryway, which is consistent with previous studies that reported that the kitchen area is the location of greatest contamination.17, 25 Additionally, the kitchen areas of these households were most commonly in the back, in closest proximity to the sanitation facility (if sanitation facilities were onsite) (See Figure S1, Supporting Information). This makes the kitchen area the most likely first point of contact for a household member after defecation and may therefore increase the bacterial loads within this area of the house. Homes with dirt floors were also found to have higher levels of bacteria than homes with cement floors. This suggests that changing the type of household flooring from dirt to cement, which can be more easily disinfected, may interrupt transmission of fecal pathogens and protect infants from these exposures supporting the finding from a previous intervention that replacing dirt floors with cement floors may significantly improve child health.33
The sanitation facility was the household characteristic found to have the most significant and consistent relationship with the levels of bacteria on kitchen floors. These findings support the potential for sanitation interventions targeting hygienic containment of human waste to reduce exposures to fecal pathogens in the home. In the study communities, a flush toilet to a septic was a more hygienic sanitation option than the pit latrine, which was simply a hole in the ground (either covered or uncovered). Those who shared sanitation facilities were also more likely to have floors contaminated with E. coli in the kitchen area. This provides evidence in support of the definition for “shared” sanitation facilities being characterized as “unimproved” by JMP. The underlying assumption by the JMP that there is little commitment or incentive for users to keep a shared facility clean may in fact hold true in this community despite contrary evidence in other settings.17
Among homes with the same sanitation type, there was a reduction in fecal contamination when comparing unimproved (dirt) to improved (either wood or cement) floors however, the magnitude of reduction was greater among homes with improved sanitation. Interestingly, the reduction of fecal contamination was not as large with only one of the two fecal-oral transmission pathways was interrupted (improved sanitation or an improved floor). This highlights the importance of interrupting additional fecal-oral transmission pathways, such as floors, during a sanitation intervention to most effectively reduce exposures to fecal pathogens in the home
This study also found that the presence of chickens in homes significantly increased the E. coli contamination on floors. Similar to people, either pathogenic or commensal E. coli can be identified in the chicken gastrointestinal tract, and chickens can be either asymptomatic carriers or exhibit disease.31 Study staff frequently observed the presence of chicken droppings on surfaces in the home when chickens were present, suggesting the potential for direct fecal contamination from the birds.
This was the first study to use a dry electrostatic cloth as the sampling method for E. coli on floor surfaces in low-resourced settings. Other studies that sampled for E. coli either collected soil or used a cotton swab. One study in Tanzania examined household floors across different locations in the home by quantifying the number of E. coli from a layer of soil 10 cm by 10 cm by 1 cm thick.18 Another study in Cambodia sampled the floor surface around the base of household latrine and a floor surface near the kitchen sink using a swab method over the sample surface of 4 cm2.19 In comparison to these studies, the concentrations of E. coli contamination found of the dirt floors of these Peruvian homes were approximately 5 to 80 times more contaminated. 17,18 This may be due to the efficiency of the sampling method used or may additionally or alternately reflect a higher typical bacterial load among homes in this community. The climate in the Peruvian Amazon provided an ideal environment for Gram-negative bacteria with consistently hot and humid weather year round and regular precipitation with dark and shady spaces inside the houses. Dirt floors in homes further enable bacterial survival and are difficult to disinfect due to the organic material and complex matrix. Therefore, fecal pathogens that reach household floors have a high chance for survival in the environment with increased potential for transmission to children.
This study also found evidence for the consistency in the contamination of floors across the entrance floor area as evidenced by the side-by-side sampling. This finding enhances confidence that the concentrations of E. coli measured on the entrance and kitchen floors represent a spatially-typical exposure for children in those locations. It also highlights the utility of the use of a dry electrostatic cloth sampling method as reproducible. Previous research on beach sand contamination found that on a micro-spatial scale, fecal indicator bacteria can vary greatly over short distances.20 The strong correlations between the side-by-side measurements taken on the entrance floors suggest that the E. coli are evenly distributed across locations within households and these bacteria are significantly associated with that location within the household.
The main limitation of this study was that E. coli is an indicator organism for fecal contamination and may have limited accuracy for determining the presence of pathogens.35 E. coli represents a large group of fecal bacteria from both human and animal sources and may come from relatively low-risk sources of fecal pollution.36 Many E. coli are commensal, while other more pathogenic species, such as enteroviruses, norovirus, Cryptosporidium spp. and Giardia spp., have different survival rates in the environment than E. coli.37, 38 Therefore, the presence virulent strains or other pathogenic microbes may or may not be accurately indicated by the detection of E. coli. Additionally, given the finding of an association with chickens and fecal contamination on floors, future studies should incorporate fecal contamination from all animal sources. The strengths of the study were that it used a novel sampling technique of the dry electrostatic cloth with high recovery efficiencies during the elution process. As the evidence base increases for the importance of the floors pathway, this study highlights the need for rigorous methodological evaluation of household bacterial sampling strategies and methods in the context of environmental enteropathy. Another strength of the study was the analysis of within sample variability. This analysis showed high correlations between samples taken side-by-side and therefore increased confidence that the fecal contamination measured in this study is an accurate reflection of the levels of microbial pressure for that location within the home.
This study demonstrated that household floors are a potential pathway for transmission of fecal pathogens and demonstrated that households with unimproved sanitation facilities and shared facilities had higher loads of E. coli bacteria. The high loads of E. coli bacteria suggest that this route of exposure is especially important for children less than 12 months of age who spend most of their time on the floor and partake in hand-to-mouth activity. These results suggest that interventions, such as covering dirt floors with cement and excluding chickens from contact with surfaces in the home, hold promise to reduce chronic exposure to fecal pathogens that may be implicated in diseases such as environmental enteropathy. This study also highlights the importance of a multidisciplinary approach to the reduction of fecal contamination that extends current drinking water interventions to interrupt the transmission of pathogens in the environment by other pathways.
Supplementary Material
ACKNOWLEDMENTS
We would like to thank the families of the community of Santa Clara enrolled in the MAL-ED study for allowing us to visit and collect the samples we have analyzed here, as well as to all of the members of the PRISMA team working with JHSPH in Iquitos. We would also like to thank the members of the National Science Foundation Integrative Graduate Education and Research Traineeship (IGERT) team who generated pilot data in the summer of 2014, Christopher Kelley and Douglas Findeisen. The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the National Institutes of Health, Fogarty International Center. While conducting this work, NGE was supported by The NSF IGERT Grant 1069213, The Osprey Foundation of Maryland Grant 1602030014, the Johns Hopkins Water Institute, Johns Hopkins Fisher Center Discovery Program Grant 010KOS2015, The Kazuyoshi Kawata fund in Sanitary Engineering and Science, and the Dr. C. W. Kruse Memorial Fund Scholarship. KJS received funding from The Bill and Melinda Gates Foundation Grant 1607050014, MWH-Global Grant 90055959, The Osprey Foundation of Maryland Grant 1602030014, NIEHS Grant R01ES025135, and NIH (Grant Pending). MFD received funding from NIH Grant 1K010D019918–01A1, Johns Hopkins NIOSH Education and Research Center Pilot Award, Morris Animal Foundation Pilot Award Grant D15CA-802, Faculty Innovation Award, AKC Canine Health Foundation Grant CHF02241, Johns Hopkins Fisher Center Discovery Program Grant 004MAT2014. CDH received funding from NIOSH K01 Grant 1K010H010193–01A1, E.W. “Al” Thrasher Award 10287, and NSF-NIH-USDA Ecology and Evolution of Infectious Diseases program Grant 1316318. MPO, PPY and MNK received funding from the NIH- NIDDK, NCI and The Bill and Melinda Gates Foundation. MNK received funding from the Center for Global Health of Johns Hopkins University and Johns Hopkins Fisher Center Discovery Program Grant 010K0S2015.
Footnotes
Supporting Information
Details on the floor plan of a typical household in the study communities and a graph of the Pearson correlation coefficient of log10 E. coli CFU per 900 cm2 from entrance floor duplicate samples taken side by side.
REFERENCES
- 1.Liu L; Johnson HL; Cousens S, Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000 (vol 379, pg 2151, 2012). Lancet 2012, 380, (9850), 1308–1308. [DOI] [PubMed] [Google Scholar]
- 2.Black RE; Allen LH; Bhutta ZA; Caulfield LE; de Onis M; Ezzati M; Mathers C; Rivera J; Maternal; Child Undernutrition Study, G., Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008, 371, (9608), 243–60. [DOI] [PubMed] [Google Scholar]
- 3.Kosek M; Guerrant RL; Kang G; Bhutta Z; Yori PP; Gratz J; Gottlieb M; Lang D; Lee G; Haque R; Mason CJ; Ahmed T; Lima A; Petri WA; Houpt E; Olortegui MP; Seidman JC; Mduma E; Samie A; Babji S; Investigators M-EN, Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis 2014, 59 Suppl 4, S239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rah JH; Cronin AA; Badgaiyan B; Aguayo VM; Coates S;Ahmed S, Household sanitation and personal hygiene practices are associated with child stunting in rural India: a cross-sectional analysis of surveys. BMJ open 2015, 5, (2), e005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickering AJ; Djebbari H; Lopez C; Coulibaly M; Alzua ML, Effect of a community-led sanitation intervention on child diarrhoea and child growth in rural Mali: a cluster-randomised controlled trial. Lancet Glob Health 2015, 3, (11), e701–11. [DOI] [PubMed] [Google Scholar]
- 6.Ngure FM; Reid BM; Humphrey JH; Mbuya MN; Pelto G; Stoltzfus RJ, Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Annals of the New York Academy of Sciences 2014, 1308, 118–28. [DOI] [PubMed] [Google Scholar]
- 7.Kawata K, Water and other environmental interventions--the minimum investment concept. Am J Clin Nutr 1978, 31, (11), 2114–23. [DOI] [PubMed] [Google Scholar]
- 8.Austin C, Investigation of in-house water chlorination and its effectiveness for rural areas of the Gambia [dissertation]. New Orleans: Tulane University School of Public Health and Tropical Medicine 1993. [Google Scholar]
- 9.Clasen T; Pruss-Ustun A; Mathers CD; Cumming O; Cairncross S; Colford JM Jr., Estimating the impact of unsafe water, sanitation and hygiene on the global burden of disease: evolving and alternative methods. Tropical Medicine & International Health 2014, 19, (8), 884–893. [DOI] [PubMed] [Google Scholar]
- 10.Colford JM Jr.; Wade TJ; Sandhu SK; Wright CC; Lee S; Shaw S; Fox K; Burns S; Benker A; Brookhart MA; van der Laan M; Levy DA, A randomized, controlled trial of in-home drinking water intervention to reduce gastrointestinal illness. Am J Epidemiol 2005, 161, (5), 472–82. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhoff LV; McClelland KE; Do Carmo Pinho M; Araujo JG; De Sousa MA; Guerrant RL, Feasibility and efficacy of in-home water chlorination in rural North-eastern Brazil. JHyg (Lond) 1985, 94, (2), 173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engell RE; Lim SS, Does clean water matter? An updated metaanalysis of water supply and sanitation interventions and diarrhoeal diseases. The Lancet 2013, 381, S44. [Google Scholar]
- 13.Black K; Shalat SL; Freeman NC; Jimenez M; Donnelly KC; Calvin JA, Children’s mouthing and food-handling behavior in an agricultural community on the US/Mexico border. J Expo Anal Environ Epidemiol 2005, 15, (3), 244–51. [DOI] [PubMed] [Google Scholar]
- 14.Teunis PF; Reese HE; Null C; Yakubu H; Moe CL, Quantifying Contact with the Environment: Behaviors of Young Children in Accra, Ghana. The American journal of tropical medicine and hygiene 2016, 94, (4), 920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsou MC; Ozkaynak H; Beamer P; Dang W; Hsi HC; Jiang CB; Chien LC, Mouthing activity data for children aged 7 to 35 months in Taiwan. J Expo Sci Environ Epidemiol 2015, 25, (4), 388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue J; Zartarian V; Tulve N; Moya J; Freeman N; Auyeung W; Beamer P, A meta-analysis of children’s object-to-mouth frequency data for estimating non-dietary ingestion exposure. J Expo Sci Environ Epidemiol 2010, 20, (6), 536–45. [DOI] [PubMed] [Google Scholar]
- 17.Exley JL; Liseka B; Cumming O; Ensink JH, The sanitation ladder, what constitutes an improved form of sanitation? Environ Sci Technol 2015, 49,(2), 1086–94. [DOI] [PubMed] [Google Scholar]
- 18.Pickering AJ; Julian TR; Marks SJ; Mattioli MC; Boehm AB; Schwab KJ; Davis J, Fecal Contamination and Diarrheal Pathogens on Surfaces and in Soils among Tanzanian Households with and without Improved Sanitation. Environ Sci Technol 2012, 46, (11), 5736–5743. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair RG; Gerba CP, Microbial contamination in kitchens and bathrooms of rural Cambodian village households. Lett ApplMicrobiol 2011, 52, (2), 144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonilla TD; Nowosielski K; Cuvelier M; Hartz A; Green M; Esiobu N; McCorquodale DS; Fleisher JM; Rogerson A, Prevalence and distribution of fecal indicator organisms in South Florida beach sand and preliminary assessment of health effects associated with beach sand exposure. Mar Pollut Bull 2007, 54, (9), 1472–82. [DOI] [PubMed] [Google Scholar]
- 21.Stauber CE; Walters A; Fabiszewski de Aceituno AM; Sobsey MD, Bacterial contamination on household toys and association with water, sanitation and hygiene conditions in Honduras. International journal of environmental research and public health 2013, 10, (4), 1586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julian TR; MacDonald LH; Guo Y; Marks SJ; Kosek M; Yori PP; Pinedo SR; Schwab KJ, Fecal indicator bacteria contamination of fomites and household demand for surface disinfection products: a case study from Peru. The American journal of tropical medicine and hygiene 2013, 89, (5), 869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Investigators M-EN, The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis 2014, 59 Suppl 4, S193–206. [DOI] [PubMed] [Google Scholar]
- 24.Yori PP; Lee G; Olortegui MP; Chavez CB; Flores JT; Vasquez AO; Burga R; Pinedo SR; Asayag CR; Black RE; Caulfield LE; Kosek M, Santa Clara de Nanay: the MAL-ED cohort in Peru. Clin Infect Dis 2014, 59 Suppl 4, S310–6. [DOI] [PubMed] [Google Scholar]
- 25.Kosek M; Yori PP; Pan WK; Olortegui MP; Gilman RH; Perez J; Chavez CB; Sanchez GM; Burga R; Hall E, Epidemiology of highly endemic multiply antibiotic-resistant shigellosis in children in the Peruvian Amazon. Pediatrics 2008, 122, (3), e541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MF; Baron P; Price LB; Williams DL; Jeyaseelan S; Hambleton IR; Diette GB; Breysse PN; McCormack MC, Dry collection and culture methods for recovery of methicillin-susceptible and methicillin- resistant Staphylococcus aureus strains from indoor home environments. Applied and environmental microbiology 2012, 78, (7), 2474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusin P; Orosz-Coughlin P; Gerba C, Reduction of faecal coliform, coliform and heterotrophic plate count bacteria in the household kitchen and bathroom by disinfection with hypochlorite cleaners. J Appl Microbiol 1998, 85, (5), 819–28. [DOI] [PubMed] [Google Scholar]
- 28.USEPA, Method 1604: Total Coliforms and Escherichia coli in water by membrane filtration using a simultaneous detection technique (MI Medium). In USEPA, Ed. Washington, DC, 2002. [Google Scholar]
- 29.UNICEF W, WHO Joint Monitoring Programme for Water Supply and Sanitation. Progress on drinking water and sanitation 2012. [Google Scholar]
- 30.Akaike H, A new look at the statistical model identification. Automatic Control, IEEE Transactions on 1974, 19, (6), 716–723. [Google Scholar]
- 31.Team RC, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2013. [Google Scholar]
- 32.Wickham H, ggplot2: elegant graphics for data analysis. Springer Science & Business Media: 2009. [Google Scholar]
- 33.Cattaneo MD; Galiani S; Gertler PJ; Martinez S; Titiunik R, Housing, health, and happiness. American Economic Journal: Economic Policy 2009, 75–105. [Google Scholar]
- 34.Alexander D; Calnek B; Barnes H; Beard C; McDougald L, Diseases of poultry. Diseases of poultry 2003, 11. [Google Scholar]
- 35.Abdelzaher AM; Wright ME; Ortega C; Solo-Gabriele HM; Miller G; Elmir S; Newman X; Shih P; Bonilla JA; Bonilla TD, Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Applied and environmental microbiology 2010, 76, (3), 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boehm AB; Ashbolt NJ; Colford JM Jr.; Dunbar LE; Fleming LE; Gold MA; Hansel JA; Hunter PR; Ichida AM; McGee CD; Soller JA; Weisberg SB, A sea change ahead for recreational water quality criteria. J Water Health 2009, 7, (1), 9–20. [DOI] [PubMed] [Google Scholar]
- 37.Feachem R; Mara DD; Bradley DJ, Sanitation and disease. John Wiley & Sons; Washington DC, USA: 1983. [Google Scholar]
- 38.Schwab KJ, Are existing bacterial indicators adequate for determining recreational water illness in waters impacted by nonpoint pollution? Epidemiology 2007,18,(1), 21–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


