Abstract
Background:
Robotic complex abdominal wall reconstruction (r-AWR) using transversus abdominis release (TAR) is associated with decreased wound complications, morbidity, and length of stay compared with open repair. This report describes a single-institution experience of r-AWR.
Methods:
A retrospective chart review was performed on patients who underwent r-AWR by a single surgeon (D.H.) from August 2015 through October 2018.
Results:
Fifty-five patients underwent r-AWR (16 males [29%] and 39 females [71%]) with a mean age of 60.2 (range 33 to 87) years and a mean body mass index of 34.6 (range 23 to 54) kg/m2. Forty-one patients presented with an initial ventral hernia (74.5%) and 14 with a recurrent hernia (25.5%). Five patients had a grade 1 hernia (9.1%), 46 had a grade 2 hernia (83.6%), and 4 had a grade 3 hernia (7.3%) according to the Ventral Hernia Working Group system. Thirty-four (62%) patients underwent TAR, 21 (38%) patients underwent bilateral retrorectus release, and 10 (18.2%) patients underwent concomitant inguinal hernia repair. Mean operative time with TAR was 294 (range 106 to 472) minutes and 183 (range 126 to 254) minutes without TAR. Mean length of stay was 1.5 (range 0 to 10) days. Mean follow-up was 10.7 (range 1 to 52) weeks with no hernia recurrences. Seromas occurred in 6 (10.9%) patients, with 2 (3.6%) requiring drainage. Two (3.6%) 30-day readmissions occurred with no conversions to open or 30-day mortalities.
Conclusions:
r-AWR with and without TAR is a safe and feasible procedure associated with a short LOS, low complication rate, and low recurrence even within the surgeon's learning curve experience.
Keywords: Abdominal wall reconstruction, Complex ventral hernia, Robotic hernia repair, Transversus abdominis release, Ventral hernia
INTRODUCTION
Abdominal hernia repair has been reported as the most common surgical procedure, of which 30% are ventral hernia repairs,1 with >300,000 performed annually.2 Ventral hernias include incisional, epigastric, umbilical, parastomal, and Spigelian hernias, and the modalities for repair are open, laparoscopic, or robot-assisted approaches. Before 1993, all ventral hernia repairs were performed as open procedures.3 Open posterior component separation with transverses abdominis muscle release (TAR), a modification of the Rives-Stoppa technique, was first reported in 2012 and offers a solution for complex ventral hernias.4 Morbidities associated with the open approach were shown to be significantly lowered with the advent of the laparoscopic approach.5–7 Subsequently, the dexterity-related challenges encountered with laparoscopic ventral hernia repair during complex abdominal wall reconstruction (AWR) were addressed by the improved range of robotic instruments, which in turn led to the increasing attractiveness of robotic abdominal wall reconstruction (r-AWR).8 A recent study demonstrated that minimally invasive AWR had a significantly decreased length of stay compared with open AWR.9 In addition, robotic retromuscular ventral hernia repair was shown to be associated with decreased length of stay (LOS) compared with open repair10 and robotic TAR was associated with decreased wound complication rates, morbidity, and LOS compared with open repair.10–12 This report describes the early experience and short-term outcomes of robotic TAR and r-AWR for repair of complex ventral hernias performed by a single surgeon.
METHODS
An institutional review board–approved retrospective chart review was performed on all patients who underwent r-AWR for abdominal wall defects from August 2015 through October 2018 by a single surgeon (D.H.) at a tertiary-care institution. Patient characteristics, operative details, and outcomes were assessed. Patient characteristics included gender, age, body mass index (BMI), smoking history, comorbidities, hernia grade, and abdominal surgical history. Operative details included operative time, American Society of Anesthesiologists classification, defect size, procedure performed, occurrence of concomitant inguinal hernia repair, mesh use, and estimated blood loss (EBL). Outcomes included LOS, 30-day mortality, 30-day readmissions, 30-day reoperations, conversions to open surgery, seroma occurrence, hernia recurrence, and operative times.
The decision of whether to use TAR in addition to the retrorectus technique was based on the following factors: defect size, presence or absence of associated diastasis, multiplicity of defects, abdominal wall compliance, defect location (i.e., lateral wall vs midline), and degree of fibrosis and contracture of the rectus muscles. In general, midline defects <8 cm in width, without rectus musculature contracture or narrowing, were repaired solely with the retrorectus approach. Defects >8 cm, located on the lateral wall, accompanied by a wide diastasis (>8 cm), with multiple orifices, or defects associated with narrowed, thickened, and fibrotic rectus muscles required release beyond the semilunaris (i.e., TAR).
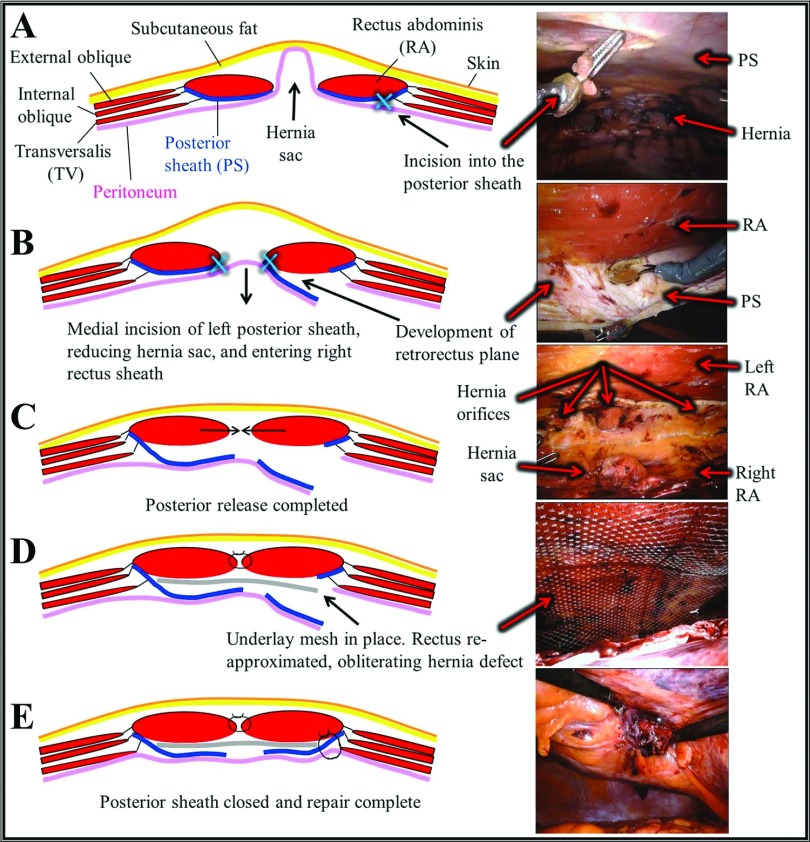
Robotic retromuscular complex abdominal wall repair was performed by using the technique depicted in Figure 1. Three ports were placed in the abdomen lateral to the anterior axillary line and the robot was docked. An assist port was used on the contralateral side. An incision was made in the ipsilateral posterior sheath, and a wide posterior flap composed of ipsilateral posterior sheath, hernia sac, and contralateral posterior sheath was developed across the midline. The rectus muscles were medialized and reapproximated by using a running, unidirectional barbed suture. A mesh device was selected and placed to fit the dimensions of the retromuscular space. The initial incision in the posterior sheath was closed with an absorbable suture, thereby excluding the mesh from the peritoneal cavity and completing the repair.
Figure 1.
Robotic retromuscular abdominal wall reconstruction. (A) Incision into the lateral apect of the left posterior sheath. (B) Medial dissection of the left posterior sheath, development of a preperitoneal plane across the midline and incision into the right posterior sheath. (C) Posterior release completed (D) Reapproximation of the rectus muscles and obliteration of the hernia defect. Placement of retrorectus synthetic mesh. (E) Closure of the initial left posterior sheath incision, completing the repair.
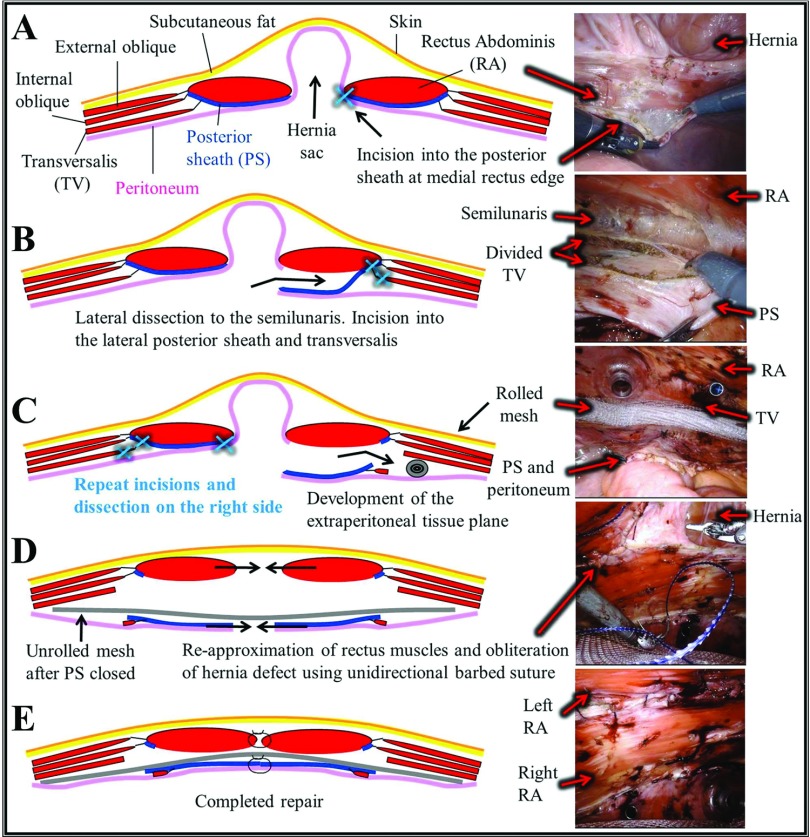
Robotic abdominal wall reconstruction with TAR was performed using a double-dock technique as described by Carbonell13 and Ballecer and Parra-Davila.14 Figure 2 depicts the key steps of the procedure. Three ports were placed lateral to the anterior axillary line and the robot was docked. After reduction of the contents of the hernia sac, dissection began on the medial aspect of the contralateral posterior sheath and continued laterally with TAR, developing a wide flap composed of posterior sheath, divided transversalis, and peritoneum. A rolled mesh was then positioned above the flap and secured to the transversalis using interrupted 2–0 absorbable, braided, synthetic sutures. Three ports were then placed on the contralateral abdomen lateral to the anterior axillary line. The robot was then redocked over the contralateral side, and a mirror image dissection was performed. The posterior sheath was then reapproximated and the mesh was unfurled. The repair was completed using a running, unidirectional barbed suture to bring together the edges of the fascia in the midline.
Figure 2.
Robotic abdominal wall reconstruction with transversus abdominis release (TAR). (A) Incision in the posterior sheath at the medial edge of the rectus muscle. (B) Retromuscular dissection laterally to the semilunaris, then incision into the lateral posterior sheath and division of the transversalis with preservation of the innervating nerves. (C) Development of the extraperitoneal tissue plane and placement of secured, rolled mesh. Redock and repeat dissection on the contralateral side. (D) Posterior sheath is closed and mesh is unfurled. Rectus muscles reapproximated, repairing the hernia defect and any associated diastasis. (E) Repair complete.
Patients were scheduled for outpatient follow-up visits with the operating surgeon at the following time points: 1 to 2 weeks, 6 weeks, 3 months, 6 months, and yearly. Additional visits were scheduled as needed and patients were encouraged to call or return for any questions or concerns. Hernia recurrence was assessed by history and physical examination.
RESULTS
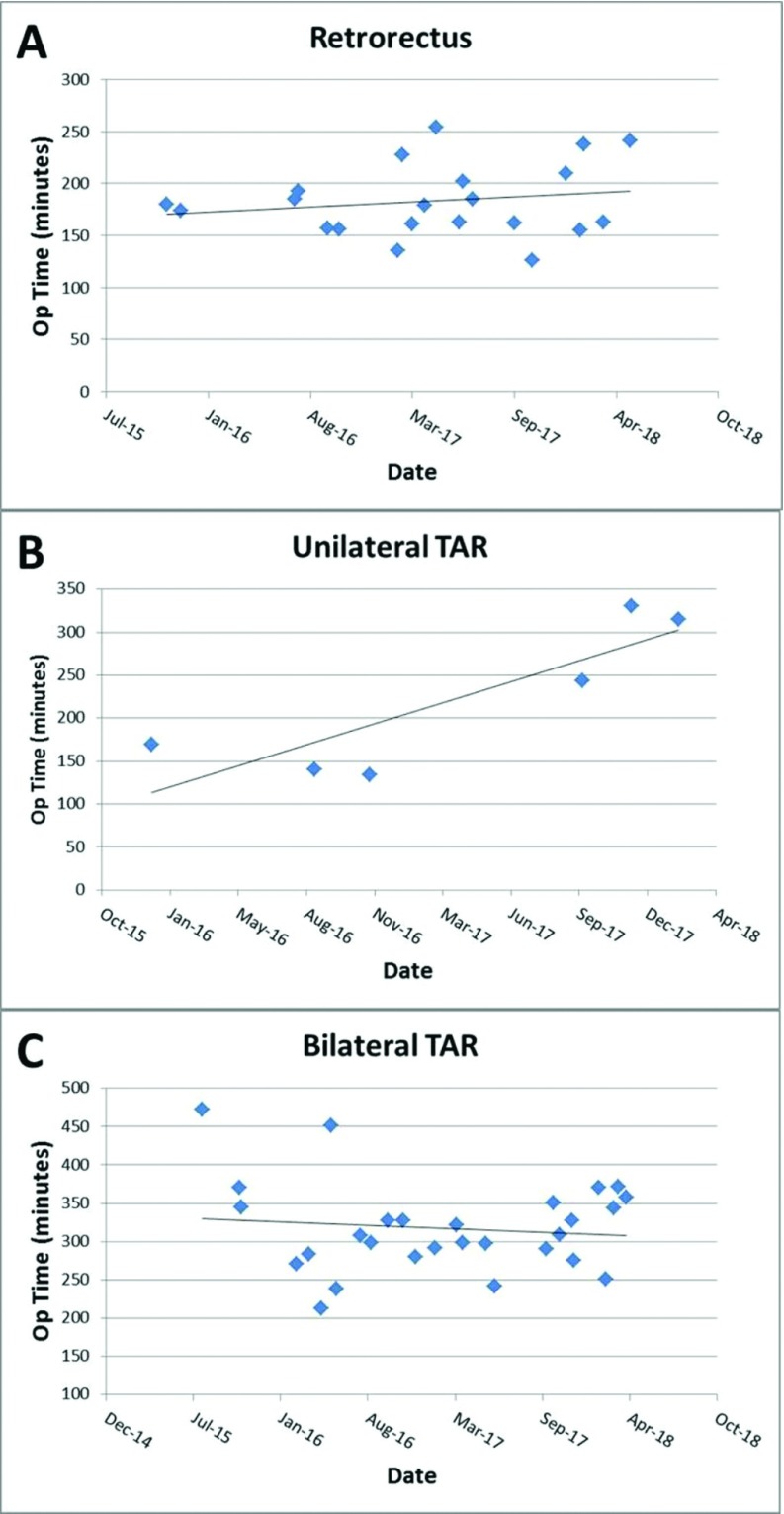
Complex r-AWR was successfully performed in 55 patients. Patient demographics are shown in Table 1. Of note, a majority (72.7%) of the patients were obese (BMI >30 kg/m2) with a positive smoking history (51%) and multiple comorbidities (98%). Hernias were classified according to the Ventral Hernia Working Group grading system,15 and 90% of patients were classified as hernia grade 2 or 3. All 55 operations were CDC class I (clean) with defect sizes ranging from 3 to 15 cm in width. Table 2 demonstrates the operative details of the 55 cases. Mean operative times were notably longer with TAR versus without TAR (294 vs 183 minutes), which is not unexpected. Of note, hernia defects repaired with unilateral TAR ranged from 4.5 to 8 cm in width, while hernia defects repaired with bilateral TAR ranged from 6 to 15 cm in width. Figure 3A–C shows the operative times of the cases with and without TAR. Outcomes are shown in Table 3, with no hernia recurrences noted. Seromas occurred in 6 patients, with 2 requiring drainage. Two 30-day readmissions occurred – 1 after inadvertent drain displacement and 1 for operative drainage of an infected seroma.
Table 1.
Patient Demographics (N = 55)
| Retrorectus (n = 21) |
TAR (n = 34) |
|||
|---|---|---|---|---|
| Mean (range) | SD | Mean (range) | SD | |
| Age (years) | 55.8 (36 to 74) | 11.1 | 62.9 (33 to 87) | 13.0 |
| BMI (kg/m2) | 34.2 (25 to 54) | 6.6 | 34.8 (23 to 50) | 6.8 |
| n | % | n | % | |
|---|---|---|---|---|
| Gender | ||||
| Male | 8 | 38.1 | 8 | 23.5 |
| Female | 13 | 61.9 | 26 | 76.5 |
| BMI (kg/m2) | ||||
| <25 | 1 | 4.8 | 1 | 2.9 |
| 25 to <30 | 3 | 14.3 | 10 | 29.4 |
| 30 to <40 | 13 | 61.9 | 14 | 41.2 |
| ≥40 | 4 | 19.0 | 9 | 26.5 |
| Smoking history | ||||
| Former | 3 | 14.3 | 3 | 8.8 |
| Current | 9 | 42.9 | 13 | 38.2 |
| Never | 9 | 42.9 | 18 | 52.9 |
| Hernia history | ||||
| Initial | 15 | 71.4 | 26 | 76.5 |
| Recurrent | 6 | 28.6 | 8 | 23.5 |
| Hernia grade | ||||
| 1 | 2 | 9.5 | 3 | 8.8 |
| 2 | 18 | 85.7 | 28 | 82.4 |
| 3 | 1 | 4.8 | 3 | 8.8 |
| 4 | 0 | 0 | 0 | 0 |
| Prior abdominal surgery | 20 | 95.2 | 33 | 97.1 |
| Diabetes | 5 | 23.8 | 12 | 35.3 |
| ≥1 Comorbidity | 21 | 100 | 33 | 97.1 |
BMI, body mass index; SD, standard deviation.
Table 2.
Operative Details
| Retrorectus (n = 21) |
TAR (n = 34) |
|||
|---|---|---|---|---|
| Mean (range) | SD | Mean (range) | SD | |
| Operative time (min) | 183 (126 to 254) | 79.4 | 294 (106 to 472) | 85.5 |
| Defect width (cm), mean, (range) | 5.3 (3 to 8) | 9.2 (5 to 15) | ||
| n | % | n | % | |
|---|---|---|---|---|
| ASA | ||||
| Class 1 | 1 | 4.8 | 1 | 2.9 |
| Class 2 | 11 | 52.4 | 17 | 50.0 |
| Class 3 | 9 | 42.9 | 15 | 44.1 |
| Class 4 | 0 | 0 | 1 | 2.9 |
| Concomitant inguinal hernia repair | 1 | 4.8 | 9 | 26.5 |
| Mesh | ||||
| Polypropylene | 6 | 28.6 | 33 | 97.1 |
| Polyethylene | 15 | 71.4 | 1 | 2.9 |
ASA, American Society of Anesthesiologists.
Figure 3.
Duration of operation over time. (A) Retrorectus procedure. (B, C) Retrorectus with unilateral and bilateral transversus abdominis release, respectively.
Table 3.
Outcomes
| Retrorectus (n = 21) |
TAR (n = 34) |
|||
|---|---|---|---|---|
| Mean (range) | Median | Mean (range) | Median | |
| Length of stay (days) | 1 (0 to 3) | 1 | 1.8 (0 to 10) | 1 |
| Follow-up (weeks) | 10 (1 to 29) | 8 | 28 (1 to 90) | 23 |
| n | % | n | % | |
|---|---|---|---|---|
| Seromas requiring intervention | 0 | 0 | 2 | 5.9 |
| Seromas managed expectantly | 1 | 4.8 | 3 | 8.8 |
| 30-day mortality | 0 | 0 | 0 | 0 |
| Recurrences* | 0 | 0 | 0 | 0 |
| 30-day readmission | 0 | 0 | 2 | 5.9 |
| 30-day reoperation | 0 | 0 | 0 | 0 |
Assessed at follow up.
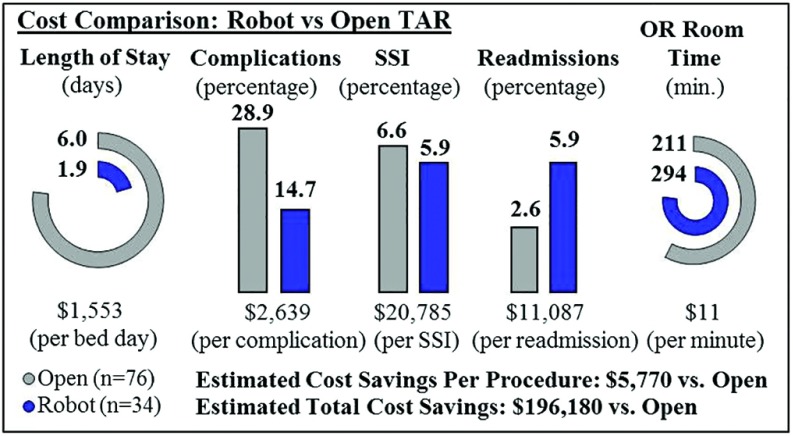
A focused cost analysis was performed of robotic TAR compared with open TAR11 (Figure 4). We were unable to obtain cost data from our institution. Costs associated with LOS,16 complications,17 surgical site infections (SSIs),18 readmissions,19 and operating room (OR) time20 were obtained from the literature. These same metrics were applied to both robotic TAR and open TAR and used to calculate overall differences. Compared with open TAR, the robotic TARs performed in this study (n = 34) demonstrated an estimated cost savings of $5,770 per procedure and an estimated total savings of $196,180.
Figure 4.
Cost comparison of robotic vs. open transversus abdominis release. Cost metrics associated with length of stay,16 complications,17 surgical site infections (SSIs),18 readmissions,19 and operating room (OR) time20 were applied to robotic TAR data from this study and a previously published open TAR11 study.
Of note in Figure 4, the readmission rate for robotic TAR was found to be higher than that of open TAR. This was due to 2 readmissions among a small subset of patients. One readmitted patient was an obese female with a large hernia sac who underwent operation early in our experience. Due to the size of the hernia sac, a subcutaneous drain was placed. However, the drain was inadvertently displaced after discharge, and the patient subsequently developed a large, symptomatic seroma and cellulitis that was managed with interventional radiology (IR) drainage and antibiotic therapy, respectively. The second readmission was an obese female with multiple prior abdominal surgeries and wound infections after each procedure. She returned to the hospital on postoperative day (POD) 13 with an infected seroma of the native hernia sac and underwent IR drainage followed by operative wound debridement on POD 15 with placement of a wound vac. Her wound was noted to be healed at the 2-month follow-up visit. Neither patient developed a mesh infection.
DISCUSSION
Complex AWR has evolved significantly during the past several decades. For medium-sized abdominal wall defects, the Rives Stoppa retromuscular repair was considered the standard.21 Difficulty and controversy arose regarding the best method to extend the dissection beyond the semilunaris in order to repair larger defects. One method, the anterior abdominal component separation technique, was first described by Ramirez et al in 1990.22 This technique added incision of the external oblique and separation from the internal oblique in addition to the Rives dissection, which separated the rectus muscle and anterior sheath from the posterior sheath. The resultant compound flap (composed of rectus muscle, anterior sheath, internal oblique, and transversus abdominis muscle) was then advanced medially to close defects ranging in size from 4 × 4 to 18 × 35 cm. The component separation procedure proved quite effective and reproducible; yet, it had the disadvantage of creating large flaps of skin and subcutaneous tissue. Wound complications, such as skin necrosis, were reported to be as high as 41%.23 Repair methods such as the perforator-sparing techniques introduced by Saulis and Dumanian resulted in decreased rates of tissue ischemia.24 In addition, endoscopic approaches to external oblique incision were introduced with the intent to decrease the incidence of soft tissue infections.25,26
With the increasing methods and modalities available for ventral hernia repair, a growing interest developed in posterior dissection beyond the semilunaris. Unfortunately, early techniques of extending the dissection between the oblique muscles denervated the rectus abdominis by dividing the neuromuscular bundle. In 2012, Yuri Novitsky published a landmark article describing posterior component separation beyond the semilunaris with TAR.4 The TAR technique obviated the need for the creation of large skin flaps and enabled placement of a large, extraperitoneal, retromuscular mesh. Furthermore, innervation to the rectus muscles was preserved during the dissection. The complexity of the dissection and the difficulty of suturing under tension made the development of a laparoscopic approach formidable. For many years, the only minimally-invasive approach to medium-sized abdominal wall defects was laparoscopic intraperitoneal onlay mesh (IPOM).27 The IPOM technique fails to close the native hernia orifice, can lead to ballooning of the mesh through the hernia defect, and requires placement of an intraperitoneal prosthesis, which can lead to adhesion formation and mesh fistulization. Larger abdominal wall defects were best approached with an open repair and component separation. However, the advent of the robotic platform facilitated the feasibility of the minimally invasive TAR technique. In 2015, Carbonell performed the first live-broadcast robotic TAR at the Global Symposium on Robotic-assisted and Minimally Invasive Hernia Repair in New York City, catapulting the birth of robotic abdominal wall reconstruction.28
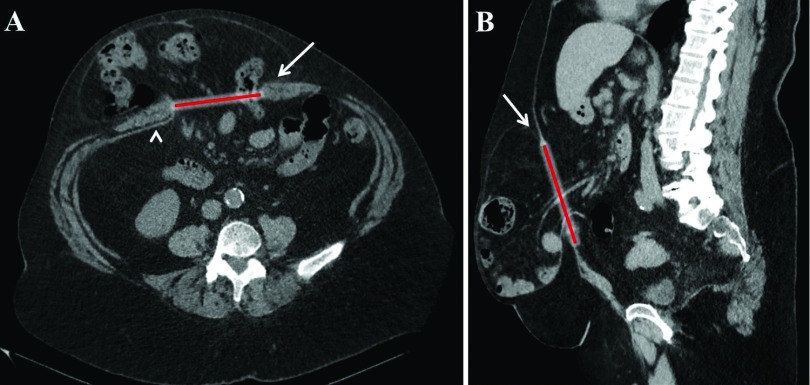
The r-AWR has marked benefits compared with both open techniques and laparoscopic IPOM. First and foremost, the native abdominal anatomy is restored by obliteration of the hernia defect, medialization of the rectus muscles, and repair of associated diastasis, if present. Second, the repair is buttressed by a retromuscular mesh. In theory, a retromuscular placement is advantageous because force generated during abdominal wall contraction is transmitted inwardly onto the mesh, versus the same force pushing an overlay mesh outward. Inward force should result in decreased hernia recurrence rates. In contrast, an IPOM repair, which lacks defect closure, is effectively a hybrid of an underlay and interposition mesh. The outward force against the interposition portion of the mesh bridging the defect is responsible for the mesh ballooning through the hernia orifice and hernia recurrences associated with IPOM repairs. Figure 5 shows the computed tomography (CT) of a patient with a recurrent incisional hernia that was previously repaired using the laparoscopic IPOM technique.
Figure 5.
Recurrent incisional hernia after laparoscopic intraperitoneal onlay mesh repair (IPOM). (A) Coronal and (B) sagittal view of a 10 × 9 cm recurrent incisional hernia that was previously repaired with an open onlay mesh technique, followed by a laparoscopic IPOM repair. The current repair was performed with a bilateral transversus abdominis release and 28 × 30 cm mesh. Arrows and red line indicating defect and arrowhead in (A) showing previously placed mesh.
Another advantage of r-AWR is the placement of mesh outside of the peritoneal cavity. The extraperitoneal position eliminates the potential for mesh adhesions and mesh fistula to intra-abdominal contents. The minimally invasive approach also obviates the need for the large tissue flaps created during open repair, thereby decreasing the risk of wound complications, such as seroma formation and mesh infection. Decreased soft tissue disruption also obviates the need for drains. Patients, particularly those with obesity, often require drains to remain in place for up to 6 weeks postoperatively with the open technique. In this single-surgeon experience, the routine use of drains during r-AWR was replaced with selective use after the first few cases with no subsequent change in the incidence of seroma. In an effort to decrease seroma formation, bites of the hernia sac are incorporated into the closure of the abdominal wall defects while suturing. In rare cases in which a massive hernia sac cannot be sufficiently obliterated during closure of the abdominal wall, a drain is placed into the hernia sac.
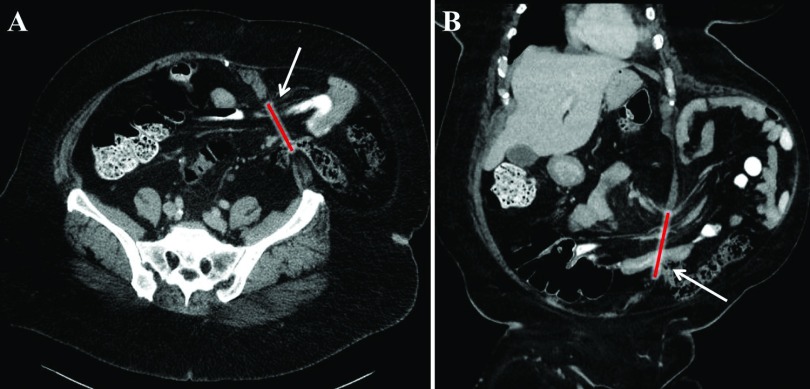
In addition to midline defects, r-AWR was found to be useful for the repair of complex lateral abdominal wall defects. In this series, a single-dock technique with unilateral TAR dissection was used to successfully repair 6 lateral abdominal wall defects. Figure 6 illustrates the preoperative CT of a patient with a lateral wall defect repaired with a unilateral TAR approach. Furthermore, caudal dissection of the preperitoneal flap to the level of the symphysis pubis and Cooper's ligament offered excellent exposure to the myopectinate orifice. This exposure facilitated the concomitant repair of ten incidental inguinal hernias at the time of r-AWR in this series. Notably in this study, 98.2% of patients had ≥1 comorbidity and 72.7% had BMI values ≥30 kg/m2. The decreased morbidity associated with the robotic approach may be beneficial for patients with an increased surgical risk due to comorbidities such as obesity and diabetes. Also, r-AWR may be performed in patients with obesity who are unable to undergo weight loss procedures before hernia repair.
Figure 6.
Lateral wall defect. (A) Coronal view and (B) sagittal view of a 6 × 5 cm lateral wall defect (arrows and red line). The defect was repaired using a unilateral transversus abdominis release approach and a 15 × 25 cm synthetic mesh.
The data presented here reflect the first 55 cases of r-AWR performed by a single surgeon. With inguinal repair, laparoscopic minimally-invasive techniques have a steep learning curve, which has been an impedance to widespread adoption of these techniques. While the robotic platform has facilitated the adoption of other minimally invasive surgeries, r-AWR should be adopted with caution. The learning curve of r-AWR is unique in that there is no true laparoscopic equivalent. Although the technique is novel and exciting, there is potential for significant injury and denervation of the abdominal wall. Resulting hernias and deformities from such injuries can be difficult to repair and leave the surgeon with limited options. We suggest a reserved, honest, and thoughtful application of these techniques. For defects <5 to 6 cm, robotic transabdominal preperitoneal repair should be considered first as allowed by the integrity of the peritoneum and the compliance of the abdominal wall. Patients with larger defects (>15 cm), associated stomas or fistulas, or poor skin integrity or those desiring scar revision are best served with open surgery.
A thorough understanding of abdominal wall anatomy is a prerequisite to r-AWR. Extensive experience with advanced laparoscopic techniques is suggested and experience with open abdominal wall reconstruction, including all facets of abdominal wall component separation is imperative. The learning curve is further facilitated by direct observation of a surgeon experienced with these techniques, as there are many nuances to be imparted that will improve efficiency. Proctoring of the first few cases is also suggested. Surprisingly, the surgeon in this series found the first few retrorectus repairs more technically challenging than TAR as the working space is more confined and final closure of the posterior sheath may be difficult due to proximity of the camera. To this regard, the author's perception may be partially skewed as most of the surgeries in this series were performed on the da Vinci Si platform (Intuitive Surgical®, Sunnyvale, CA). The Xi platform is preferable to Si and should be used whenever available as the robotic arms have a longer reach and are more facile, trocars can be spaced closer together when lack of torso length becomes an issue, and switching the camera from 30° up to 30° down is simplified.
Of note, operative times were found to increase minimally for retrorectus repair and unilateral TAR as the surgeon moved through the learning curve in this series, while operative times for bilateral TAR slightly decreased. Within 5 to 10 cases, the dissection of tissue planes began to occur with proficiency, efficiency, and confidence. Although ideal trocar positioning will vary with body habitus and defect location, positioning of trocars also improved within the first 5 to 10 cases, thereby minimizing both internal and external collisions. Patients with short torsos, higher BMI, complex recurrent hernias with prior intra-abdominal mesh in place, or a CT demonstrating retracted, narrow rectus muscles can be technically challenging early in the learning curve. Patients with longer torsos, lower BMI, wide, flat rectus muscles, and native ventral or incisional defects allow for easier dissection and more leeway in port placement. Such cases are ideal for one operating early within the learning curve. The increase in operative time as the series progressed is likely due to the fact that more complex cases were performed later in the series, sometimes requiring lengthy adhesiolysis or explantation of mesh.
Although r-AWR operative times are longer compared with open techniques, the increased costs may be offset by both the decreased LOS, SSIs, and complication rates associated with the robotic approach, as shown in Figure 4. Moreover, as surgeons move beyond the learning curve, operative times are expected to decrease. The cost analysis performed in this study was limited to focus on the 5 categories depicted in Figure 4 and may not fully account for additional hospital or OR costs. Additionally, the cost data in our study were obtained from literature review and limited to TAR alone, as we were not able to obtain actual cost data at our institution. Future studies with an emphasis on extensive review and comparison of varying techniques' cost effectiveness can further delineate the true monetary differences. Compared with laparoscopic IPOM, the robotic platform also allows for mesh fixation with sutures, eliminating the need for costly hernia tackers. Because mesh is not placed in the peritoneal cavity during r-AWR, noncomposite meshes with adhesion barriers that are usually placed during laparoscopic IPOM and are more expensive can be avoided, adding to the overall cost benefit of r-AWR. Limitations of this study include the small sample size and limited follow-up time. Future, larger studies comparing open and robotic approaches within our institution would provide further insight into the differences in both short-term and long-term outcomes, recurrence, and cost.
CONCLUSION
r-AWR with and without TAR is a safe and feasible procedure even within the learning curve experience of the surgeon. It is associated with a short LOS, low complication rate, and low recurrence rate, all of which translate into r-AWR as a more economical approach compared with open repair. Less tissue disruption associated with robotic repair may lead to decreased seroma formation, even without the use of surgical drains. The decreased morbidity associated with r-AWR is particularly beneficial to those patients with a BMI-dependent surgical risk who are unable to undergo a weight loss procedure before hernia repair, as well as patients with an increased risk of postoperative wound complications, such as those with a history of smoking or diabetes. The adoption of this complex procedure presents unique challenges to the learner. Application of mentorship and proctorship programs, experience with open component separation, proficiency with advance laparoscopic skills, and expertise in abdominal wall anatomy will assist the surgeon in ascending the learning curve. The operative times and complication rates are expected to decrease as surgeons move beyond their learning curve.
Contributor Information
David K. Halpern, Department of Surgery, NYU Winthrop Hospital, Mineola, New York, USA..
Raelina S. Howell, Department of Surgery, NYU Winthrop Hospital, Mineola, New York, USA..
Harika Boinpally, Department of Surgery, NYU Winthrop Hospital, Mineola, New York, USA..
Cristina Magadan-Alvarez, Department of Surgery, NYU Winthrop Hospital, Mineola, New York, USA..
Patrizio Petrone, Department of Surgery, NYU Winthrop Hospital, Mineola, New York, USA..
Collin E. M. Brathwaite, Department of Surgery, NYU Winthrop Hospital, Mineola, New York, USA..
References:
- 1. Oviedo RJ, Robertson JC, Desai AS. Robotic ventral hernia repair and endoscopic component separation: outcomes. JSLS J Soc Laparoendosc Surg. 2017;21(3):e2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davila DG, Parikh N, Frelich MJ, Goldblatt MI. The increased cost of ventral hernia recurrence: a cost analysis. Hernia. 2016;20:811–817. [DOI] [PubMed] [Google Scholar]
- 3. Vorst AL, Kaoutzanis C, Carbonell AM, Franz MG. Evolution and advances in laparoscopic ventral and incisional hernia repair. World J Gastrointest Surg. 2015;7:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Novitsky YW, Elliott HL, Orenstein SB, Rosen MJ. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg. 2012;204:709–716. [DOI] [PubMed] [Google Scholar]
- 5. Misiakos EP, Patapis P, Zavras N, Tzanetis P, Machairas A. Current trends in laparoscopic ventral hernia repair. JSLS J Soc Laparoendosc Surg. 2015;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itani KMF, Hur K, Kim LT, et al. ; Veterans Affairs Ventral Incisional Hernia Investigators. Comparison of laparoscopic and open repair with mesh for the treatment of ventral incisional hernia. Arch Surg. 2010;145:322–328. [DOI] [PubMed] [Google Scholar]
- 7. Kurmann A, Visth E, Candinas D, Beldi G. Long-term follow-up of open and laparoscopic repair of large incisional hernias. World J Surg. 2011;35:297–301. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez A, Escobar E, Romero R, et al. Robotic-assisted ventral hernia repair: a multicenter evaluation of clinical outcomes. Surg Endosc. 2017;31:1342–349. [DOI] [PubMed] [Google Scholar]
- 9. Belyansky I, Weltz AS, Sibia US, et al. The trend toward minimally invasive complex abdominal wall reconstruction: is it worth it? Surg Endosc. 2018;32:1701–1707. [DOI] [PubMed] [Google Scholar]
- 10. Carbonell AM, Warren JA, Prabhu AS, et al. Reducing length of stay using a robotic-assisted approach for retromuscular ventral hernia repair: a comparative analysis from the Americas Hernia Society Quality Collaborative. Ann Surg. 2018;267:210–217. [DOI] [PubMed] [Google Scholar]
- 11. Martin-del-Campo LA, Weltz AS, Belyansky I, Novitsky YW. Comparative analysis of perioperative outcomes of robotic versus open transversus abdominis release. Surg Endosc. 2018;32:840–845. [DOI] [PubMed] [Google Scholar]
- 12. Bittner JG, Alrefai S, Vy M, Mabe M, Del Prado PAR, Clingempeel NL. Comparative analysis of open and robotic transversus abdominis release for ventral hernia repair. Surg Endosc. 2018;32:727–734. [DOI] [PubMed] [Google Scholar]
- 13. Carbonell AM. Lecture Series and Case Observation. Greenville, SC: Intuitive Surgical, Inc; 2015. [Google Scholar]
- 14. Ballecer C, Parra-Davila E. Robotic ventral hernia repair. In: Hernia Surgery. Cham: Springer International Publishing; 2016:273–286. [Google Scholar]
- 15. Breuing K, Butler CE, Ferzoco S, t a;. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544–558. [DOI] [PubMed] [Google Scholar]
- 16. Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. [DOI] [PubMed] [Google Scholar]
- 17. Vonlanthen R, Slankamenac K, Breitenstein S, et al. The impact of complications on costs of major surgical procedures. Ann Surg. 2011;254:907–913. [DOI] [PubMed] [Google Scholar]
- 18. Zimlichman E, Henderson D, Tamir O, et al. Health care–associated infections. JAMA Intern Med. 2013;173:2039. [DOI] [PubMed] [Google Scholar]
- 19. Agency for Healthcare Research and Quality. HCUPnet: A Tool for Identifying, Tracking, and Analyzing National Hospital Statistics. All Patient Readmissions Within 30 Days. National Statistics, 2012. Available at https://hcupnet.ahrq.gov/. [Google Scholar]
- 20. Chatterjee A, Payette MJ, Demas CP, Finlayson SRG. Opportunity cost: a systematic application to surgery. Surgery. 2009;146:18–22. [DOI] [PubMed] [Google Scholar]
- 21. Stoppa RE, Rives JL, Warlaumont CR, Palot JP, Verhaeghe PJ, Delattre JF. The use of Dacron in the repair of hernias of the groin. Surg Clin North Am. 1984;64:269–285. [DOI] [PubMed] [Google Scholar]
- 22. Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–526. [DOI] [PubMed] [Google Scholar]
- 23. Bougard H, Coolen D, De Beer R, et al. HIG (SA) Guidelines for the Management of Ventral Hernias. South African Journal of Surgery. 2016;54:1–32. [Google Scholar]
- 24. Saulis AS, Dumanian GA. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in “separation of parts” hernia repairs. Plast Reconstr Surg. 2002;109:2275–2280, 2002; discussion 2281–222. [DOI] [PubMed] [Google Scholar]
- 25. Lowe JB, Garza JR, Bowman JL, Rohrich RJ, Strodel WE. Endoscopically assisted “components separation” for closure of abdominal wall defects. Plast Reconstr Surg. 2000;105:720–729; quiz 730. [DOI] [PubMed] [Google Scholar]
- 26. Harth KC, Rosen MJ. Endoscopic versus open component separation in complex abdominal wall reconstruction. Am J Surg. 2010;199(3):342–347. [DOI] [PubMed] [Google Scholar]
- 27. Fitzgibbons RJ, Salerno GM, Filipi CJ, Hunter WJ, Watson P. A laparoscopic intraperitoneal onlay mesh technique for the repair of an indirect inguinal hernia. Ann Surg. 1994;219:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warren JA, Cobb WS, Ewing JA, Carbonell AM. Standard laparoscopic versus robotic retromuscular ventral hernia repair. Surg Endosc Other Interv Tech. 2017;31:324–432. [DOI] [PubMed] [Google Scholar]