Abstract
Aims/Introduction
The present study aimed to investigate the effects of low‐intensity resistance training with slow movement and tonic force generation (LST) on muscular function and glucose metabolism in older patients with type 2 diabetes.
Materials and Methods
A total of 10 patients with type 2 diabetes (age 68.2 ± 9.7 years) engaged in LST training twice a week for 16 weeks. Before the long‐term intervention, they were subjected to the measurement of acute changes in blood factors relating to glycemic control as a result of a bout of LST. Body composition, muscular size and strength, and glycated hemoglobin were measured before and after the intervention.
Results
The magnitudes of the acute changes in the blood factors were all small and were not considered harmful for glucose metabolism. The 16‐week LST training caused significant increases in thigh muscle thickness and strength, and decreases in body fat mass and glycated hemoglobin. The change in glycated hemoglobin showed a significant negative correlation with the change in the isokinetic knee extension peak torque measured at a high angular velocity (180°/s).
Conclusions
The LST training was shown to be effective for gaining muscular size and strength, and improving glycemic control in older patients with type 2 diabetes. The mechanisms underlying this effect might involve the improvement of contractile function in fast glycolytic fibers.
Keywords: Fast muscle fibers, Glycated hemoglobin, Resistance training
Introduction
Aerobic exercise training has generally been used as exercise therapy of type 2 diabetes patients. Recently, however, resistance training (RT) has been found to be as effective for improving glycemic control. The American Diabetes Association recommends that patients with diabetes carry out ≥150 min of moderate‐to‐vigorous intensity aerobic exercise per week, as well as two to three sessions/week of RT1. Furthermore, a recent meta‐analysis concluded that RT improves glycemic control2. Although its exact mechanism remains unclear, it has been suggested that increases in muscle mass and glucose transporter content (GLUT4) lead to an increase in the capacity of glucose uptake3, 4.
In general, RT at middle‐to‐high intensity (−80% one‐repetition maximum [1RM]) has been regarded as optimal for gaining muscular size and strength, whereas RT at intensities <65% 1RM has been considered far less effective5. For patients with type 2 diabetes, previous studies showed that RT at middle‐to‐high intensity significantly improved glycemic control and insulin resistance6, 7, whereas low‐intensity RT (40–50% 1RM) was not effective8. However, RT at −80% 1RM has strong, acute effects on systolic blood pressure, sympathetic nerve function and endocrine activities related to glycemic control9. Given the additional risks for orthopedic injury, RT at middle‐to‐high intensity might not be appropriate for all, particularly older patients with type 2 diabetes.
Low‐intensity resistance training with slow movement and tonic force generation (LST) was found to be effective for gaining muscular size and strength in young untrained men, even at an intensity lower than −50% 1RM10, 11. In addition, a recent study showed that LST led to significant increases in muscle size and strength in healthy older participants12, 13. Based on studies with young participants, the mechanism underlying the muscle‐hypertrophic effect of LST has been thought to involve the lowered intramuscular oxygenation by sustained tension, the subsequent changes in muscle fiber recruitment and the local accumulation of metabolic products10, 11. If the same mechanism operates, LST would cause hypertrophy in fast glycolytic muscle fibers, thereby effectively promoting the glucose metabolism. Actually, a recent study showed that LST improved glucose and lipid metabolisms in obese patients with type 2 diabetes14. However, the association between changes in muscular function and the improvement of glycemic control induced by LST has not been clarified.
In the present study, we investigated both the acute and the long‐term (16 weeks) effects of LST on glucose metabolism in older patients with type 2 diabetes to obtain an insight into the mechanism underlying the effects of LST on glucose metabolism.
Methods
Participants and study design
From the outpatients with treated (diet and medication) type 2 diabetes at the Kitasato Institute Hospital (Tokyo, Japan), 15 eligible patients (estimated number for adequate intervention study at the hospital) aged 55–80 years were recruited by posters. They were sedentary (persons inexperienced in RT) and their glycated hemoglobin (HbA1c) range was 6.5–8.0%. Their participation in this study was regarded as appropriate at the discretion of the attending physician. Patients with severe osteoarthritis, history of stroke in the past 3 months, coronary artery disease in the past 1 year or an increase in blood creatine phosphokinase level in the past 1 year were excluded. Eventually, 10 patients (mean age 68.2 ± 9.7 years; 50% women) participated in the period from May 2012 to December 2013. Written informed consent was obtained from each patient, and the protocol of this study was approved by the institutional review board of the Kitasato Institute Hospital (Study No. 11059) and registered by University Hospital Medical Information Network Clinical Trials Registry (UMIN000020249). The study was carried out in accordance with the Declaration of Helsinki.
The study was a 16‐week clinical trial including acute study in the initial period. The participants carried out LST exercises twice a week using the fitness facility of the Diabetes Center at the Kitasato Institute Hospital, in addition to receiving their usual care during the 16 weeks (2‐week preparation and 14‐week intervention). Figure 1 is a schematic representation of the study protocol.
Figure 1.
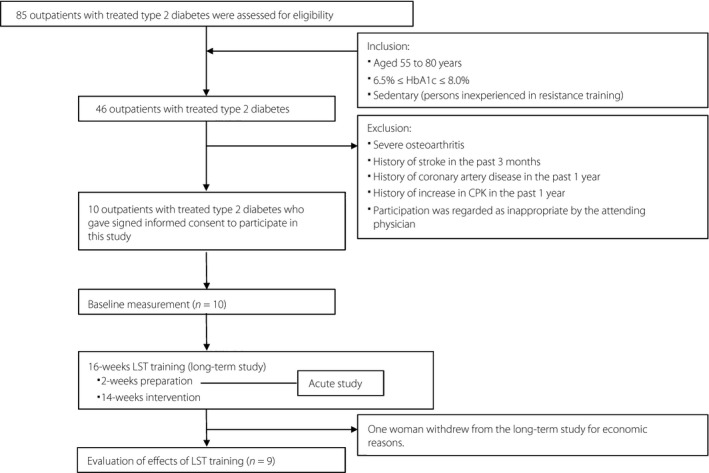
Schematic representation of the study protocol. CPK, blood creatine phosphokinase; HbA1c, glycated hemoglobin; LST, low‐intensity resistance training with slow movement and tonic force generation.
In the initial study protocol, we planned to set a control group with the same number of patients who are subjected to standard treatment without LST exercise using a random assignment method. However, it could not be carried out, because we could not obtain participation agreements from patients assigned to the control group. Therefore, the study design was changed to the single group intervention test without a control group.
Among participants in the acute study, one woman withdrew from the long‐term study at the early stage of the 16‐week intervention period for financial reasons. Therefore, the data of 10 participants were used for the analysis of the acute study, and the data from nine participants (mean age 69.8 ± 8.8 years; 5 men and 4 women) who completed the 16‐week intervention were used for the analysis of the long‐term study. For one of the participants in the long‐term study, we could not obtain the body composition data after the 16‐week intervention (i.e., post‐data). The missing body composition data of this participant were compensated for by analyzing the baseline data (baseline observation carried forward analysis).
Exercise training
The participants completed a 16‐week LST program consisting of three resistance exercises using bodyweight; that is, squats, scissor squats (squats with an open stance) and crunches (trunk flexion), twice a week under the instruction of skilled physiotherapists. The resistance exercise was based on the LST method; that is, slow movement and tonic force generation (3 s for concentric, 3 s for eccentric and 1 s for isometric actions with no rest between each repetition). For each exercise, they carried out two to three sets with the repetition maximum (RM: 8–15 repetitions), until which they could continue exercise movement at the proper speed and range of motion. Although the exercise volume in a session was finely adjusted to the physical condition on the day of exercise, it was basically unchanged in each participant throughout the intervention. The rest periods between sets and exercises were 1 min and 2 min, respectively. A 5‐min walk on a treadmill, and stretching for trunk and lower extremities were also carried out before and after the LST exercises.
Acute study
LST has been shown to give rise to acute increases in blood lactate, growth hormone and cortisol concentrations10, 15. These responses might not only be related to the chronic adaptation of muscle, but also affect the glucose metabolism in diabetes patients. Thus, to see whether the present LST program is associated with any undesirable effect on older diabetes patients, we investigated acute changes in blood factors after a bout of exercise at the time between the fourth and eighth training sessions during the 16‐week intervention period. Two experiment protocols (protocols 1 and 2) separated by >2 days were carried out randomly.
Protocol 1
All participants were instructed to fast, except for drinking water, during the 10 h before the experiment to minimize any acute influences of nutritional status. After arriving at the fitness facility (08.30 hours), they were allowed to rest on a chair for 30 min, and at 09.00 hours, blood lactate concentration was measured, and venous blood samples were taken for the measurements of fasting plasma glucose and hormone concentrations (serum insulin, growth hormone, and cortisol, plasma adrenaline and noradrenaline). Next, the participants carried out LST for approximately 15 min (14–16 min), and blood samples were taken immediately, and 15 min and 30 min afterwards. Blood lactate concentration was measured using an enzymatic method (Lactate Pro; Arkray Inc., Kyoto, Japan). Serum and plasma samples were stored at 4°C and −20°C, respectively. Concentrations of growth hormone, cortisol, insulin, adrenaline and noradrenaline were determined using conventional methods.
Protocol 2
The participants were subjected to the same procedure as in protocol 1, but took rest for 15 min in place of doing LST.
Long‐term study
Body composition and muscle thickness
Bodyweight, fat mass and lean body mass were measured with dual‐energy X‐ray absorptiometry using a DPX‐L densitometer (iDXA; GE Healthcare Japan Inc., Tokyo, Japan). The muscle thickness of the front portion of the right thigh was measured using B‐mode ultrasound imaging. While the participants were standing, the midpoint between the lateral epicondyle of the femur and the greater trochanter was determined and marked as the measurement point. Transverse images were obtained using a real‐time liner electronic scanner with a 7.5‐MHz scanning head (SSD‐500; Aloka Inc., Tokyo, Japan). The measurements were taken three times, and the mean values from the three measurements were used for analysis. The intraclass correlation coefficient and the mean coefficient of variance for the repeated measurements were 0.986 and 1.6%, respectively.
Lower limb muscle strength
Knee extension and flexion strengths were measured with an isokinetic dynamometer (Cybex2010; Medica Co. Ltd., Fukuoka, Japan). The isokinetic peak torques for both knee extension and flexion were measured at an angular velocity of 60°/s and 180°/s. The measurement at each angular velocity was taken for three reciprocal motions of knee extension and flexion with the range of knee angle between 135° and 0°. After conditioning trials at submaximal effort, the measurement at maximal effort was taken at each angular velocity, first at 60°/s and then at 180°/s, with a 30‐s rest period. The mean values for both the right and left legs at each angular velocity were used for analyses. In addition, the maximum strength for leg press was also measured with a pneumatic leg press machine (Cybex; Proavance Inc., Tokyo, Japan). The maximum strength was evaluated by using “3RM strength”; that is, the maximal weight that can be lifted three times per set.
Blood examination
Venous blood samples were taken after a 10‐h overnight fast. The primary objective was to evaluate the changes in HbA1c level from the original level at the end of the 16‐week intervention period. The secondary variables to measure included fasting plasma glucose and the lipid levels (total cholesterol, triglycerides, high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol calculated using the Friedewald equation16). For HbA1c, the changes during the 16‐week period before the 16‐week intervention period were used as an internal control for the same participant.
Nutritional information
A 3‐day food record was obtained to assess changes in nutrient intake. All nutritional information obtained from the food records was analyzed using the Foodworks nutrient analysis software program (Excel Eiyou‐kun; Kenpakusha, Tokyo, Japan).
Statistical analysis
All values are expressed as mean ± standard deviation. To assess the acute effect of LST, a two‐way analysis of variance (anova) with repeated measures followed by post‐hoc analysis (Bonferroni's test) was used to examine the effects of occasion and time, and their interaction in all variables after a single bout of LST exercise or rest. To assess the long‐term effect of LST, the Wilcoxon signed‐rank test was applied to evaluate changes in all variables. For all statistical tests, P < 0.05 was considered significant. The P‐value was reported with an effect size of d.
Results
Baseline characteristics
Baseline physical and clinical characteristics of the participants are summarized in Table 1. Because one woman dropped out during the long‐term study, the data of 10 participants were used for the analysis of the acute study and the data of nine participants were used for the analysis of the long‐term study.
Table 1.
Baseline physical and clinical characteristics
| Acute study | Long‐term study | |
|---|---|---|
| n | 10 | 9 |
| Sex (men/women) | 5/5 | 5/4 |
| Age (years) | 68.2 ± 9.7 | 69.8 ± 8.8 |
| Height (cm) | 162.9 ± 6.7 | 163.2 ± 7.0 |
| Weight (kg) | 64.8 ± 8.2 | 65.0 ± 8.7 |
| Body mass index (kg/m2) | 24.4 ± 2.4 | 24.4 ± 2.6 |
| Retinopathy | ||
| None | 9 | 8 |
| Simple | 1 | 1 |
| Preproliferative | 0 | 0 |
| Proliferative | 0 | 0 |
| Albuminuria | ||
| Normo | 9 | 8 |
| Micro | 1 | 1 |
| Macro | 0 | 0 |
| Treatment | ||
| Untreated | 0 | 0 |
| Oral hypoglycemic agents | 9 | 8 |
| Insulin | 1 | 1 |
| Anticholesteremic agents | 5 | 4 |
| Antihypertensive agents | 3 | 3 |
Age, height, weight and body mass index are expressed as mean ± standard deviation. Other variables are shown as the number of participants.
Most participants (9/10) were taking oral hypoglycemic agents, and one of them was receiving additional insulin therapy. In addition, five and three participants were taking lipid‐lowering drugs and antihypertensive agents, respectively. No participant increased the daily dose of hypoglycemic agents during the study period.
Acute study
The concentrations of fasting plasma glucose and hormones were measured at rest (pre), immediately after a single bout of exercise (post), and 15 min and 30 min after the exercise (post 15 min, post 30 min) with protocol 1 (exercise) and protocol 2 (control, i.e., rest in place of exercise).
For fasting plasma glucose, a two‐way anova showed no significant main effects of group or time, which were superseded by a significant interaction effect of group by time (P < 0.01). Fasting plasma glucose after exercise (128 ± 21 mg/dL at post, 130 ± 23 mg/dL at post 15 min and 131 ± 25 mg/dL at post 30 min) was significantly higher than at rest (125 ± 18 mg/dL; P < 0.05). In control trials, fasting plasma glucose after rest gradually decreased from 127 ± 23 mg/dL to 124 ± 22 mg/dL at post 30 min (P < 0.05). Even though these changes were statistically significant, the magnitudes of change were far smaller than what would be considered problematic.
The concentration of serum insulin showed no significant main effect of group, a significant main effect of time (P < 0.05), and no significant interaction effect of group by time. LST exercise caused slight, but significant, increases in the concentration of serum insulin, from 6.1 ± 2.1 μIU/mL at rest to 8.1 ± 3.4 μIU/mL (P < 0.01) at post 15 min.
The concentrations of blood lactate and plasma noradrenaline showed significant main effects of group (P < 0.01) and time (P < 0.01), and also significant interaction effects of group by time (P < 0.01). LST exercise caused significant increases in the concentrations of blood lactate and plasma noradrenaline from rest to the peaks at post (from 1.2 ± 0.3 mmol/L to 4.4 ± 2.0 mmol/L, and from 514 ± 210 pg/mL to 824 ± 433 pg/mL, respectively, P < 0.01). In the control group, blood lactate and plasma noradrenaline did not show significant changes throughout the protocol. The magnitudes of change in lactate and noradrenaline after LST exercise were similar to those reported for healthy older participants in the previous study12. No significant differences were seen in the concentrations of serum growth hormone, plasma adrenaline, and cortisol between the LST exercise and control trials.
Long‐term study
Changes in body composition and muscle size and strength
After the 16‐week intervention of LST training, bodyweight, body fat mass and body fat percentage decreased significantly, whereas body mass index tended to decrease. Muscle thickness of the front portion of the thigh increased significantly. Both the ratio of total lean body mass to bodyweight and the ratio of lean body mass of trunk to bodyweight increased significantly. With regard to muscle strength, all of the related parameters measured increased significantly (Table 2).
Table 2.
Changes in participant characteristics
| Pre‐training | Post‐training | P | ES | |
|---|---|---|---|---|
| Changes in measures of body composition | ||||
| Bodyweight (kg) | 65.0 ± 8.7 | 64.1 ± 8.4 | 0.0499 | 0.10 |
| Body mass index (kg/m2) | 24.4 ± 2.6 | 24.1 ± 2.5 | 0.069 | 0.12 |
| Body fat mass (kg) | 21.2 ± 5.0 | 20.2 ± 5.0 | 0.012 | 0.20 |
| Body fat percentage (%) | 32.8 ± 6.8 | 31.5 ± 6.6 | 0.012 | 0.18 |
| Total LBM (kg) | 41.3 ± 6.7 | 41.4 ± 5.9 | 0.866 | 0.02 |
| LBM of lower limbs (kg) | 13.3 ± 2.3 | 13.3 ± 2.3 | 0.889 | 0.01 |
| LBM of trunk (kg) | 19.6 ± 2.9 | 20.0 ± 2.7 | 0.161 | 0.12 |
| Total LBM/bodyweight (%) | 63.6 ± 6.6 | 64.7 ± 6.5 | 0.012 | 0.18 |
| LBM of lower limbs/bodyweight (%) | 20.4 ± 1.9 | 20.7 ± 2.0 | 0.674 | 0.15 |
| LBM of trunk/bodyweight (%) | 30.3 ± 3.5 | 31.4 ± 4.1 | 0.017 | 0.27 |
| Muscle thickness of front portion of the thigh (mm) | 42.3 ± 6.0 | 44.3 ± 7.5 | 0.021 | 0.29 |
| Changes in glycemic control | ||||
| HbA1c (%) | 7.1 ± 0.5 | 6.8 ± 0.4 | 0.066 | 0.54 |
| Fasting plasma glucose (mg/dL) | 124.8 ± 16.5 | 129.6 ± 31.2 | 0.767 | 0.19 |
| Changes in muscle strength | ||||
| Isokinetic knee extension peak torque (N·m) | ||||
| 60°/s | 72.9 ± 32.1 | 80.4 ± 28.4 | 0.015 | 0.25 |
| 180°/s | 46.3 ± 17.7 | 52.7 ± 19.6 | 0.021 | 0.34 |
| Isokinetic knee flexion peak torque (N·m) | ||||
| 60°/s | 42.7 ± 20.7 | 50.9 ± 20.2 | 0.012 | 0.40 |
| 180°/s | 25.3 ± 12.4 | 36.2 ± 12.3 | 0.008 | 0.88 |
| Maximum leg press strength (kg) | 62.4 ± 19.9 | 101.2 ± 22.7 | 0.008 | 1.82 |
Values are expressed as mean ± standard deviation. ES, effect size; LBM, lean body mass; HbA1c, glycated hemoglobin.
Changes in HbA1c, Glucose and Lipid Levels
After the 16‐week intervention of LST training, HbA1c tended to decrease from 7.06 ± 0.46% to 6.82 ± 0.41% (P = 0.07; Table 2). For internal control, the mean value of HbA1c in the same nine participants did not change during the period of 16 weeks before the intervention (6.90 ± 0.71 to 7.06 ± 0.46; P = 0.31).
A close examination of the change in each participant showed that the value increased markedly in only one participant, mainly as a result of environmental changes (see Discussion), but decreased in all of the remaining eight participants (Figure 2). When the values from this participant were withdrawn, the mean HbA1c significantly decreased after the intervention period (7.11 ± 0.46 to 6.80 ± 0.43; P < 0.05).
Figure 2.
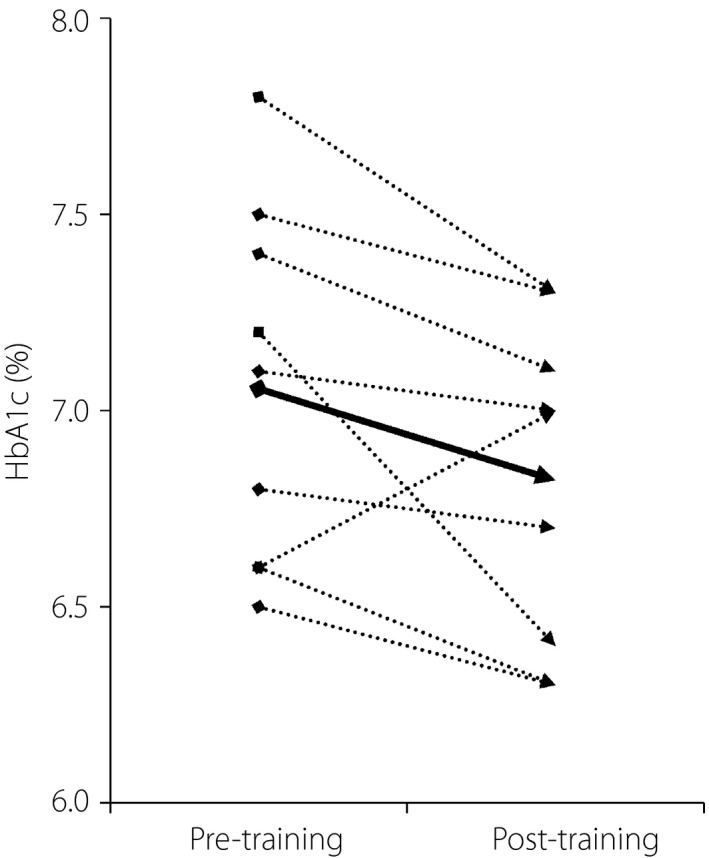
Changes in glycated hemoglobin (HbA1c) in the long‐term study. Dotted lines show the changes for each participant. The solid bold line shows the change in mean value for all (9) participants.
No significant changes were seen in fasting plasma glucose (Table 2) and lipid levels (Table S1).
Relationships between changes in HbA1c and Muscle strength
A significant correlation was found between the change in HbA1c and the change in the isokinetic knee extension peak torque at the angular velocity of 180°/s (Figure 3a). In contrast, no such association was found between the change in HbA1c and the change in the isokinetic knee extension peak torque at the angular velocity of 60°/s (Figure 3b). In the isokinetic knee flexion peak torque, such an association with the change in HbA1c was found at the angular velocity of neither 180°/s nor 60°/s.
Figure 3.
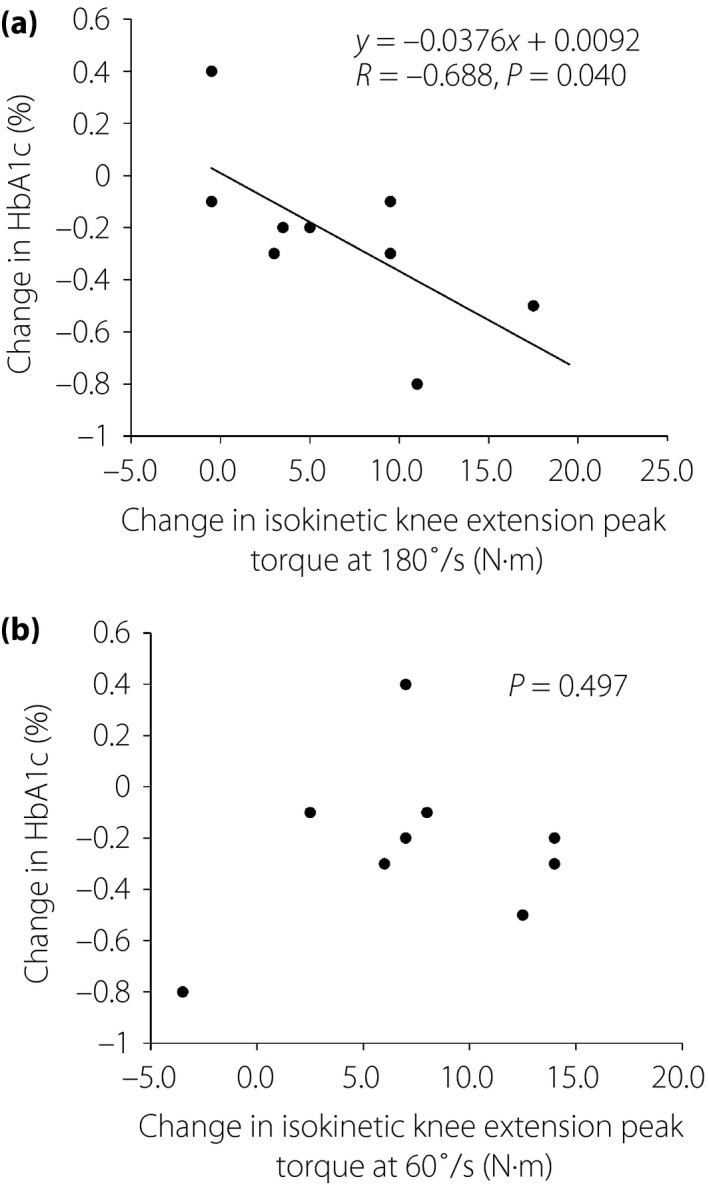
Change in glycated hemoglobin (HbA1c) versus changes in isokinetic knee extension peak torque. (a) Isokinetic knee extension peak torque at 180°/s. (b) Isokinetic knee extension peak torque at 60°/s.
Discussion
The present study on acute effects showed that LST caused increases in the concentrations of plasma glucose, serum insulin and plasma noradrenaline, suggesting that LST causes secretion of noradrenaline through sympathetic activation, and there is a subsequent increase in blood glucose and secretion of insulin. These changes are physiologically normal responses to resistance exercise, and the magnitude of changes would not be harmful for glucose metabolism15. To our knowledge, this is the first study to show the acute effect of LST on glucose metabolism in older patients with type 2 diabetes. Based on these results, it was confirmed that the present LST program causes no undesirable, acute effects on glycemic control in older patients with type 2 diabetes.
In addition, the present results showed a significant increase in blood lactate after LST, and the magnitude of increase was comparable with that reported for LST with healthy, older adults12. Because lactate is mainly produced by fast‐twitch muscle fibers17 with faster contraction speed and higher glycolytic activity than slow‐twitch muscle fibers18, this LST program is expected to recruit a considerable number of the fast glycolytic muscle fibers.
After the 16‐week intervention with the LST program, HbA1c was significantly decreased in the remaining eight participants, excluding one who was judged as having a problem (7.11 ± 0.46 to 6.80 ± 0.43; P < 0.05), though it tended to decrease in all nine participants (7.06 ± 0.46 to 6.82 ± 0.41; P = 0.07). The participant was only one who showed an increase of HbA1c after the intervention, probably due to a large change in the daily nutrient condition (a hospitalization of his wife during the intervention). The participant's daily energy intake increased by 12.4% at the end of the intervention period, and this change was the largest among the participants.
A recent study showed that a 12‐week intervention with a training program similar to the present one gave rise to a significant decrease in HbA1c in obese patients with type 2 diabetes (8.6 ± 2.4 to 7.2 ± 1.2; P = 0.001)14. In contrast, we failed to show a marked decrease in HbA1c. One of the possible reasons for this discrepancy would be the difference in the initial HbA1c level; that is, 8.6% versus 7.1%. According to a recent meta‐analysis, a larger effect size was observed in studies of participants with diabetes with a high baseline HbA1c (≥7.5% vs <7.5%; P = 0.01)19.
The present study showed a significant negative correlation between the change in HbA1c and the change in the isokinetic knee extension peak torque at a high angular velocity (180°/s). As changes in muscular strength at high velocity might readily reflect functional changes in fast motor units, this suggests that the improvement of contractile function in fast‐twitch fibers relates to the improvement of glycemic control. It is plausible that hypertrophy and/or preferred recruitment of fast‐twitch fibers with higher glycolytic activity led to an increase in the capacity of glucose uptake from blood18. The reason why no such association was found between the change in HbA1c and the change in the isokinetic knee flexion peak torque at the angular velocity of 180°/s might be that the knee flexor muscle contains a smaller percentage of fast fibers than does extensor muscle20.
However, the “size principle” for motor unit recruitment21 predicts that low‐intensity RT preferentially recruits small motor units with slow‐twitch fibers. As fast‐twitch fibers have a larger capacity for hypertrophy than slow‐twitch fibers, low‐intensity RT has been considered less effective for muscle hypertrophy than high‐intensity RT that readily causes the recruitment of large motor units with fast‐twitch fibers5. By contrast, recent studies have shown that low‐intensity RT is also highly effective to gain muscle size and strength when either mechanical impulse (force × time) or exercise volume (force × repetition) is sufficiently large10, 22. Although the mechanisms underlying such effects of low‐intensity RT remain unclear, it has been speculated that particular mechanical conditions, such as prolonged exertion of small force with slow movement, facilitates the additional recruitment of fast‐twitch fibers through muscular fatigue23, 24.
In the present study, a bout of LST training caused a significant increase in blood lactate, suggesting the recruitment of fast‐twitch fibers during the exercise. After the period of training, a significant correlation was found between the strength gain measured at fast movement and the decrease in HbA1c. These suggest that the mechanisms underlying the effect of LST on glycemic control involve hypertrophy and/or improved contractile function of fast‐twitch fibers. In addition, a greater decrease in HbA1c was found in relatively young participants, probably due to the aging‐related decrease in the relative number of fast‐twitch fibers25.
There were several limitations to interpreting the present effects of LST training. First, the absence of an untrained control group for measurement other than HbA1c weakens the evidence for the effects of long‐term intervention of LST. Therefore, further study with randomized controlled trials is to be carried out with a sufficiently large sample size. Second, despite the increase in muscle size (i.e., muscle thickness) after the intervention of LST training, the change in muscle size did not show a significant correlation with the change in HbA1c, as did muscle strength. This is probably because muscle strength is also related to neural factors; that is, the ability to recruit motor units, in addition to muscle size26. It has been shown that, in older people, the contribution of neural factors predominates that of muscular size in strength gain after RT27. Thus, muscular strength reflecting both neural factors and muscle size might show a clearer association with the change in HbA1c than does muscle size alone. Alternatively, the gain in muscle strength might directly affect the daily life of the participants so as to make them more active.
In conclusion, the 16‐week LST training might be effective for improving glycemic control in older patients with type 2 diabetes. The mechanisms underlying this effect involve the improvement of contractile function in fast glycolytic fibers. Owing to its low exercise intensity, it should be useful as a countermeasure against diabetes in older patients, particularly those associated with sarcopenia.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 ¦ Changes in lipid levels.
Acknowledgments
The authors thank all of the participants for their cooperation. This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
J Diabetes Investig 2019; 10: 331–338
Clinical Trial Registry
University Hospital Medical Information Network Clinical Trials Registry
UMIN000020249
References
- 1. American Diabetes Association . Lifestyle management: standards of medical care in diabetes‐2018. Diabetes Care 2018; 41(suppl 1): S38–S50. [DOI] [PubMed] [Google Scholar]
- 2. Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: A systematic review and meta‐analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med 2010; 40: 397–415. [DOI] [PubMed] [Google Scholar]
- 3. Baldi JC, Snowling N. Resistance training improves glycaemic control in obese type 2 diabetic men. Int J Sports Med 2003; 24: 419–423. [DOI] [PubMed] [Google Scholar]
- 4. Gaster M, Vach W, Beck‐Nielsen H, et al GLUT4 expression at the plasma membrane is related to fibre volume in human skeletal muscle fibres. APMIS 2002; 110: 611–619. [DOI] [PubMed] [Google Scholar]
- 5. McDonagh MJ, Davies CT. Adaptive response of mammalian skeletal muscle to exercise with high loads. Eur J Appl Physiol Occup Physiol 1984; 52: 139–155. [DOI] [PubMed] [Google Scholar]
- 6. Wycherley TP, Noakes M, Clifton PM, et al A high‐protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care 2010; 33: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black LE, Swan PD, Alvar BA. Effects of intensity and volume on insulin sensitivity during acute bouts of resistance training. J Strength Cond Res 2010; 24: 1109–1116. [DOI] [PubMed] [Google Scholar]
- 8. Kwon HR, Han KA, Ku YH, et al The effects of resistance training on muscle and body fat mass and muscle strength in type 2 diabetic women. Korean Diabetes J 2010; 34: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyachi M. Effects of resistance training on arterial stiffness: a meta‐analysis. Br J Sports Med 2013; 47: 393–396. [DOI] [PubMed] [Google Scholar]
- 10. Tanimoto M, Ishii N. Effects of low‐intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Appl Physiol 2006; 100: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 11. Tanimoto M, Sanada K, Yamamoto K, et al Effects of whole‐body low‐intensity resistance training with slow movement and tonic force generation on muscular size and strength in young men. J Strength Cond Res 2008; 22: 1926–1938. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe Y, Tanimoto M, Ohgane A, et al Increased muscle size and strength from slow‐movement, low‐intensity resistance exercise and tonic force generation. J Aging Phys Act 2013; 21: 71–84. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe Y, Madarame H, Ogasawara R, et al Effect of very low‐intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin Physiol Funct Imaging 2014; 34: 463–470. [DOI] [PubMed] [Google Scholar]
- 14. Hamasaki H, Kawashima Y, Tamada Y, et al Associations of low‐intensity resistance training with body composition and lipid profile in obese patients with type 2 diabetes. PLoS ONE 2015; 10: e0132959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goto K, Ishii N, Kizuka T, et al Hormonal and metabolic responses to slow movement resistance exercise with different durations of concentric and eccentric actions. Eur J Appl Physiol 2009; 106: 731–739. [DOI] [PubMed] [Google Scholar]
- 16. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18(6): 499–502. [PubMed] [Google Scholar]
- 17. Brooks GA. Intra‐ and extra‐cellular lactate shuttles. Med Sci Sports Exerc 2000; 32: 790–799. [DOI] [PubMed] [Google Scholar]
- 18. Essén B, Jansson E, Henriksson J, et al Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand 1975; 95: 153–165. [DOI] [PubMed] [Google Scholar]
- 19. Ishiguro H, Kodama S, Horikawa C, et al In search of the ideal resistance training program to improve glycemic control and its indication for patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. Sports Med 2016; 46: 67–77. [DOI] [PubMed] [Google Scholar]
- 20. Prietto CA, Caiozzo VJ. The in vivo force‐velocity relationship of the knee flexors and extensors. Am J Sports Med 1989; 17(5): 607–611. [DOI] [PubMed] [Google Scholar]
- 21. Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 1965; 28: 560–580. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell CJ, Churchward‐Venne TA, West DW, et al Resistance exercise load does not determine training‐mediated hypertrophic gains in young men. J Appl Physiol 2012; 113(1): 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burd NA, West DW, Staples AW, et al Low‐load high volume resistance exercise stimulates muscle protein synthesis more than high‐load low volume resistance exercise in young men. PLoS ONE 2010; 5: e12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burd NA, Andrews RJ, West DW, et al Muscle time under tension during resistance exercise stimulates differential muscle protein sub‐fractional synthetic responses in men. J Physiol 2012; 590: 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand 1983; 117: 469–471. [DOI] [PubMed] [Google Scholar]
- 26. Ikai M, Fukunaga T. A study on training effect on strength per unit cross‐sectional area of muscle by means of ultrasonic measurement. Int Z Angew Physiol 1970; 28: 173–180. [DOI] [PubMed] [Google Scholar]
- 27. Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 1979; 58: 115–130. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 ¦ Changes in lipid levels.


