Abstract
This work presents fabrication and characterization of flexible three-dimensional (3D) multi-electrode arrays (MEAs) capable of high signal-to-noise (SNR) electromyogram (EMG) recordings from the expiratory muscle of a songbird. The fabrication utilizes a photoresist reflow process to obtain 3D structures to serve as the electrodes. A polyimide base with a PDMS top insulation was utilized to ensure flexibility and biocompatibility of the fabricated 3D MEA devices. SNR measurements from the fabricated 3D electrode show up to a 7x improvement as compared to the 2D MEAs.
I. Introduction
Recent advances in data analysis methods in neuroscience have provided new insights on how a nervous system controls complex behaviors such as vocal learning and song production in songbirds [1-2]. Recent evidence [3,4] has pointed to the importance of precise timing of individual motor units for controlling behavior and showed that EMG activity can be used to understand how nervous systems produce behaviors.
Understanding how nervous systems produce behaviors requires recording devices and algorithms that can identify individual motor events, called muscle potentials. Among the challenges involved with obtaining high fidelity recordings suitable for neural analyses are: biological compliance of recording devices [5] and the signal to noise ratio. In addition, characterizing single motor unit activity requires a stable, reliable EMG recording for a duration long enough to produce sufficient data for advanced computational analyses [6,7].
Polyimide, PDMS and parylene-C have been widely used for the fabrication of high-density mutli-electrode arrays [3, 8-13]. To increase the signal fidelity, three-dimensional neural and muscular recording devices have also been explored [9-13]. However, these involve complex processing methodologies increasing the fabrication complexity, cost and time.
To address these challenges, this work presents fabrication and characterization of a flexible 3D MEA utilizing a simple photoresist reflow process [14] to obtain the 3D electrodes. Polyimide is the base substrate for better metal adhesion and PDMS is the top insulation layer as it is more affordable, easier to etch and can be diluted to obtain thin top insulation layer. The height of the 3D electrodes can easily be modulated by changing the film thickness of the spin coated photoresist. In vivo EMG measurements from an anesthetized songbird are also presented. The fabricated 3D MEAs provide up to 7x SNR improvement over the 2D [15] array allowing detection of small units which can otherwise get lost in noise.
II. Fabrication of 3D Multi-Electrode Arrays
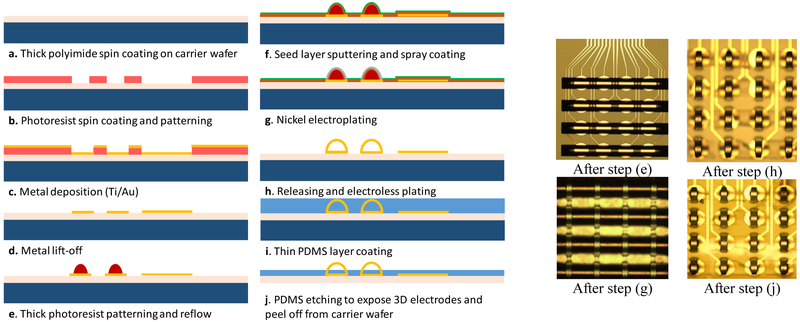
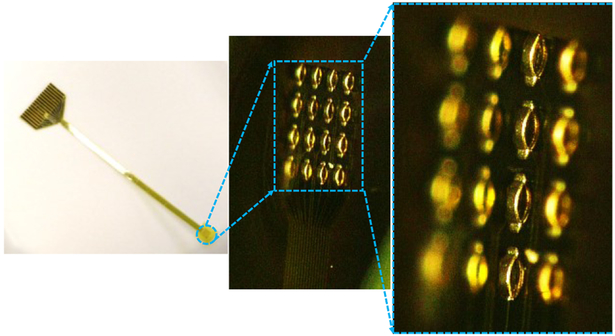
The fabrication process of the 3D MEAs is outlined in Fig. 2. Polyimide (PI-2611 from HD Microsystems) is spin coated @ 450 rpm on a carrier wafer and subsequently cured to get a thick polyimide film. Photoresist is then spin coated and patterned followed by metallization and a lift-off process. A Ti/Au layer of 30nm / 200nm is deposited using an evaporation process. Thick photoresist (AZ-40XT) is then spin coated and patterned using photolithography which is then reflowed to form the hemispherical structures as shown in Fig. 2 (e). Double-reflow process described in [14] can also be utilized here to obtain multi-height 3D electrodes in the same fabrication flow. An electroplating seed layer consisting of 50 nm of Ti and 300 nm of Cu is subsequently sputtered. Photoresist is then spray coated and the electroplating mold is formed. Nickel electroplating (10 μm thick) is then performed followed by the removal of the underlying photoresist and the seed layer to give the free-standing 3D MEAs. Electroless gold plating is then performed to passivate the electrode surface and prevent oxidation. To obtain the top insulation layer, a thin coating of PDMS (Sylgard 184, 1:10 ratio) diluted with toluene (0.9% weight ratio) is obtained and then cured. A reactive-ion etching (RIE) process is then used to etch the PDMS to expose the 3D electrodes. SF6 and O2 were used as the etching gases with a flow rate of 90 and 6 sccm respectively while the RF power was 300 watts. The etch rate obtained for the PDMS was ~ 170 nm/ min. The final 3D electrodes obtained are shown in Fig. 4.
Fig. 2.
Fabrication process flow for the flexible 3D MEAs
Fig. 4.
3D optical images of the fabricated 3D MEAs
III. EMG Measurements and SNR Comparison
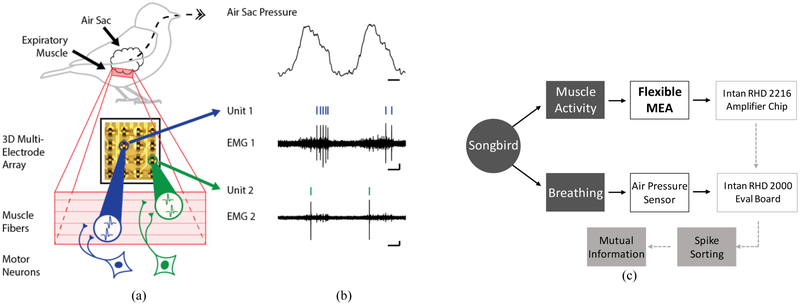
EMG and air pressure data were collected as outlined in Fig. 1(c). Rhythmic muscle activity generates air pressure during breathing. Analog signals are detected by the flexible MEA and an air pressure sensor. EMG activity from the flexible MEA is amplified and digitized by an Intan RHD2216 bipolar amplifier chip. Air pressure data was also simultaneously collected using a pressure sensor connected to a tube inserted into the air sac of the anesthetized songbird. The Intan RHD 2000 evaluation board records digital signals for both EMG and air pressure data for analysis. Spike sorting is used to distinguish individual motor units (Fig. 1) from background noise and mutual information is then used to determine the correlation between neural activity and behavior. Both 3D and 2D [15] arrays were used to record EMG activity from the expiratory muscles of anesthetized songbirds. All procedures were approved by the Emory University Institutional Animal Care and Use Committee. EMG recordings for the flexible MEA devices were collected using 16 contacts arranged in a 4×4 matrix. Example EMG units recorded on one of the 16 contacts were chosen based on their physiological properties including the type of spiking exhibited during breathing cycles (i.e., spiking at a constant, moderate firing rate versus only a few spikes at a higher firing rate) and the relative amplitude in comparison to other EMG units that were simultaneously recorded.
Fig. 1.
Schematic of experimental set up and EMG activity recorded during breathing. (a) The exhaling phase of breathing in songbirds is controlled by expiratory muscles that contract around an air sac. Motor neurons excite individual muscle fibers that cause the expiratory muscle to contract. Multielectrode arrays are used to record electromyography (EMG) activity, (b) Increases in air pressure occur when the expiratory muscles contract. Spike sorting algorithms are used to detect individual spikes (Unit 1, Unit 2) from recorded muscle activity (EMG 1, EMG 2). Tick marks above physiological traces indicate spike times. Time scale: 100 ms. Vertical scale: 30 μV, (c) Data collection flow chart; the EMG data from the flexible MEA is amplified by the using the intan RHD 2216 chip which is then fed into the evaluation board along with the air pressure data.
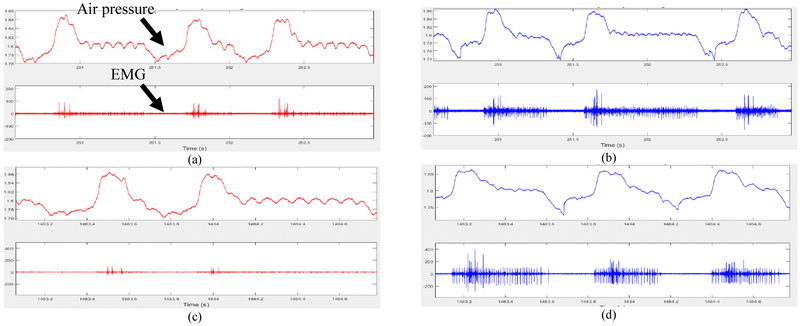
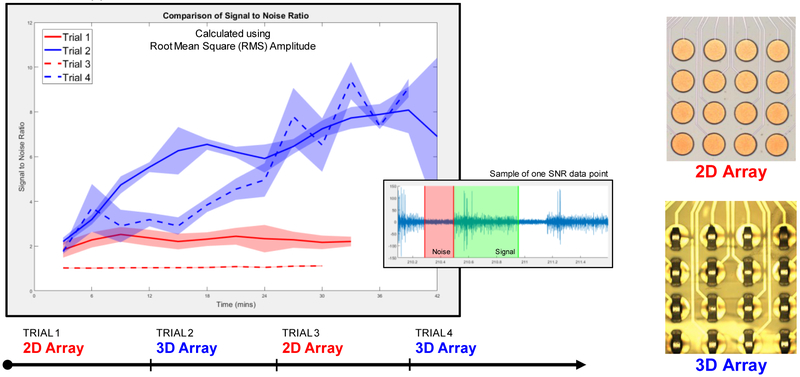
The data collection sequence of experiments for comparison of the SNR was conducted as follows: A total of four recordings of at least 30 minutes each were carried out alternating between 2D and 3D MEAs. After each recording and before the placement of the next MEA, a drop of saline was poured on the exposed expiratory muscle. Care was taken to ensure that the electrode placement was roughly over the same area of the expiratory muscle of the bird. Fig. 5 shows the EMG measurements along with the pressure data from the air sac of the bird. As seen from the figure, the 3D MEAs pick up more activity from the muscle as compared to the 2D MEA and give a more consistent recording across trials. On the other hand, the 2D array’s signal deteriorates from one trial to the next; this can be attributed to poor contact with the muscle due to protein or other unwanted material build up as the EMG recording duration increases; this is also exacerbated by the fact that the electrodes are inset below the surface of the top PDMS insulation layer. SNR calculations were performed by taking the ratio of the root-mean-square (RMS) amplitude of the waveforms during periods of activity with large or small units (signal) over periods of noise. Fig. 6 shows the SNR for the 2D and 3D MEAs over the course of the four different trials that were carried out. An average SNR was calculated in 3-minute intervals with 4 measurements during each interval used to determine the standard error of the mean (shaded region in Fig. 6).
Fig. 5.
Example air pressure and electromyograph recordings. (a,b) EMG recording after 5 minutes of array placement on the expiratory muscle of the songbird with (a) flexible 2D MEA, and (b) flexible 3D MEA. (c,d) EMG recording after 25 minutes of array placement on the expiratory muscle of songbird with (c) flexible 2D MEA, and (d) flexible 3D MEA.
Fig. 6.
Comparison of signal-to-noise ratio (SNR) using multi-electrode arrays with either 2D (red) or 3D (blue) electrode sites. Recordings for each trial were collected over at least 30-minute periods and alternated between 2D and 3D MEAs to control for non-stationary factors of the in vivo preparation. An average (solid or dashed line) SNR was calculated every 3 minutes with 4 measurements during each minute to determine a standard error of the mean (shaded regions).
Table I summarize the SNR measurements for the 2D and 3D electrodes over the different trials carried out for small and large amplitude unit activity; the 3D MEAs provide significant improvement in SNR for both small and large units with a 3.5x SNR improvement 5 minutes after the array placement and more than 7x SNR improvement 25 minutes into the recording for the larger unit. Improvement of SNR within a trial over time can be explained by better contact of the electrodes with the muscle as the saline dries out.
Table I.
SNR comparison of EMG signal recorded using the 2D and 3D MEAs. N/A is listed where signal was not discernable from noise.
| Trial # | Contact Type | Time Since Array placement (mins) |
Large Unit SNR | Small Unit SNR |
|---|---|---|---|---|
| 1 | 2D | 5 | 3.76 | 1.74 |
| 25 | 3.58 | 1.79 | ||
| 2 | 3D | 5 | 4.68 | 2.77 |
| 25 | 8.99 | 5.70 | ||
| 3 | 2D | 5 | 1.02 | N/A |
| 25 | 1.18 | N/A | ||
| 4 | 3D | 5 | 3.59 | 2.12 |
| 25 | 8.45 | 6.82 |
IV. Conclusion
A flexible 3D MEA for high SNR in vivo EMG recordings is presented. The process flow allows for easy height modulation of 3D electrodes by changing the film thickness of the photoresist. An Intan RHD2000 evaluation board and an RHD2216 amplifier chip is used to record expiratory muscle EMG activity and air pressure data. The 3D MEAs yielded higher SNR measurements over a longer duration of time as compared a 2D array. This is particularly important for detecting and analyzing smaller units which are otherwise lost in noise. Using the 3D arrays, an SNR of up to 7x was achieved and some of the improvement may have been due to better electrical isolation as excess liquid dried around the recording site. With better signal fidelity, individual units can be identified more reliably and for longer periods of time, which will allow more advanced analysis techniques that can be used to understand how nervous systems control behavior.
Fig. 3.
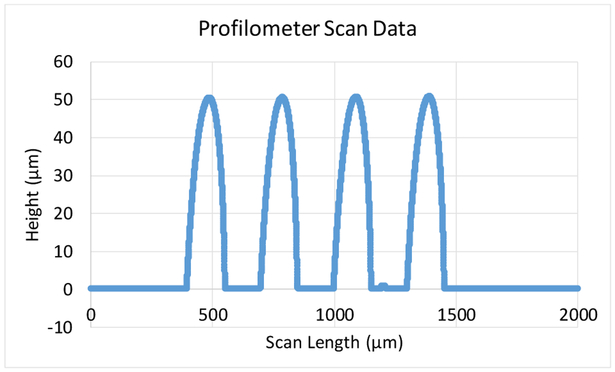
Profilometer scan data after reflow of photoresist to form the hemispherical domes (step (e) in the process flow). A 50 μm dome height was obtained for the 3D MEAs and can be modulated by changing the photoresist film thickness.
Acknowledgment
The authors would like to acknowledge HD Microsystems for providing the polyimide used for the fabrication of the 3D MEAs described in this paper. The authors would also like to acknowledge Mingu Kim for helpful discussion for the process development.
References
- [1].Mackavicius EL, Fee MS. Building a state space for song learning. Current Opinion in Neurobiology, vol. 49, pp. 59–68, April 2018. [DOI] [PubMed] [Google Scholar]
- [2].Srivastava KH, Elemans CPH, Sober SJ. Multifunctional and context- dependent control of vocal acoustics by individual muscles. J Neurosci, vol. 35, iss. 42, pp. 14183–14194, October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Srivastava KH, et al. Motor control by precisely timed spike patterns. PNAS, vol. 114, iss. 5, pp. 1171–1176, January 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tang C, Chehayeb D, Srivastava K, Nemenman I, Sober SJ. Millisecond-scale motor encoding in a cortical vocal area. PLoS Biology, vol. 12, iss. 12, December 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weltman Ahuva, Yoo James and Meng Ellis, “Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Fabrication”, Micromachines 2016, 7(10), 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kraskov A, Stogbauer H, Grassberger P. Estimating mutual information. Phys Rev E Stat Nonlin Soft Matter Phys, vol. 69, iss. 6, pt. 2, 066138, June 2004. [DOI] [PubMed] [Google Scholar]
- [7].Nemenman I, Bialek W, de Ruyter van Steveninck. Entropy and information in neural spike trains: Progress on the sampling problem. Physical Review E, vol. 69, iss. 5, 056111, May 2004. [DOI] [PubMed] [Google Scholar]
- [8].Kim O, et al. “Novel neural interface electrode array for the peripheral nerve,” in Rehabilitation Robotics (ICORR), 2017 International Conference on, IEEE, pp. 106–1072, July 2017. [DOI] [PubMed] [Google Scholar]
- [9].Lin K, Wang X, Zhang X, Wang B, Huang J, Huang F. An FPC based flexible dry electrode with stacked double-micro-domes array for wearable biopotential recording system. Microsystem Technologies, vol. 23, iss. 5, pp.1443–1451, May 2017. [Google Scholar]
- [10].Guvanasen GS, et al. A stretchable microneedle electrode array for stimulating and measuring intramuscular electromyograhpic activity Neural Systems and Rehabilitation Engineering, Transactions on, IEEE, no. 9, pp. 1440–1452, September 2017. [DOI] [PubMed] [Google Scholar]
- [11].Kim JM, Im C, Lee WR. Plateau-shaped flexible polymer microelectrode array for neural recordings. Polymers, vol. 9, iss. 12, p. 690, December 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Metallo C, White RD, Trimmer BA. Flexible parylene-based microelectrode arrays for high resolution EMG recordings in freely moving small animals. J Neurosci Methods, vol. 195, iss. 2, pp. 176–184, February 2011. [DOI] [PubMed] [Google Scholar]
- [13].Nandra MS, Lavrov IA, Edgerton VR, Tai YC. “A parylene-based microelectrode array implant for spinal cord stimulation in rats,” in Micro Electro Mechanical Systems (MEMS), 2011 IEEE 24th International Conference on, IEEE, pp. 1007–1010, January 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang C, Yang HS, and Bakir MS, “A double-lithography and double-reflow process and application to multi-pitch multi-height mechanical flexible interconnects,” J. Micromech. Microeng, vol. 27, no. 2, p. 025014, 2017. [Google Scholar]
- [15].Zia M, Chung B, Sober SJ and Bakir MS, “In Vivo EMG recording from breathing muscle of Songbird using hybrid polyimide-PDMS flexible multi-electrode arrays”, to be published. [Google Scholar]