Abstract
The appropriate spatial and temporal regulation of canonical Wnt signaling is vital for eye development. However, the literature often conflicts on the distribution of canonical Wnt signaling in the eye. Here, using a sensitive mouse transgenic reporter line, we report a detailed re-evaluation of the spatiotemporal dynamics of canonical Wnt signaling in the developing eye. Canonical Wnt activity was dynamic in the optic vesicle and later in the retina, while it was absent from the ectodermal precursors of the lens and corneal epithelium. However, later in corneal development, canonical Wnt reporter activity was detected in corneal stroma and endothelium precursors as they form from the neural crest, although this was lost around birth. Interestingly, while no canonical Wnt signaling was detected in the corneal limbus or basal cells at any developmental stage, it was robust in adult corneal wing and squamous epithelial cells. While canonical Wnt reporter activity was also absent from the postnatal lens, upon lens injury intended to model cataract surgery, it upregulated within 12 hours in remnant lens epithelial cells, and co-localized with alpha smooth muscle actin in fibrotic lens epithelial cells from 48 hours post-surgery onward. This pattern correlated with downregulation of the inhibitor of canonical Wnt signaling, Dkk3. These data demonstrate that canonical Wnt signaling is dynamic within the developing eye and upregulates in lens epithelial cells in response to lens injury. As canonical Wnt signaling can collaborate with TGFβ to drive fibrosis in other systems, these data offer the first evidence in a lens-injury model that canonical Wnt may synergize with TGFβ signaling to drive fibrotic posterior capsular opacification (PCO).
Introduction
Precise control of canonical Wnt signaling is necessary for ocular morphogenesis (Fujimura, 2016). Early in development, suppression of canonical Wnt signaling in the head ectoderm is necessary for lens placode specification (Kreslova et al., 2007; Smith et al., 2005). Similarly, canonical Wnt signaling in the dorsal optic cup is necessary for retinal pigmented epithelial commitment (Hagglund et al., 2013), while canonical Wnt signaling at the margin of the optic cup is necessary to repress neural retina differentiation allowing for the morphogenesis of the ciliary body/iris (Esteve et al., 2011; Liu et al., 2007). Later in eye development, canonical Wnt signaling has been reported to influence the development of many ocular cell types including the ocular neural crest (Zacharias and Gage, 2010), corneal keratocytes (Zhang et al., 2015), corneal limbus/epithelium (Han et al., 2014; Lee et al., 2017), retinal amacrine cells (Liu et al., 2006; Sukhdeo et al., 2014), retinal ganglion cells (Liu et al., 2006; Patel et al., 2017), retinal vasculature (Zhou et al., 2014), and early lens epithelium (Cain et al., 2008; Martinez et al., 2009).
In the adult, canonical Wnt signaling has been reported in retinal ganglion cells (Alldredge and Fuhrmann, 2016) as well as the amacrine and horizontal cells of the inner nuclear layer of the retina (Alldredge and Fuhrmann, 2016), and has been proposed to be necessary for the maintenance of ocular stem cells (Das et al., 2008; Dziasko and Daniels, 2016). Further, misregulation of canonical Wnt signaling has been implicated in the pathogenesis of many ocular diseases including age related macular degeneration (Tuo et al., 2015), myopia (Ma et al., 2014), limbal stem cell deficiency (Han et al., 2014; Lee et al., 2017), familial exudative vitreoretinopathy (Pau et al., 2015), proliferative vitreoretinopathy (Tamiya and Kaplan, 2016), diabetic retinopathy (Chen and Ma, 2017), glaucoma (Villarreal et al., 2014), and posterior capsular opacification (Bao et al., 2012; Wei et al., 2017).
However, despite the likely importance of canonical Wnt signaling in both normal eye development and disease, the unambiguous identification of canonical Wnt signaling and assessment of its function has been challenging as its downstream mediator, β-catenin, has dual functions. While the Wnt-mediated stabilization of “free” β-catenin, its nuclear translocation, and subsequent binding to TCF/Lef factors, co-activates transcription of Wnt target genes (MacDonald et al., 2009), it also binds to the cytoplasmic tail of cadherins and is important to tether them to the actin cytoskeleton (Valenta et al., 2012).
As it is experimentally challenging to distinguish the three pools of β-catenin found in cells, reporter constructs have been developed which express heterologous reporter genes when β-catenin is translocated to the nucleus and heterodimerizes with TCF/Lef transcription factors in response to canonical Wnt signaling (Barolo, 2006). Several different transgenic mouse lines, which are designed to express reporter genes when canonical Wnt signaling is active, have been used by multiple laboratories to map canonical Wnt signaling during in vivo eye development (Alldredge and Fuhrmann, 2016; Carpenter et al., 2015; Dawes et al., 2013; Grisanti et al., 2016; Kreslova et al., 2007; Liu et al., 2003; Liu et al., 2007; Miller et al., 2006; Smith et al., 2005). However, the conclusions from these reports are sometimes conflicting, likely in part because they largely used reporter strains which express cytoplasmically localized β-galactosidase, which can suffer from low sensitivity, high background, and difficulties in assigning expression to single cells. In order to overcome this challenge, we re-evaluated the distribution of canonical Wnt activity in the developing eye using an advanced, sensitive reporter mouse capable of revealing canonical Wnt activation on a single cell basis by utilizing a nuclear localized green fluorescent protein (cWnt-nlGFP) reporter (Ferrer-Vaquer et al., 2010), and tested the hypothesis that canonical Wnt signaling is activated during the formation of posterior capsular opacification (PCO), the most prevalent side effect of cataract surgery. We provide an extended discussion of these new findings in the context of previously published data on Wnt signaling in specific tissues in eye development.
Methods
Animals
All animal experiments described in this article conform to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the University of Delaware Institutional Animal Care and Use Committee. All mice were maintained and bred under specific pathogen free conditions at the University of Delaware animal facility. Wnt reporter mice (STOCK Tg(TCF/Lef1-HIST1H2BB/EGFP)61Hadj/J ), harboring a canonical Wnt reporter gene consisting of six copies of the T cell factor (TCF)/lymphoid enhancer-binding factor (Lef) binding site upstream of a hsp68 minimal promoter driving the expression of a histone 2b/green fluorescent protein (GFP) fusion protein (cWnt-nlGFP mice) (Ferrer-Vaquer et al., 2010), were obtained from the Jackson Laboratory (Bar Harbor, Maine) and crossed to wild-type (C57BL/6J, Stock #000664) mice (Jackson Laboratory). Embryos were obtained from timed matings, with noon on the day that the copulatory plug was found defined as embryonic day (E) 0.5. For genotyping, DNA was isolated from tail snips using the PureGene Tissue and Mouse Tail kit (Gentra Systems, Minneapolis, MN) followed by PCR analysis using the primers and protocol recommended by Jackson Laboratory (https://www2.jax.org/protocolsdb/f?p=116:5:0::NO:5:P5_MASTER_PROTOCOL_ID,P5_JRS_CODE:29499,013752).
Surgical Removal of Lens Fiber Cells (Murine Cataract Surgery)
Surgical removal of lens fiber cells to mimic human cataract surgery was performed in adult mice as previously described (Call et al., 2004; Desai et al., 2010; Manthey et al., 2014b). Mice were anesthetized and their pupils dilated. Then, a scalpel was used to generate a 2–3mm penetrating central corneal incision, going through the anterior lens capsule. Normal saline solution was then injected into the lens to separate the lens capsule from the lens fiber cells, and the entire lens fiber cell mass was carefully removed by fine forceps, leaving behind an intact lens capsule. The corneal incision was sutured with 10–0 nylon corneal suture (Ethicon Inc., Somerville, NJ), and normal saline was injected into the anterior chamber to inflate the eye, which regained its original size and shape. Mice were sacrificed at 0 hour, 3 hours, 6 hours, 12 hours, 18 hours, 24 hours, 48 hours, 5 days and 9 days after surgery, and eyes isolated for analysis. At least three biological replicates were analyzed for each time point.
Immunofluorescence (IF) staining and confocal imaging
All immunofluorescence analyses were performed as previously described (Reed et al., 2001). Briefly, eyes were collected and embedded directly in Optimum Cutting Temperature (OCT, Tissue Tek, Torrance, CA) and stored at −80°C prior to sectioning. 16μm-thick sections were obtained with a Leica CM3050 cryostat (Leica Microsystems, Buffalo Grove, IL), and mounted on ColorFrost Plus slides (Fisher Scientific Hampton, NH). For GFP and keratin 8 immunodetection, slides were fixed with 1:1 acetone-methanol for 20 minutes at −20°C, and blocked with 2% BSA in PBS for 1 hour at room temperature. Then, blocked sections were incubated with primary antibody (1:200 dilution, Anti-GFP, Catalog #A11122, Life Technologies or 1:100 dilution of a rat monoclonal antibody against keratin 8, TROMA-I, Developmental Studies Hybridoma Bank) for 1 hour at room temperature. The specificity of the anti-GFP antibody was confirmed by the complete lack of staining observed in tissues harvested from mice lacking the GFP reporter construct. For Dkk3 immunodetection, slides were fixed in 4% PFA for 15 minutes, washed in PBS then incubated overnight at 4°C with a 1:100 dilution of a rabbit polyclonal anti-Dkk3 antibody (Thermofisher, Catalog # PA5–21325). For all experiments, after primary antibody incubation, slides were washed in PBS, and incubated with a solution consisting of Alexa Fluor 488 or Alex Fluor 568 labeled secondary antibody (1:200 dilution; Invitrogen, Grand Island, NY), Draq-5 (1:2000 dilution, Biostatus Limited, Shepshed, Leicestershire, UK), and FITC-labeled anti-αSMA (for post-surgery samples only, 1:200 dilution, F3777, clone 1A4, Sigma-Aldrich, Sigma-Aldrich, St. Louis, MO) for 1 hour at room temperature in the dark. Sections were washed in PBS, and then mounted with mounting media (10ml of PBS with 100mg of phenylenediamine added to 90ml of glycerol; final pH 8.0).
Slides were imaged with a Zeiss LSM 780 confocal microscope (Carl Zeiss, Inc., Gottingen, Germany). For each experiment/comparison, all sections were stained simultaneously and imaged using identical configurations to ensure the validity of staining intensity comparisons. Under some circumstances, images were processed to optimize the brightness and/or contrast for optimal viewing on diverse computer screens. However, in all cases, any such adjustments were applied identically to both control and experimental images.
Results
Several studies have indicated that appropriate modulation of canonical Wnt signaling is critical for eye development. However, the present understanding of this pathway has discrepancies in the localization of canonical Wnt signaling as reported by different groups, perhaps due to the difficulty in unambiguously detecting nuclear β-catenin in epithelial cells with high levels of cadherin/β-catenin engagement, reporter sensitivity, experimental conditions, mouse strain differences, or the design of the canonical Wnt responsive promoters driving reporter expression (Barolo, 2006). Therefore, we re-examined the distribution of canonical Wnt signaling in the eye using a “third generation” canonical Wnt reporter that drives nuclear localized GFP expression under the control of 6 TCF/Lef binding sites placed upstream of the hsp68 basal promoter (cWnt-nlGFP) which allows for the sensitive localization of canonical Wnt activity to single cells in tissue sections (Ferrer-Vaquer et al., 2010).
Re-evaluation of the dynamics of canonical Wnt signaling during early eye development
At E9.5 in mouse, GFP staining (green) in the eye was brightest in the periocular mesenchyme and regions of the optic vesicle fated to become the retinal pigmented epithelium and optic stalk. In contrast, very low, albeit still detectable, levels of GFP staining are observed in the lens placode/peri-ocular surface ectoderm as delineated by co-localization with keratin 8, a known ectodermal/pre-placodal protein (Manthey et al., 2014a), (Figure 1A, B). At E10.5, minimal GFP expression was found in the keratin 8 positive lens pit or presumptive corneal epithelium although occasional cells associated with the lens pit exhibited high levels of GFP staining. The most posterior region of the outer optic cup fated to become the retinal pigmented epithelium (RPE), optic stalk, and periocular mesenchyme still exhibit strong GFP staining at this stage, although the inner layer of the optic cup fated to form the neural retina exhibits lower GFP levels (Figure 1C, D).
Figure 1.
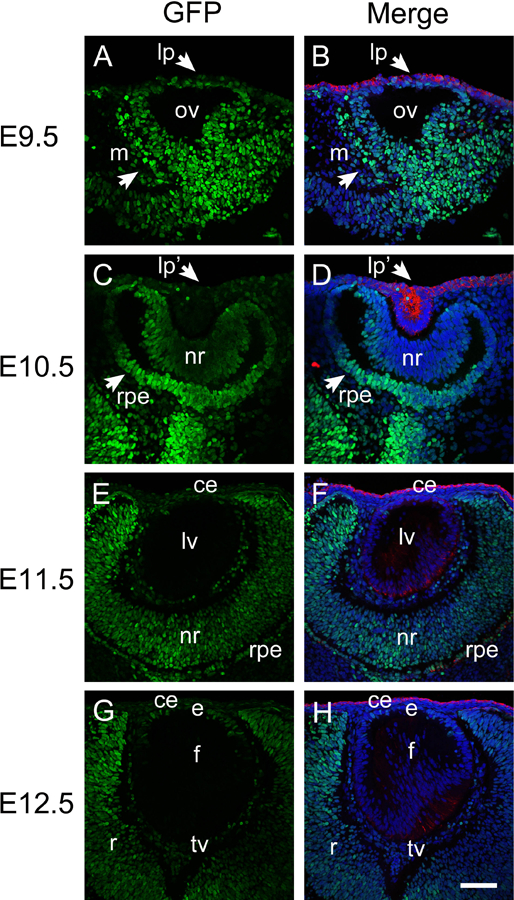
Activation of canonical Wnt signaling (revealed by GFP expression) during early embryonic eye development. Immunofluorescent staining for GFP (green) and keratin 8 (red) in E9.5 (A-B), E10.5 (C-D), E11.5 (E-F) and E12.5 (G-H) embryonic mouse eye. Panels A, C, E, and G, are GFP only, panel B, D, F, and H are merged images. Blue-DNA, Green-GFP, Red-keratin 8, lp, lens placode; ov, optic vesicle; m, periocular mesenchyme; lp, lens placode; lp’, lens pit; nr, neuroretina; rpe, presumptive retinal pigmented epithelium; ce, cornea epithelium; lv, lens vesicle; e, lens epithelium; f, lens fibers; tv, tunica vasculosa; scale bar = 70μm.
At E11.5, GFP levels were very low in the presumptive corneal epithelium which still stains strongly for keratin 8, the lens vesicle which exhibits greatly reduced keratin 8 staining at this stage, and the migratory neural crest arriving between the corneal epithelium and early lens. In contrast, appreciable GFP staining was observed in both the inner optic cup forming the neural retina and outer optic cup forming the RPE, although staining was stronger at the optic margin than the remainder of the optic cup. GFP staining was also prominent in cells surrounding the lens that are fated to form the tunica vasculosa lentis (TV) (Figure 1E, F). At E12.5, the distribution of GFP positive nuclei is similar to that observed at E11.5 although staining is weaker in the neural retina, while some GFP positive nuclei are detected in the lens epithelium (Figure 1G, H).
Canonical Wnt signaling is dynamic during corneal development
While little to no GFP is detected in either neural crest precursors to the corneal stroma or endothelium, or the head ectoderm derived corneal epithelium (Figure 1G, H), this changes dramatically at E14.5 as robust GFP staining is seen throughout the developing corneal stroma and endothelium although the presumptive corneal epithelium, marked by keratin 8 staining, still lacks GFP labeling (Figure 2A, B). At E16.5, the corneal epithelium was still both GFP-negative and keratin 8 positive, while staining in the developing corneal stroma was greatly diminished. Notably though, the forming corneal endothelium was still highly GFP positive at this stage (Figure 2C, D). This pattern was largely maintained at postnatal day zero (P0) (Figure 2E, F) and P3 (Figure 2G, H), although occasional GFP positive cells were detected in the corneal epithelium at these stages. However, by P13, GFP expression was absent from the corneal stroma and endothelium, but became prominent in the outer-most layer of the stratifying corneal epithelium, which also lost keratin 8 expression (Figure 2I, J). This pattern became more prominent in the central cornea in adulthood where keratin 8 expression was not detected, while strong GFP labeling was seen in the squamous and wing cells of the corneal epithelium. However, the basal layer of the epithelium was only weakly GFP positive. In contrast, keratin 8 positive cells indicative of limbal niches (Pajoohesh-Ganji et al., 2012, 2016) were still detected in the peripheral cornea at both P13 (Figure 3A-D) and adult (Figure 3E-H), however no GFP was detected in either these cells nor the less stratified peripheral corneal epithelium, showing that the reporter for canonical Wnt signaling used in this study preferentially labels the most differentiated corneal epithelial cells in the post-natal cornea.
Figure 2.
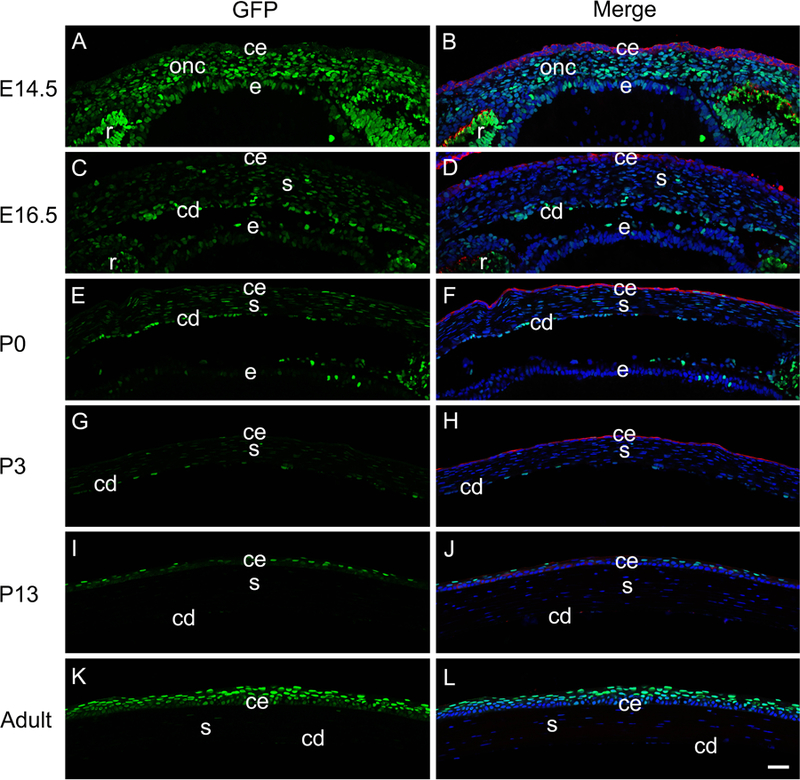
The activation of canonical Wnt signaling (represented by GFP) during cornea development. Immunofluorescent staining for GFP (green) and keratin 8 (red) in E14.5 (A-B), E16.5 (C-D), P0 (E-F), P3 (G-H), P13 (I-J) and adult (K-L) mouse cornea. Panels A, C, E, G, I and K are GFP only, panel B, D, F, H, J and L are merged images. Blue-DNA; Green-GFP Red-keratin 8; ce-cornea epithelium; cd-corneal endothelium; onc-ocular neural crest; s-stroma, e-lens epithelium, r-retina, scale bar = 35µm. *Images were taken using the tiling feature (three 3X3 matrix) of the Zeiss 780 confocal microscope. Individual images were taken and then stitched together, which results in a subtle inconsistency near the edges of two adjacent images.
Figure 3.
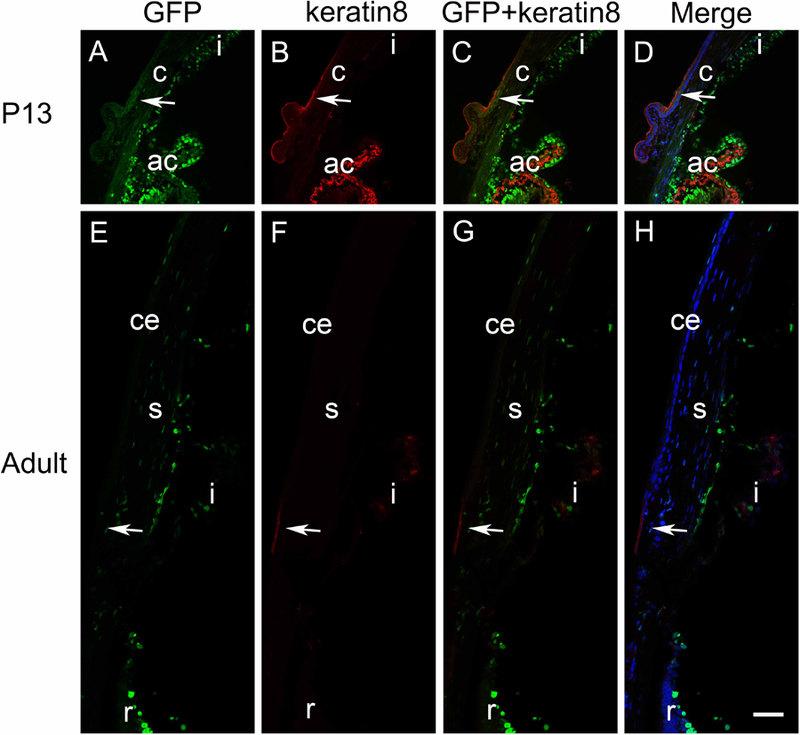
The pattern of canonical Wnt signaling (represented by GFP) at the corneal limbus. Immunofluorescent staining for GFP (green) and keratin 8 (red) at the P13 (A-D) and adult (E-H) limbal region. No obvious GFP expression was found to colocalize with keratin 8, an adult limbal marker, at the corneal limbus (arrows), in either P13 or adult mice. Panels A and E are GFP only, panels B and F are keratin 8 only, panels C and G are the merge of the GFP and keratin 8 images, and panels D and H are the merge of the DNA, GFP, and keratin 8 images. Blue-DNA, Green-GFP, Red-keratin 8, c-cornea; ce-corneal epithelium; s-corneal stroma; i-iris; ac-anterior chamber angle, r-retina, scale bar = 35µm. *Images were taken using the tiling feature (three 3X3 matrix) of the Zeiss 780 confocal microscope. Individual images were taken and then stitched together, which results in a subtle inconsistency near the edges of two adjacent images.
At P13, robust GFP expression was found in pigmented epithelium of the iris as well as pigmented and non-pigmented epithelium of the ciliary processes. Minimal signals were found in the cornea and conjunctiva (Figure 3A). As expected, keratin 8 was detected in the limbal region, indicating the presence of limbal stem cells. Strong keratin 8 signals were also found in the stroma of ciliary processes, which did not overlap with GFP expression found in pigmented and non-pigmented epithelium (Figure 3B). The colocalization images revealed that minimal GFP-positive cells reside in the limbal region (Figure 3C-D).
The Activation of Canonical Wnt Signaling During Retina Development
We detected canonical Wnt reporter activity throughout the optic vesicle at E9.5 (Figure 1A, B), and optic cup at E10.5, although the GFP staining was higher in cells of the posterior outer optic cup than the cells of the optic margin and inner optic cup fated to become the iris pigmented epithelium and neural retina respectively (Figure 1C,D). In contrast, modest GFP expression was observed throughout both the inner and outer optic cup at E11.5 and E12.5 (Figure 1E-H). By E14.5, GFP labeling was diminished in keratin 8 positive developing RPE, but still robust throughout the neural retina, although labeling was more intense in the inner neuroblastic layer which is producing retinal ganglion cells compared to the outer neuroblastic layer (Figure 4A,B). The pattern was more established at E16.5 with little to no GFP staining detected in the RPE or outer neuroblastic layer, but GFP was still detectable in the inner neuroblastic layer (Figure 4C, D).
Figure 4.
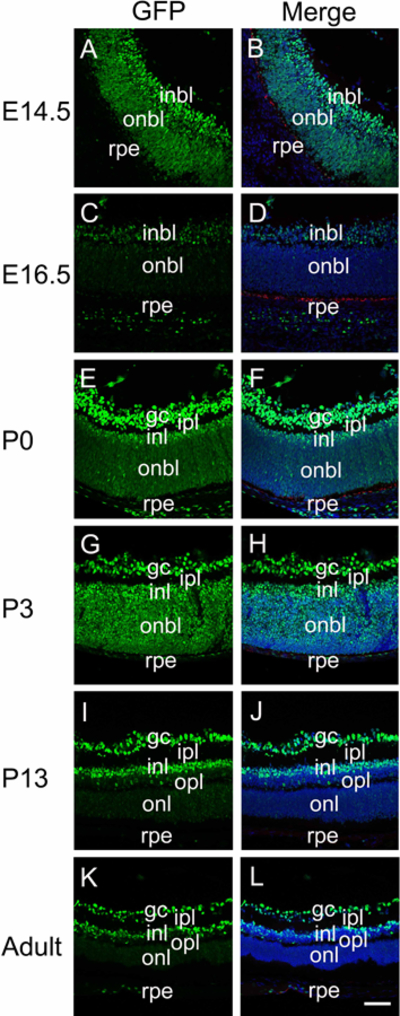
The distribution of canonical Wnt signaling (represented by GFP) during retina development. Immunofluorescent staining for GFP (green) and keratin 8 (red) in E14.5 (A-B), E16.5 (C-D), P0 (E-F), P3 (G-H), P13 (I-J) and adult (K-L) mouse retina. Panels A, C, E, G, I and K are GFP plus keratin 8, panels B, D, F, H, J and L are merged images including the DNA channel. Blue-DNA, Green-GFP, Red-keratin 8; inbl, inner neuroblastic layer; onbl, outer neuroblastic layer; Gc, ganglion cell; rpe, retinal pigmented epithelium; ipl, inner plexiform layer; inl, inner nuclear layer; opl, outer plexiform layer; onl, outer nuclear layer; scale bar = 70μm.
At P0 in mice, the retinal ganglion cells have formed and exhibit bright GFP staining, while the outer neuroblastic layer exhibits weakly GFP positive cells throughout although the staining is brighter at the most anterior aspect fated to give rise to the inner nuclear layer (Figure 4E, F). A similar staining pattern is observed at P3, although the staining in the outer neuroblastic layer/inner nuclear layer is more intense than seen at P0 (Figure 4G, H). By P13 (Figure 4I, J), the adult (Figure 4K, L) GFP distribution was established with prominent labeling of the retinal ganglion cells and inner nuclear layer, while the outer nuclear layer largely lacked detectable GFP.
Canonical Wnt signaling is detected transiently in the embryonic lens
Consistent with prior reports, the nuclear localized GFP Wnt signaling reporter mouse exhibited little to no GFP expression in either the developing lens placode (Figure 1A, B), lens pit (Figure 1C, D) or lens vesicle (Figure 1E, F). However, as primary fiber cell elongation obliterates the lens vesicle lumen at E12.5, low levels of GFP are detected in the anterior lens epithelium (Figure 1G, H), which are more obvious at E14.5 (Figure 2A, B; Figure 5E, F). GFP expression in lens epithelial cells diminishes by E16.5 (Figure 2C, D; Figure 5G, H) and is largely absent from the lens from birth onward (Figure 5I-P; data not shown).
Figure 5.
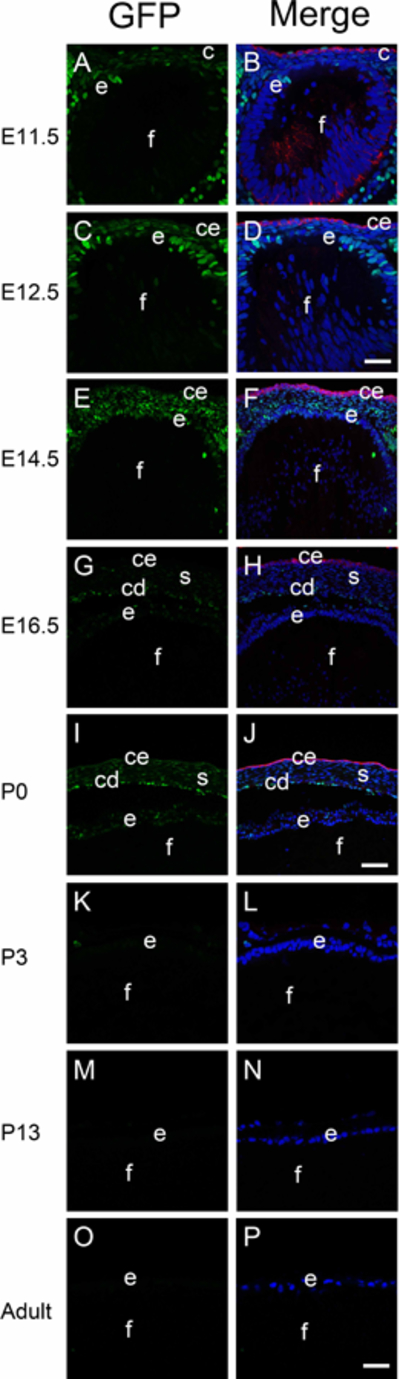
The distribution of canonical Wnt signaling (represented by GFP) during lens development. Immunofluorescent staining for GFP (green) and keratin 8 (red) in E11.5 (A-B), E12.5 (C-D), E14.5 (E-F), E16.5 (G-H), P0 (I-J), P3 (K-L), P13 (M-N) and adult (O-P) mouse lens. Panels A, C, E, G, I, K, M and O are GFP only, panels B, D, F, H, J, L, N and P are merged images. c-cornea; l-lens; ce-cornea epithelial cells; e-lens epithelial cells; f-fiber cells; s-cornea stroma; cd-cornea endothelial cells. Blue-DNA, Green- GFP, Red- keratin 8, A-D and K-P, scale bar = 35μm, E-J, scale bar = 70μm.
Canonical Wnt Signaling is activated in lens epithelial cells in a mouse model of cataract surgery
Cataracts, which are defined as the loss of lens transparency, are treated by surgical removal of the lens fiber cell mass, while the lens capsule with attached lens epithelial cells is retained to tether the artificial intraocular lens that is implanted to restore patient vision (Ashwin et al., 2009). However, the remnant lens epithelial cells subsequently undergo a wound healing response, which produces a combination of dysgenic lens fiber cells and scar tissue-forming myofibroblasts. If these cells reach the optic axis, they occlude patient vision, a condition denoted as posterior capsular opacification (PCO) (Wormstone et al., 2009). While it is established that transforming growth factor beta (TGFβ) plays an important role in PCO progression (Wormstone et al., 2009), it is likely not the only player. Notably, activation of the canonical Wnt pathway has been reported to drive fibrosis in other tissues (Akhmetshina et al., 2012b), and there are intriguing reports suggesting that inhibition of the canonical Wnt pathway after cataract surgery can ameliorate PCO pathogenesis (Liu et al., 2017), although a recent study conflicted with these findings that canonical Wnt signaling plays a role in PCO (Taiyab et al., 2016). Here, we investigated whether canonical Wnt signaling was activated in lens epithelial cells during PCO pathogenesis by removing the lens fiber cells from cWnt-nlGFP reporter mice and following the onset of GFP expression compared to the onset of expression of the commonly used fibrotic marker, alpha smooth muscle actin (SMA).
Consistent with our observations in intact adult lenses (Figure 5O, P), no GFP was detected in remnant lens epithelial cells either immediately following lens fiber cell removal (post-cataract surgery, PCS) (Figure 6A) or six hours later (Figure 6B). By 12 hours PCS, GFP was detected in occasional lens epithelial cells (figure 6C) while GFP positive cells became progressively more common at 18 and 24 hours PCS, which is 24–30 hours prior to the onset of elevated SMA expression (Figure 6F). From 48 hours through 9 days PCS (Figure 6F-H), we find that most cell nuclei in SMA positive fibrotic plaques stain intensely for GFP (Figure 6F-H) suggesting that activation of canonical Wnt signaling is associated with fibrotic PCO.
Figure 6.
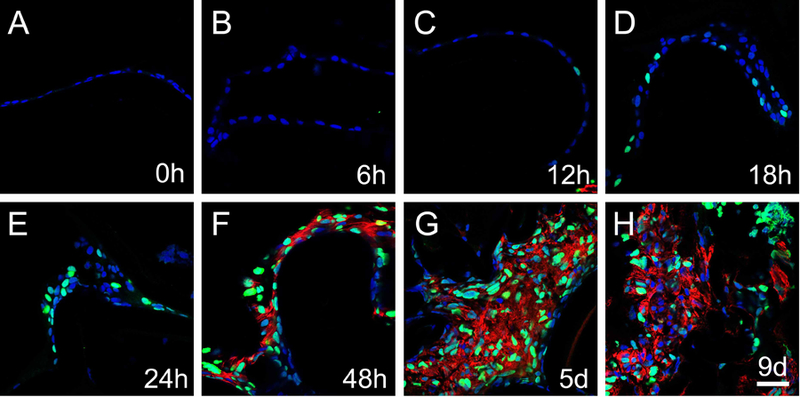
The activation of canonical Wnt signaling (represented by GFP) in lens epithelial cells remaining behind in a mouse model of cataract surgery. Immunofluorescent staining for GFP (green) and αSMA (red) in 0h, 6h, 12h, 18, 24h, 48h, 5 days and 9 days post-surgery samples. A) GFP expression was not detected at 0h post-surgery, B) GFP expression was not detected at 6h post-surgery, C) GFP expression was detected in occasional lens epithelial cell nuclei at 12h post-surgery, D) GFP expression becomes more prevalent in lens epithelial cells at 18h post-surgery, E) GFP expression continues to upregulate at 24h post-surgery, F) robust GFP and αSMA expression co-localize in remnant lens cells at 48h post-surgery, G) robust GFP and αSMA expression co-localize in remnant lens cells at 5 days post-surgery, H) robust GFP and αSMA expression persist in remnant lens cells at 9 days post-surgery. Blue-DNA, Green-GFP, Red-αSMA, scale bar = 35μm
DKK3, an inhibitor of canonical Wnt signaling, is expressed in the lens throughout its development and downregulates in LECs prior to the onset of canonical Wnt signaling post-cataract surgery
During early eye development, it has been proposed that canonical Wnt signaling is inhibited in the head ectoderm via the Pax6 mediated expression of inhibitors of the canonical Wnt signaling pathway (Fujimura, 2016; Machon et al., 2010). Consistent with this, it has been reported that the embryonic lens expresses numerous inhibitors of the canonical Wnt pathway including Dkk1, Dkk2, Dkk3, Wif1, Sfrp1 and Sfrp2 (Ang et al., 2004; Martinez et al., 2009). In order to ascertain a potential mechanism for the induction of the canonical Wnt pathway in LECs PCS, we mined our recently completed transcriptomic analysis of the 24 hours PCS lens epithelium and compared it to the transcriptome of LECs isolated immediately after lens fiber cell removal (manuscript in preparation). This analysis revealed that Dkk3 is the 15th most abundant mRNA (1469 reads per kilobase per million (rpkm)), and most abundant known Wnt inhibitor, expressed in adult lens epithelial cells, while its mRNA levels decrease three-fold in LECs by 24 hours PCS.
In order to relate these data to the dynamics of canonical Wnt signaling in lens development, we determined the pattern of Dkk3 protein expression both during lens development and in lens epithelial cells PCS. At the lens vesicle stage (E11.5), Dkk3 protein was detected throughout the developing eye including the presumptive corneal epithelium, lens vesicle and inner optic cup (Figure 7A), a pattern maintained through E12.5 (Figure 7B). By E14.5, Dkk3 expression is diminished in the central lens fibers, but is maintained in the lens epithelium and newly formed peripheral lens fibers (Figure 7C). At 16.5, Dkk3 expression is diminished throughout the lens (Figure 7D), but it upregulates at birth, with intense Dkk3 labeling in LECs, with lesser levels in lens fibers (Figure 7E). By P14, Dkk3 protein distribution in the lens is largely restricted to the lens epithelium (Figure 7F), and this pattern is maintained into adulthood (Figure 7G). Consistent with this, intense Dkk3 labeling was detected in adult LECs immediately after lens fiber cell removal (Figure 7H, O). However, this labeling was obviously diminished by 3 hours PCS (Figure 7I, P), and Dkk3 immunolabeling remained weak in the LECs remaining behind after fiber cell removal through 5 days PCS (Figure 7J-N; Q-U) consistent with the reduction in Dkk3 mRNA observed at 24 hours PCS and the upregulation of canonical Wnt signaling observed at 12 hours PCS (Figure 6).
Figure 7.
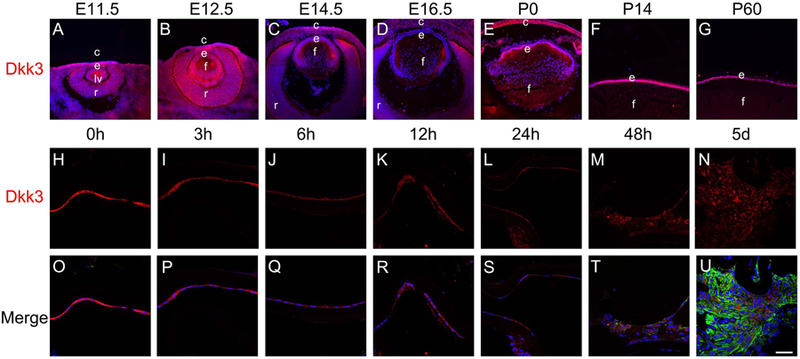
The distribution of Dkk3 protein in the developing lens and in lens epithelial cells remaining in the eye after lens fiber cell removal in a mouse model of cataract surgery. A-G) Immunofluorescent staining for Dkk3 (red) during mouse lens development from A) E11.5 B) E12.5 C) E14.5 D) E16.5 E) P0 F) P14 G) P60 showing that Dkk3 expression becomes restricted to the lens epithelium around birth. H-U) Immunofluorescent staining for Dkk3 (red) and αSMA (green) in 0h, 3h, 6h, 12h, 24h, 48h, and 5 days post-surgery samples. H, O) Dkk3 protein levels are high in lens epithelial cells (LECs) immediately after surgery, I, P) Dkk3 protein levels appear to have downregulated in lens epithelial cells (LECs) by 3 hours post-surgery J, Q) By six hours post surgery, Dkk3 protein levels have decreased in LECs compared to those analyzed immediately after surgery. At 12 (K, R) and 24 (L, S) hours post surgery, Dkk3 levels are still reduced and αSMA is still absent from remnant LECs. M, T) At 48 hours post surgery, Dkk3 levels are still modest in LECs compared to those analyzed immediately after surgery while αSMA levels have begun to upregulate in remnant LECs N, U) At 5 days post surgery, Dkk3 levels remain low in LECs compared to those analyzed immediately after surgery while αSMA levels are high in remnant LECs Blue-DNA, Green- αSMA, Red-Dkk3, scale bar = 36μm
Discussion
There are numerous prior studies using Wnt reporter mouse strains and/or immunostaining for the nuclear translocation of β-catenin to visualize canonical Wnt signaling during eye development. However, the results of some of these studies contradict functional data (or even differ between laboratories), likely due to difficulties visualizing nuclear β-catenin in tissues with robust cadherin/catenin interactions, and experimental limitations associated with sensitive detection of cytoplasmic β-galactosidase activity. Thus, we chose to use a third generation canonical Wnt reporter mouse (cWnt-nlGFP) which can sensitively reveal canonical Wnt signaling at a single cell level via GFP expression (Ferrer-Vaquer et al., 2010) for our ongoing investigations of the role of canonical Wnt signaling in ocular fibrosis. In this report, we re-evaluated the distribution of canonical Wnt signaling in the eye during its development (see Table 1 for a summarry of results) and review the state of the literature in this area below.
Table 1.
A summary of the dynamic changes in canonical Wnt signaling observed during murine eye development
| Developmental time point | Distribution of Wnt signaling in the eye |
|---|---|
| E9.5 | periocular mesenchyme, optic vesicle (most intense in regions fated to become the posterior optic cup/optic stalk) |
| E10.5 | periocular mesenchyme, optic cup (higher in posterior layer rated to form RPE), optic stalk, |
| E11.5 | inner and outer optic cup (intense at margin), and cells fated to form the tunica vasculosa lentis (TV) |
| E12.5 | inner and outer optic cup (intense at margin), tunica vasculosa lentis, and weak staining in anterior lens epithelium |
| E14.5 | developing corneal stroma and endothelium, neural retina (particularly the inner neuroblastic layer), and anterior lens epithelium |
| E16.5 | developing corneal stroma and corneal endothelium, inner neuroblastic layer, some weak signal in lens epithelium |
| P0 | forming corneal endothelium, retinal ganglion cells, and outer neuroblastic layer |
| P3 | forming corneal endothelium, retinal ganglion cells, and outer neuroblastic layer |
| P13 | Outer layer of stratifying corneal epithelium, pigmented epithelium of the iris, pigmented and non-pigmented epithelium of the ciliary processes, retinal ganglion cells and inner nuclear layer |
| Adult | Central corneal epithelium (most intense in wing and squamous cells), retinal ganglion cells and inner nuclear layer |
Canonical Wnt signaling activity in the optic cup and developing retina
Some studies using the TOPGAL mouse reporter of canonical Wnt signaling suggest that canonical Wnt signaling is absent from the optic vesicle/early optic cup (Miller et al., 2006) and later embryonic retina (Grisanti et al., 2016). However, the TCF/Lef-LacZ reporter mouse revealed canonical Wnt signaling in the neuroepithelium of the dorsal-nasal aspect of the optic vesicle at E9.5 and intense β-galactosidase labeling of the ciliary margin from E11.5 to E15.5. In the neural retina, patchy TCF/Lef-LacZ reporter activity was detected in a small subset of cells at E11.5 and from E12.5 until E17.5, this staining was seen in the developing inner neuroblastic layer that produces the retinal ganglion cells, along with more patchy staining in the outer neuroblastic layer. In the mature retina, canonical Wnt signaling was detected in retinal ganglion and amacrine cells (Liu et al., 2003; Liu et al., 2006). Overall, our investigation of canonical Wnt signaling in the developing retina using cWnt-nlGFP reporter mice largely mirror these prior studies, consistent with the known role for canonical Wnt signaling in lamination of the developing retina (Fu et al., 2006).
cWnt-nlGFP reporter mice also revealed putative canonical Wnt reporter activity in the posterior optic vesicle and outer optic cup/optic stalk between E9.5 and 11.5, cells fated to become the retinal pigmented epithelium (RPE) and outer sheath of the optic nerve, although this labeling is less prominent as the RPE begins to differentiate (as measured by the onset of pigment production) at E12.5. This detection of putative canonical Wnt signaling in the precursors of the RPE is consistent with the results from axin2-lacz (Alldredge and Fuhrmann, 2016), and BATGAL (Maretto et al., 2003; Song et al., 2007) canonical Wnt reporter mice although it revealed a wider extent of canonical Wnt signaling in the putative RPE than TOPGAL reporter mice (Fuhrmann et al., 2009). Overall, the broad distribution of putative canonical Wnt signaling in the posterior optic cup of cWnt-nlGFP reporter mice supports functional data showing that canonical Wnt signaling is critical for RPE determination in early eye development (Bharti et al., 2012; Fujimura et al., 2009).
Canonical Wnt signaling during corneal development
The role of canonical Wnt signaling during corneal development is an area of active investigation. Some reports have concluded that canonical Wnt signaling does not play a role in corneal development due to the absence of detectable TOPGAL or TCF/Lef-Lacz reporter activity as well as axin 2 expression in any portion of the cornea from eye induction until adulthood (Carpenter et al., 2015; Dawes et al., 2013; Gage et al., 2008; Grisanti et al., 2016; Liu et al., 2007; Smith et al., 2005). However, other investigations have proposed that canonical Wnt signaling is active in, and important for, the programming of the neural crest derived peri-ocular mesenchyme (Alldredge and Fuhrmann, 2016; Zhao and Afshari, 2016). Other studies suggest that canonical Wnt signaling is also important for the subsequent development of all corneal cell types including the endothelium, stromal keratocytes, and limbal stem cells/epithelium (Zhang et al., 2015). Overall, our data obtained from the cWnt-nlGFP canonical Wnt reporter line does support prior functional data indicating that canonical Wnt signaling is active, albeit at modest levels, during the development of all corneal subtypes.
We detected cWnt-nlGFP reporter activity in the ocular neural crest at E9.5 which is consistent with the functional requirement for canonical Wnt signaling to program ocular neural crest from eye field stem cells (Zacharias and Gage, 2010; Zhao and Afshari, 2016) and the detection of axin2 lacZ reporter activity in periocular mesenchyme (Alldredge and Fuhrmann, 2016). Canonical Wnt reporter activity was also detected in neural crest derived cells that are establishing the corneal stroma and endothelium at E14.5 (Figure 2A, B; Figure 5E, F), while this becomes more restricted to the developing corneal endothelium from E16.5 until P3, after which labeling is lost. This correlates with the proposed role for stromal canonical Wnt signaling in preventing the premature stratification of the developing corneal epithelium (Zhang et al., 2015), the ability of ectopic canonical Wnt signaling to reprogram corneal stromal stem cells into a corneal endothelial fate (Hatou et al., 2013), and the observation that the ectopic activation of the canonical Wnt pathway in adult corneal endothelial cells induces these normally terminally quiescent cells to proliferate (Lee and Heur, 2015). Notably, all of these reports and our observations are consistent with a recent genome wide association study that found significant associations between the loci encoding many genes that regulate the canonical Wnt pathway and central corneal thickness (Benson et al., 2017).
Our observation that cWnt-nlGFP reporter activity is largely absent from head ectoderm fated to become the corneal epithelium is consistent with prior observations using other canonical Wnt reporters (Kreslova et al., 2007; Smith et al., 2005) and functional experiments that have demonstrated that inhibition of canonical Wnt signaling in the head ectoderm is required for eye development (Kreslova et al., 2007; Miller et al., 2006). Consistent with this, we also detected no canonical Wnt reporter activity in presumptive corneal epithelial cells that stain for the simple epithelial marker, keratin 8. However, as keratin 8 levels diminish in the corneal epithelium after birth, we detected cWnt-nlGFP reporter activity in the outer most layer of the newly stratifying corneal epithelium. This presages the absence of GFP labeling in the undifferentiated limbal and basal epithelial cells of the adult cornea, in contrast to the apparently robust canonical Wnt signaling detected in the postmitotic wing and squamous layers of the central cornea.
While our results suggest that terminally differentiated corneal epithelial cells exhibit robust canonical Wnt signaling, the role of canonical Wnt signaling in the establishment and maintenance of the stratified corneal epithelium is unclear. First, the cornea never exhibits canonical Wnt activity as measured in TOPGAL transgenic mice (Grisanti et al., 2016; Mukhopadhyay et al., 2006), although the extensive evidence provided above that canonical Wnt signaling is active in the corneal stroma and endothelium and an immunolocalization study (Fuhrmann et al., 2009) suggests that detection of the TOPGAL reporter by β-galactosidase enzyme activity is not sensitive enough to detect bona fide Wnt signaling in the cornea. Second, the literature on the role of canonical Wnt signaling in corneal epithelial cells in the maintenance of the ocular surface is complex. Many investigations into the dynamics of the corneal limbus and/or its differentiation from stem cells provide evidence that canonical Wnt signaling is critical for maintenance of corneal epithelial stem cells in the limbus. A qRT-PCR study showed that several Wnts and Wnt inhibitors are preferentially expressed in the limbus compared to central cornea, while nuclear β-catenin was observed in a small subset of basal epithelial cells in the limbus. Functionally, this investigation found that activation of canonical Wnt signaling can facilitate limbal stem cell proliferation and colony-formation (Nakatsu et al., 2011), while another study found that limbal cells express high levels of Wnt7a, while its knockdown in cultured limbal stem cells leads to their conversion to an epidermal fate (Ouyang et al., 2014). This correlates with a report finding that the clonal growth of cultured limbal stem cells is fostered when canonical Wnt signaling is activated (Han et al., 2014) and the requirement for frizzled 7 in maintaining the undifferentiated state of limbal stem cells (Mei et al., 2014).
However, a comparative microarray study of the human corneal limbus and congenital limbal dermoids suggested that the limbus features comparatively high level expression of the Wnt inhibitors Dkk1 and Sfrp1 (Li et al., 2015). Further, our inability to detect canonical Wnt signaling in the limbus or basal cells of the corneal epithelium in cWnt-nlGFP reporter mice is consistent with the report showing that deletion of the gene for the Wnt inhibitor, Dkk2, leads to the transdifferentiation of the corneal epithelium into epidermis associated with the presence of ectopic TOPGAL activity in the corneal limbus (Mukhopadhyay et al., 2006). Our detection of canonical Wnt signaling in the wing and squamous layers of the corneal epithelium is novel, although it is intriguing that the expression of some Wnt family members is elevated in the central cornea compared to the limbus (Nakatsu et al., 2011). While it is possible that some of these differences between studies represent species specific differences, overall, the function of canonical Wnt signaling in the corneal epithelium is still an open question requiring additional investigation.
Canonical Wnt signaling in the lens
It is accepted that canonical Wnt signaling must be suppressed in the surface ectoderm for lens placode formation (Machon et al., 2010; Smith et al., 2005) which correlates with our detection of little to no GFP expression in the lens placode, lens pit or lens vesicle of embryonic cWnt-nlGFP reporter mice. The role of canonical Wnt signaling during the later stages of lens development is more controversial. BATGAL Wnt reporter mice exhibit no β-galactosidase staining in the lens from E9.5–13.5 (Kreslova et al., 2007; Machon et al., 2010), TOPGAL mice did not exhibit β-galactosidase activity between E9.5 and E16.5 (Fuhrmann et al., 2009; Smith et al., 2005) although this may reflect the low sensitivity of β-galactosidase enzyme labeling as one of these studies also observed β-galactosidase immunostaining in the lens epithelium of TOPGAL mice at E14.5 (Fuhrmann et al., 2009). In contrast, TCF/Lef-LacZ reporter mice exhibited β-galactosidase enzyme activity in the anterior lens epithelium of E13.5 embryos (Liu et al., 2003), while this was absent largely absent at E14.5. However, in other studies by this same group using this same reporter strain, canonical Wnt signaling was observed in lens anterior epithelium from E11.5 to E14.5, which was downregulated from E15.5 onward (Liu et al., 2006; Liu et al., 2007). While TCF/Lef-LacZ show some differences in staining among studies, likely due to differences in LacZ staining sensitivity, this distribution largely agrees with our observation that cWnt-nlGFP reporter mice exhibit canonical Wnt signaling in the lens epithelium at E12.5 and 14.5. This transient activation of canonical Wnt signaling in embryonic lens development revealed by both TCF/Lef-LacZ and cWnt-nlGFP reporter mice supports reports that canonical Wnt signaling is functionally important in the early lens (Martinez et al., 2009; Stump et al., 2003).
Later in lens development, we did not detect cWnt-nlGFP reporter activity in the lens epithelium consistent with a prior report showing that TCF/Lef-LacZ activity is absent from the postnatal day 3 lens (Dawes et al., 2013). This lack of putative canonical Wnt signaling in the post-natal lens correlates with the high mRNA levels of the canonical Wnt pathway inhibitor, Dkk3 that we detected in adult LECs, and the preferential expression of Dkk3 protein in the mouse lens epithelium from birth onward.
Canonical Wnt signaling is activated in lens epithelial cells shortly after lens injury: implications for PCO pathogenesis
During cataract surgery, the central anterior lens capsule with attached lens epithelial cells (LECs) is excised, and the lens fibers removed, leaving the remaining lens capsule intact to hold an artificial intraocular lens which restores vision (Ashwin et al., 2009). Cataract surgery outcomes are compromised when the LECs remaining behind after surgery begin proliferating while also often migrating onto the posterior lens capsule (Wormstone et al., 2009). This occurs concurrently with epithelial-mesenchymal transition (EMT) leading to fibrotic posterior capsular opacification (PCO) which compromises visual acuity following cataract surgery (Montenegro et al., 2010). It is well accepted that active TGFβ drives both fibrotic PCO and the lens fibrotic disease, anterior subcapsular cataract (ASC) (Ishida et al., 2005; Saika et al., 2004). In other contexts, TGFβ and Wnt/β-catenin signaling collaborate to drive tissue fibrosis (Akhmetshina et al., 2012a; Beyer et al., 2012; Piersma et al., 2015; Zhou et al., 2012) by regulating the expression of pro-fibrotic genes (Akhmetshina et al., 2012a; Guo et al., 2012). However, the role of canonical Wnt signaling in PCO pathogenesis is currently unclear.
The induction of the Wnt/β-catenin pathway in the embryonic lens via adenomatosis polyposis coli inactivation blocks lens fiber differentiation, and induces lens epithelial multilayering and ectopic αSMA expression (Martinez et al., 2009), both indicative of LEC EMT. However, it was recently reported that β-catenin interactions with the lysine acetyltransferase CBP were important for TGFβ mediated LEC EMT, while Tcf7l2-β-catenin interactions were not, discounting a role for canonical Wnt signaling. However, the loss of Tcf7l2/Lef activity by inhibitor treatment was not confirmed, and this investigation only studied events downstream of active TGFβ signaling (Taiyab et al., 2016). Conversely, multiple Wnts and Frizzled receptors were reported to be upregulated in TGF-β induced EMT and fibrotic plaque formation in the lens (Chong et al., 2009). Further, injection of the canonical Wnt pathway inhibitor, Dkk1, reduced PCO in a rabbit model, although the molecular pathways regulated were not elucidated (Liu et al., 2017).
Here, using the cWnt-nlGFP reporter mouse, we show that putative canonical Wnt signaling is upregulated within 12–18 hours post lens injury, which is much earlier than we were able to detect the Smad3 phosphorylation indicative of canonical TGFβ pathway activation (Mamuya et al., 2014) in this model of PCO. Interestingly, we found that the mRNA expression levels of the canonical Wnt pathway inhibitor, Dkk3, are three-fold downregulated in LECs by 24 hours after lens injury, while semi-quantitative immunolocalization indicated that Dkk3 protein levels in LECs decrease by 3–6 hours after lens injury, correlating with the initial detection of cWnt-nlGFP at 12 hours post injury. In aggregate, these data suggest that canonical Wnt signaling may cooperate with TGF-β signaling to drive fibrotic PCO (Figure 8), although this assertion will require functional testing in future studies.
Figure 8.
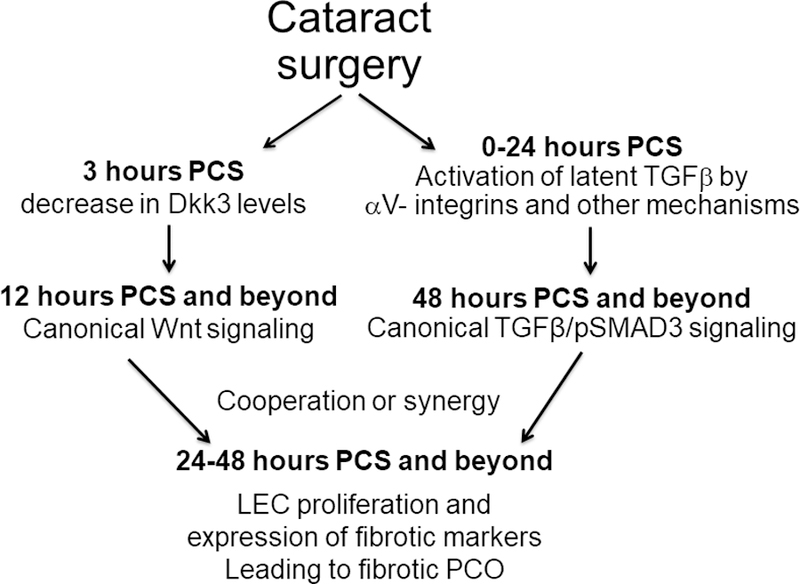
A model of the possible role that canonical Wnt signaling could play in PCO
All canonical Wnt signaling reporters are limited in their ability to detect Wnt signaling functioning independently from TCF/Lef transcription factors or leading to transcriptional repression (Barolo, 2006), and thus can generate “false negative results”. These reporters may also generate “false positive” signals due to their ability to respond to non-Wnt dependent TCF/Lef transcriptional activity(Barolo, 2006). However, the third generation canonical Wnt reporter used in this investigation did confirm many experimentally validated sites of cannonical Wnt activity during eye development such as in the optic cup(Hagglund et al., 2013), ciliary margin (Liu et al., 2007) and developing retina (Das et al., 2008). Further, the canonical Wnt reporter used in this study has been used by many laboratories and found to accurately reveal bona fide canonical Wnt signaling in non-ocular sites including the early embryo, developing lung, brain, kidney(Ferrer-Vaquer et al., 2010), hair follicle (Ahtiainen et al., 2014; Telerman et al., 2017), and teeth (Babb et al., 2017). While the novel sites of canonical Wnt signaling reported here (the stratifying central cornea and injured lens epithelium in particular) need functional confirmation, this may be a fruitful area for future study.
Acknowledgements:
We thank Dr. Salil Lachke, University of Delaware and members of the Duncan lab for their critical reading of this manuscript.
Grant information: this study was supported by grants from the National Institutes of Health (EY015279) and Aniridia Foundation International to MKD; Yichen Wang received a Fight for Sight-Aniridia Foundation International Summer Fellowship, PM received a University of Delaware Life Sciences Scholar Summer Fellowship; INBRE program grant P20 GM103446 supported the University of Delaware Core Imaging facility and a core facility access award to MKD; the University of Delaware CBI training grant (T32- GM008550-24) funded BHP, and 1S10 (RR027273-01) funded the acquisition of the confocal microscope used in this study.
References:
- Ahtiainen L, Lefebvre S, Lindfors PH, Renvoise E, Shirokova V, Vartiainen MK, Thesleff I, Mikkola ML, 2014. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev Cell 28, 588–602. [DOI] [PubMed] [Google Scholar]
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH, 2012a. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun 3, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH, 2012b. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 3, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldredge A, Fuhrmann S, 2016. Loss of Axin2 Causes Ocular Defects During Mouse Eye Development. Invest Ophthalmol Vis Sci 57, 5253–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW, 2004. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns 4, 289–295. [DOI] [PubMed] [Google Scholar]
- Ashwin PT, Shah S, Wolffsohn JS, 2009. Advances in cataract surgery. Clin Exp Optom 92, 333–342. [DOI] [PubMed] [Google Scholar]
- Babb R, Chandrasekaran D, Carvalho Moreno Neves V, Sharpe PT, 2017. Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/beta-catenin signaling in response to tooth damage. Sci Rep 7, 3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao XL, Song H, Chen Z, Tang X, 2012. Wnt3a promotes epithelial-mesenchymal transition, migration, and proliferation of lens epithelial cells. Mol Vis 18, 1983–1990. [PMC free article] [PubMed] [Google Scholar]
- Barolo S, 2006. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene 25, 7505–7511. [DOI] [PubMed] [Google Scholar]
- Benson MD, Khor CC, Gage PJ, Lehmann OJ, 2017. A targeted approach to genome-wide studies reveals new genetic associations with central corneal thickness. Mol Vis 23, 952–962. [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, Sonnylal S, de Crombrugghe B, Taketo MM, Distler O, Schett G, Distler JH, 2012. beta-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis 71, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Gasper M, Ou J, Brucato M, Clore-Gronenborn K, Pickel J, Arnheiter H, 2012. A regulatory loop involving PAX6, MITF, and WNT signaling controls retinal pigment epithelium development. PLoS Genet 8, e1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU, 2008. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol 321, 420–433. [DOI] [PubMed] [Google Scholar]
- Call MK, Grogg MW, Del Rio-Tsonis K, Tsonis PA, 2004. Lens regeneration in mice: implications in cataracts. Exp Eye Res 78, 297–299. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Smith AN, Wagner H, Cohen-Tayar Y, Rao S, Wallace V, Ashery-Padan R, Lang RA, 2015. Wnt ligands from the embryonic surface ectoderm regulate ‘bimetallic strip’ optic cup morphogenesis in mouse. Development 142, 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ma JX, 2017. Canonical Wnt signaling in diabetic retinopathy. Vision Res 139, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CC, Stump RJ, Lovicu FJ, McAvoy JW, 2009. TGFbeta promotes Wnt expression during cataract development. Exp Eye Res 88, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AV, Bhattacharya S, Zhao X, Hegde G, Mallya K, Eudy JD, Ahmad I, 2008. The canonical Wnt pathway regulates retinal stem cells/progenitors in concert with Notch signaling. Dev Neurosci 30, 389–409. [DOI] [PubMed] [Google Scholar]
- Dawes LJ, Sugiyama Y, Tanedo AS, Lovicu FJ, McAvoy JW, 2013. Wnt-frizzled signaling is part of an FGF-induced cascade that promotes lens fiber differentiation. Invest Ophthalmol Vis Sci 54, 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai VD, Wang Y, Simirskii VN, Duncan MK, 2010. CD44 expression is developmentally regulated in the mouse lens and increases in the lens epithelium after injury. Differentiation 79, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziasko MA, Daniels JT, 2016. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul Surf 14, 322–330. [DOI] [PubMed] [Google Scholar]
- Esteve P, Sandonìs A, Ibañez C, Shimono A, Guerrero I, Bovolenta P, 2011. Secreted frizzled-related proteins are required for Wnt/β-catenin signalling activation in the vertebrate optic cup. Development 138, 4179–4184. [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK, 2010. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev Biol 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Sun H, Klein WH, Mu X, 2006. Beta-catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev Biol 299, 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Riesenberg AN, Mathiesen AM, Brown EC, Vetter ML, Brown NL, 2009. Characterization of a transient TCF/LEF-responsive progenitor population in the embryonic mouse retina. Invest Ophthalmol Vis Sci 50, 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N, 2016. WNT/β-Catenin Signaling in Vertebrate Eye Development. Front Cell Dev Biol 4, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N, Taketo MM, Mori M, Korinek V, Kozmik Z, 2009. Spatial and temporal regulation of Wnt/beta-catenin signaling is essential for development of the retinal pigment epithelium. Dev Biol 334, 31–45. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, Rosenberg KI, 2008. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol 317, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanti L, Revenkova E, Gordon RE, Iomini C, 2016. Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Development 143, 2160–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiao L, Sun L, Liu F, 2012. Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61, 337–346. [DOI] [PubMed] [Google Scholar]
- Hagglund AC, Berghard A, Carlsson L, 2013. Canonical Wnt/beta-catenin signalling is essential for optic cup formation. PLoS One 8, e81158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Chen SY, Zhu YT, Tseng SC, 2014. Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells. Stem Cell Res 12, 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatou S, Yoshida S, Higa K, Miyashita H, Inagaki E, Okano H, Tsubota K, Shimmura S, 2013. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem Cells Dev 22, 828–839. [DOI] [PubMed] [Google Scholar]
- Ishida I, Saika S, Okada Y, Ohnishi Y, 2005. Growth factor deposition in anterior subcapsular cataract. J Cataract Refract Surg 31, 1219–1225. [DOI] [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z, 2007. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis 45, 157–168. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Wolosin JM, Chung SH, 2017. Divergent effects of Wnt/beta-catenin signaling modifiers on the preservation of human limbal epithelial progenitors according to culture condition. Sci Rep 7, 15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Heur M, 2015. WNT10B enhances proliferation through beta-catenin and RAC1 GTPase in human corneal endothelial cells. J Biol Chem 290, 26752–26764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Xu F, Zhu J, Krawczyk M, Zhang Y, Yuan J, Patel S, Wang Y, Lin Y, Zhang M, Cai H, Chen D, Zhang M, Cao G, Yeh E, Lin D, Su Q, Li WW, Sen GL, Afshari N, Chen S, Maas RL, Fu XD, Zhang K, Liu Y, Ouyang H, 2015. Transcription Factor PAX6 (Paired Box 6) Controls Limbal Stem Cell Lineage in Development and Disease. J Biol Chem 290, 20448–20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA, 2003. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn 227, 323–334. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA, 2006. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci 47, 5088–5097. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, Ren JC, Taketo MM, van der Kooy D, Wallace VA, 2007. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol 308, 54–67. [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang L, Wang Y, Zhang H, Li L, Bao X, 2017. Dickkopf-1 inhibits Wnt3a-induced migration and epithelial-mesenchymal transition of human lens epithelial cells. Exp Eye Res 161, 43–51. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhang Z, Du E, Zheng W, Gu Q, Xu X, Ke B, 2014. Wnt signaling in form deprivation myopia of the mice retina. PLoS One 9, e91086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X, 2009. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17, 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, Kreslova J, Ruzickova J, Vacik T, Klimova L, Fujimura N, Lachova J, Kozmik Z, 2010. Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/beta-catenin signaling in the lens surface ectoderm. Genesis 48, 86–95. [DOI] [PubMed] [Google Scholar]
- Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK, 2014. The roles of αV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med 18, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey AL, Lachke SA, FitzGerald PG, Mason RW, Scheiblin DA, McDonald JH, Duncan MK, 2014a. Loss of Sip1 leads to migration defects and retention of ectodermal markers during lens development. Mech Dev 131, 86–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey AL, Terrell AM, Wang Y, Taube JR, Yallowitz AR, Duncan MK, 2014b. The Zeb proteins δEF1 and Sip1 may have distinct functions in lens cells following cataract surgery. Invest Ophthalmol Vis Sci 55, 5445–5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S, 2003. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A 100, 3299–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, Wijesinghe M, Turner K, Abud HE, Taketo MM, Noda T, Robinson ML, de Iongh RU, 2009. Conditional mutations of beta-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest Ophthalmol Vis Sci 50, 4794–4806. [DOI] [PubMed] [Google Scholar]
- Mei H, Nakatsu MN, Baclagon ER, Deng SX, 2014. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cells 32, 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA, 2006. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro GA, Marvan P, Dexl A, Pico A, Canut MI, Grabner G, Barraquer RI, Michael R, 2010. Posterior capsule opacification assessment and factors that influence visual quality after posterior capsulotomy. Am J Ophthalmol 150, 248–253. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H, 2006. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 133, 2149–2154. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX, 2011. Wnt/β-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Invest Ophthalmol Vis Sci 52, 4734–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S, Cai H, Luo J, Zhang M, Zhang M, Yang Y, Li G, Li H, Jiang W, Yeh E, Lin J, Pei M, Zhu J, Cao G, Zhang L, Yu B, Chen S, Fu XD, Liu Y, Zhang K, 2014. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 511, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA, 2012. Corneal goblet cells and their niche: implications for corneal stem cell deficiency. Stem Cells 30, 2032–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA, 2016. K14 + compound niches are present on the mouse cornea early after birth and expand after debridement wounds. Dev Dyn 245, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Park KK, Hackam AS, 2017. Wnt signaling promotes axonal regeneration following optic nerve injury in the mouse. Neuroscience 343, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau MS, Gao S, Malbon CC, Wang HY, Bertalovitz AC, 2015. The Intracellular Loop 2 F328S Frizzled-4 Mutation Implicated in Familial Exudative Vitreoretinopathy Impairs Dishevelled Recruitment. J Mol Signal 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma B, Bank RA, Boersema M, 2015. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med (Lausanne) 2, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Oh DJ, Czymmek KJ, Duncan MK, 2001. An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods 253, 243–252. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB, 2004. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol 164, 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA, 2005. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol 285, 477–489. [DOI] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS, Lang RA, 2007. pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development 134, 1873–1885. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW, 2003. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol 259, 48–61. [DOI] [PubMed] [Google Scholar]
- Sukhdeo K, Koch CE, Miller TE, Zhou H, Rivera M, Yan K, Cepko CL, Lathia JD, Rich JN, 2014. The Lgr5 transgene is expressed specifically in glycinergic amacrine cells in the mouse retina. Exp Eye Res 119, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiyab A, Korol A, Deschamps PA, West-Mays JA, 2016. beta-Catenin/CBP-Dependent Signaling Regulates TGF-beta-Induced Epithelial to Mesenchymal Transition of Lens Epithelial Cells. Invest Ophthalmol Vis Sci 57, 5736–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya S, Kaplan HJ, 2016. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp Eye Res 142, 26–31. [DOI] [PubMed] [Google Scholar]
- Telerman SB, Rognoni E, Sequeira I, Pisco AO, Lichtenberger BM, Culley OJ, Viswanathan P, Driskell RR, Watt FM, 2017. Dermal Blimp1 Acts Downstream of Epidermal TGFbeta and Wnt/beta-Catenin to Regulate Hair Follicle Formation and Growth. J Invest Dermatol 137, 2270–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Wang Y, Cheng R, Li Y, Chen M, Qiu F, Qian H, Shen D, Penalva R, Xu H, Ma JX, Chan CC, 2015. Wnt signaling in age-related macular degeneration: human macular tissue and mouse model. J Transl Med 13, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K, 2012. The many faces and functions of beta-catenin. EMBO J 31, 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G Jr., Chatterjee A, Oh SS, Oh DJ, Kang MH, Rhee DJ, 2014. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Invest Ophthalmol Vis Sci 55, 7433–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Caty J, Whitson J, Zhang AD, Srinivasagan R, Kavanagh TJ, Yan H, Fan X, 2017. Reduced Glutathione Level Promotes Epithelial-Mesenchymal Transition in Lens Epithelial Cells via a Wnt/beta-Catenin-Mediated Pathway: Relevance for Cataract Therapy. Am J Pathol 187, 2399–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormstone IM, Wang L, Liu CS, 2009. Posterior capsule opacification. Exp Eye Res 88, 257–269. [DOI] [PubMed] [Google Scholar]
- Zacharias AL, Gage PJ, 2010. Canonical Wnt/beta-catenin signaling is required for maintenance but not activation of Pitx2 expression in neural crest during eye development. Dev Dyn 239, 3215–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh LK, Zhang S, Call M, Yuan Y, Yasunaga M, Kao WW, Liu CY, 2015. Wnt/β-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 142, 3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JJ, Afshari NA, 2016. Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Invest Ophthalmol Vis Sci 57, 6878–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS, Liebler JM, Minoo P, Crandall ED, Borok Z, 2012. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J Biol Chem 287, 7026–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J, 2014. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest 124, 3825–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]


