Abstract
OBJECTIVE:
Stage is a critical determinant of prognosis and treatment for endometrial cancer (EC) patients. Women who are status-post tubal ligation for sterilization have improved EC survival, secondary to lower stage at presentation, suggesting that transtubal spread may represent an important route of metastasis. We evaluated detection of intraluminal tumor cells (ILTCs) in relation to tumor characteristics and survival.
METHODS:
One pathologist retrospectively evaluated hematoxylin and eosin sections of routinely collected fallopian tubes for ILTCs from 295 EC patients, masked to outcome. We used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between demographic (age, race) and clinical [FIGO 2009 stage, lymphovascular space invasion (LVSI), histological subtype] characteristics and ILTCs. Cox regression was used to estimate hazard ratios (HRs) and 95% CIs for associations between ILTCs and recurrence-free survival (RFS) and EC-specific survival, overall and stratified by histological subtype or stage.
RESULTS:
In univariable logistic regression models, age (55–64 vs. ≥65: OR=3.41, 95% CI=1.48–7.84), stage (stage IV vs. stage I OR=14.58, 95% CI=5.27–40.35), LVSI (OR=2.93, 95% CI=1.42–6.04), and histological subtype (serous vs. low-grade endometrioid OR=3.21, 95% CI=1.08–9.58), were associated with ILTCs. Only age and stage remained significantly associated with ILTCs in adjusted models. ILTCs were significantly associated with lower EC-specific survival among women with serous EC or stage I disease; however, adjustment for age, stage, and histology attenuated these associations.
CONCLUSION:
Our findings suggest that ILTCs are associated with adverse EC prognostic features and reduced survival in cases of early stage or serous histology.
Keywords: Uterus Neoplasm, Fallopian Tube, disease-specific mortality, patterns of spread
INTRODUCTION
Endometrial cancer (EC) is the most common gynecological malignancy in the United States. In 2018, 63,230 new cases and 11,350 deaths are expected (1). EC stage characterizes the extent of disease and is strongly linked with prognosis, independent of histology or grade (2). Furthermore, stage is a strong determinant of adjuvant treatment selection and post-diagnosis surveillance (3). EC staging has evolved over time, reflecting advances in our understanding of the pathogenesis of this disease (4, 5). However, the current staging criteria do not capture the presence of tumor cells in the lumen of the fallopian tube, or transtubal spread, which is distinct from invasion into the stroma of the fallopian tubes and represented by stage IIIA disease.
Transtubal spread is proposed to occur when EC cells are exfoliated through the fallopian tubes into the abdominal cavity. The possibility of transtubal spread was first recognized when malignant EC cells were identified within cytology specimens from fallopian tubes (6). The presence of intraluminal tumor cells (ILTCs) – a putative histological marker of transtubal spread – is more common among women with aggressive compared to indolent EC histologic subtypes (7), and is associated with extra-uterine spread (8). Despite the recognition that EC cells have the capacity for cellular detachment and transtubal transportation, and that this mechanism co-occurs with other aggressive tumor characteristics, we lack empirical data on the independent prognostic impact of transtubal spread and whether this relationship is modified by other tumor characteristics. Thus, we cannot make evidence-based decisions as to whether EC stage criteria should be revised to incorporate information on ILTCs for improvement in prognostic accuracy. Therefore, our main objective was to extend prior analyses by evaluating the association between ILTCs and EC survival, overall and stratified by histologic subtype or stage. Secondarily, we examined relationships between ILTCs and tumor characteristics to confirm prior associations.
METHODS
Study population
We have previously described our institutional database of 896 consecutive EC patients (carcinoma and carcinosarcoma) treated at The Ohio State University Wexner Medical Center between 2007 and 2012 (9). All women included in the database underwent standard hysterectomy and bilateral salpingo-oophorectomy, with lymph node staging performed in 86% of cases based on the surgeon’s clinical judgment. For the current analysis, which required retrospective pathological review of fallopian tube sections, we sampled a subset of the cohort, chosen based on histological subtype. Based on prior work (7, 8, 10) we hypothesized that transtubal spread is most clinically relevant for women with aggressive histological subtypes. Therefore, we included all women with diagnoses of grade 3 endometrioid (n=64), serous (n=41), carcinosarcoma (n=30), clear cell (n=10), and mixed carcinoma tumors (n=59) and randomly sampled 100 women with grades 1 or 2 endometrioid histology among 686 such patients (all stages). Of the 100 women we randomly selected with grades 1 or 2 endometrioid histology, three did not have evaluable slides.
Assessment of clinical characteristics and outcomes
Information on age at diagnosis (<55, 55–64, ≥65), self-reported race (white vs. non-white), body mass index (BMI) (< 25, 25–30, ≥30 kg/m2), history of tubal ligation (no vs. yes), use of an intrauterine manipulator (no vs. yes), lymphovascular space invasion (LVSI) (no vs. yes), percent myometrial invasion (<25, 25–50, 50–74, ≥75%), stage [I, II, III, IV, according to the 2009 International Federation of Gynecologic Oncology (FIGO)] (5), histology (endometrioid, serous, carcinosarcoma, clear cell, mixed carcinoma), and grade (1, 2, 3) were collected from electronic medical records and pathology reports. We further classified women with endometrioid tumors according to grade: low-grade endometrioid (grades 1 or 2) or high-grade endometrioid (grade 3). By definition, serous, carcinosarcoma, clear cell, and mixed carcinoma are considered high grade tumors (11). We also examined EC type according to the traditional Type I/II dichotomy, with Type I including endometrioid histology (all grades) and Type II including serous, carcinosarcoma, clear cell, and mixed epithelial. Dates of diagnosis and death were also available from electronic medical records. Data registrars collect information on the cause and date of death through periodic follow-up assessments of patients remaining in care in the OSU Wexner Medical Center system.
Determination of ILTC status
At the time of EC surgery hematoxylin-eosin (H&E) stained slides from salpingectomy specimens were processed. The number of sections per woman varied based on availability, but at least one cross-section of the diameter of each fallopian tube was reviewed for each woman. For this study, one gynecologic pathologist (AAS) retrospectively reviewed all fallopian tube archival glass slides and recorded ILTCs without knowledge of clinical characteristics. ILTCs were defined histologically as unequivocally malignant viable cells, whether individually distributed or forming groups in the lumen of the tubes.
Statistical analysis
We compared characteristics of women with and without ILTCs using chi-square tests. Univariable logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between ILTCs and clinical/tumor characteristics. Variables significantly associated with ILTC detection in the univariable models (p<0.05) were included in a multivariable logistic regression model. Days between EC surgery and recurrence or EC-related death were computed and defined as recurrence-free survival (RFS) and EC-specific survival, respectively. Kaplan–Meier estimates and log-rank tests were used to compare survival distributions according to ILTCs. Patients who did not experience a recurrence or EC death were censored at the date of last contact or end of follow-up (December 31, 2014) in the respective analyses. Women who died of other causes were censored on their date of death. We used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% CIs for associations between ILTCs with RFS and EC-specific survival in models unadjusted and adjusted for age, histological subtype, and stage. We repeated these analyses stratified by histological subtype or stage. For analyses of RFS, only patients that were known to be disease-free following the primary surgery were included (e.g. stages I-III).
We also conducted a subgroup analysis comparing survival between women with stage I or II tumors with ILTCs and women with stage IIIA tumors to evaluate whether ILTCs in otherwise stage I or II disease led to reduced EC-specific survival.
The proportional hazards assumption, which was evaluated by modeling interaction terms of ILTCs with follow-up time in each model, was confirmed for all analyses. Statistical analyses were performed using SAS/STAT software (version 9.4 of the SAS System for Windows, SAS Institute, Cary, NC, USA). The Institutional Review Board at the Ohio State University approved this study.
RESULTS
Associations of ILTCs and clinical/tumor characteristics
ILTCs were detected in 35 (11.9%) of 295 EC patients studied. Distributions of tumor characteristics according to ILTCs along with univariable and multivariable-adjusted ORs are shown in Table 1. Higher odds of ILTCs were related to age (55–64 vs. ≥65: OR=3.41, 95% CI=1.48–7.84), stage (stage IV vs. stage I OR: 14.58, 95% CI=5.27–40.35), LVSI (OR: 2.93, 95% CI=1.42–6.04), EC type (Type II vs. Type I, OR: 2.13, 95% CI=1.03–4.42, p=0.04) and histological subtype (serous vs. low-grade endometrioid OR: 3.21, 95% CI=1.08–9.58). In a multivariable model including age, histological subtype, LVSI, and stage, only age and stage remained significantly associated with ILTCs.
Table 1.
Odds ratios (ORs) and 95% confidence intervals (CIs) for associations between tumor characteristics and ILTCs
| Tumor characteristics | ILTCs | ||||||
|---|---|---|---|---|---|---|---|
| Absent (n=260) | Detected (n=35) | ||||||
| n (%) | n (%) | OR (95% CI)1 | p2 | OR (95% CI)3 | p2 | ||
| Age | 0.01 | 0.009 | |||||
| <55 | 42 (16.2) | 6 (17.1) | 2.09 (0.70, 6.23) | 3.80 (1.06, 13.67) | |||
| 55–64 | 86 (33.1) | 20 (57.1) | 3.41 (1.48, 7.84) | 4.17 (1.64, 10.64) | |||
| ≥65 | 132 (50.8) | 9 (25.7) | 1.00 | 1.00 | |||
| Race | 0.96 | ||||||
| White | 237 (88.1) | 32 (11.9) | 1.00 | ---- | |||
| Non-white | 23 (88.5) | 3 (11.5) | 0.97 (0.28, 3.40) | ---- | |||
| BMI (kg/m2) | 0.48 | ---- | |||||
| Normal weight (<25) | 110 (42.3) | 12 (34.3) | 1.00 | ---- | |||
| Overweight (25–29.9) | 92 (35.4) | 16 (45.7) | 1.59 (0.72, 3.54) | ---- | |||
| Obese ( ≥30) | 58 (22.3) | 7 (20.0) | 1.11 (0.41, 2.96) | ---- | |||
| Tubal ligation | 0.54 | ---- | |||||
| No | 217 (87.1) | 32 (12.9) | 1.00 | ---- | |||
| Yes | 41 (93.2) | 3 (6.8) | 0.50 (0.15, 1.70) | ---- | |||
| Intrauterine manipulator use | 0.40 | ---- | |||||
| No | 225 (88.2) | 30 (11.8) | 1.00 | ---- | |||
| Yes | 17 (100.0) | 0 (0.0) | NE | ---- | |||
| Stage | <.0001 | 0.0003 | |||||
| I | 175 (95.6) | 8 (4.4) | 1.00 | 1.00 | |||
| II | 20 (87.0) | 3 (13.0) | 3.28 (0.81, 13.38) | 5.02 (1.07, 23.58) | |||
| III | 47 (80.0) | 12 (20.3) | 5.59 (2.16, 14.45) | 7.44 (2.21, 25.00) | |||
| IV | 18 (60.0) | 12 (40.0) | 14.58 (5.27, 40.35) | 18.98 (4.96, 72.64) | ---- | ||
| Myometrial invasion | 0.28 | ||||||
| <25% | 106 (91.4) | 10 (8.6) | 1.00 | ---- | |||
| 25–49% | 46 (86.8) | 7 (13.2) | 1.61 (0.58, 4.50) | ---- | |||
| 50–74% | 26 (78.8) | 7 (21.2) | 2.85 (0.99, 8.21) | ---- | |||
| ≥75% | 82 (88.2) | 11 (11.8) | 1.42 (0.58, 3.51) | ---- | |||
| LVSI | 0.004 | 0.72 | |||||
| No | 172 (66.2) | 14 (40.0) | 1.00 | 1.00 | |||
| Yes | 88 (33.9) | 21 (60.0) | 2.93 (1.42, 6.04) | 0.82 (0.28, 2.40) | |||
| Type | 0.04 | ||||||
| I | 145 (91.8) | 13 (8.2) | 1.00 | ---- | |||
| II | 115 (83.9) | 22 (16.1) | 2.13 (1.03, 4.42) | ---- | |||
| Histology | <0.0001 | 0.60 | |||||
| Low-grade endometrioid | 90 (34.6) | 7 (20.0) | 1.00 | 1.00 | |||
| High-grade endometrioid | 55 (21.2) | 6 (17.1) | 1.40 (0.45, 4.39) | 0.61 (0.16, 2.29) | |||
| Serous | 32 (12.3) | 8 (22.9) | 3.21 (1.08, 9.58) | 1.42 (0.36, 5.64) | |||
| Mixed epithelial | 49 (18.9) | 9 (25.7) | 2.36 (0.83, 6.73) | 1.82 (0.51, 6.43) | |||
| Carcinosarcoma | 26 (10.0) | 4 (11.4) | 1.98 (0.54, 7.29) | 0.79 (0.15, 4.06) | |||
| Clear cell | 8 (3.1) | 1 (2.9) | 1.61 (0.18, 14.75) | 0.64 (0.05, 8.74) | |||
Unadjusted ORs and 95% CIs
p-value
ORs and 95% CIs adjusted for age (<55, 55–64, ≥65), FIGO 2009 stage (I, II, III, IV), LVSI (no vs. yes), and histology (low-grade endometrioid, high-grade endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial)
Associations of ILTCs and survival in the overall study population
In the overall cohort ILTCs were unrelated to RFS (log-rank p=0.73, univariable HR=1.16, 9% CI=0.50–2.70, multivariable HR=0.52, 95% CI=0.21–1.29) (Figure 1). We observed lower EC-specific survival associated with ILTCs (log-rank p<0.0001), which was also reflected in the univariable Cox regression model (HR=2.80, 95% CI=1.57–4.98). After adjustment for age, histological subtype, and stage, the association between ILTCs and EC-specific survival was attenuated (HR= 1.39, 95% CI=0.67–2.86) (Figure 2).
Figure 1. Recurrence-free survival according to ILTCs.
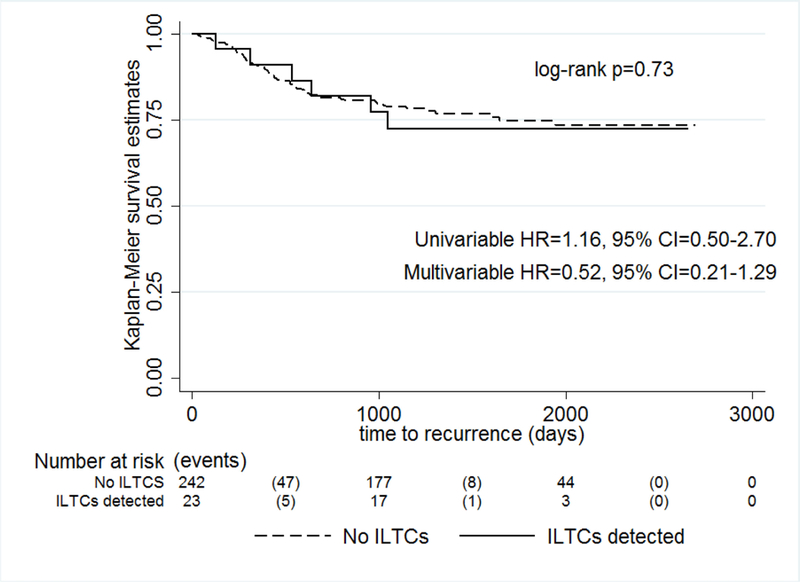
shows no significant difference in recurrence-free survival among endometrial cancer patients with or without ILTCs
Figure 2. Endometrial cancer-specific survival according to ILTCs.
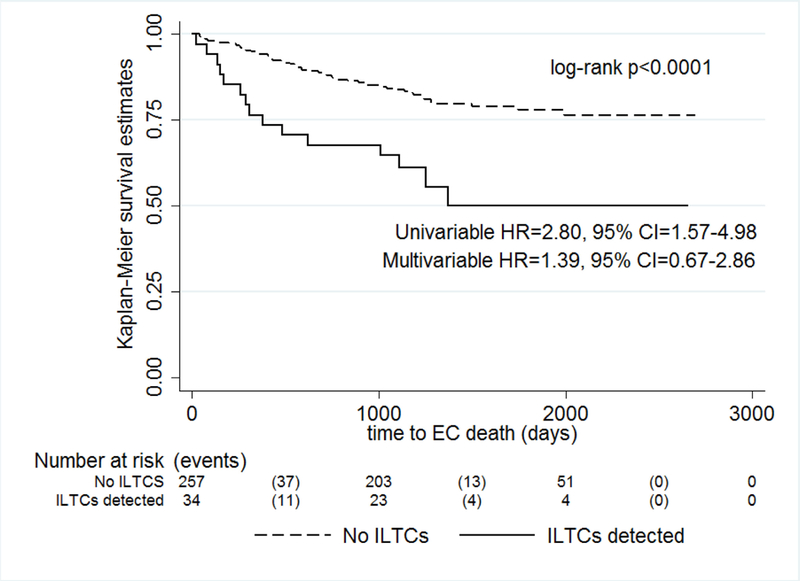
shows significantly lower endometrial cancer-specific survival among endometrial cancer patients with ILTCs compared to women without ILTCs
Associations of ILTCs and survival according to tumor characteristics
We also explored the association between ILTCs, RFS, and EC-specific survival in models stratified by histological subtype or stage. ILTCs were not associated with RFS in models stratified by histological subtype (Table 2). In unadjusted models, ILTCs were associated with EC-specific survival among women with serous EC (HR=3.19, 95% CI=1.32–7.72), but the association was attenuated after adjustment for age and stage (HR=3.38, 95% CI=0.87–13.13, data not shown). Among women with carcinosarcomas, ILTCs were associated with lower EC-specific survival, although the univariable association did not reach statistical significance (HR=2.78, 95% CI=0.76–10.14).
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between ILTCs, RFS, and endometrial cancer-specific survival by histological subtype, N=295
| ILTCs | RFS1 | ILTCs | EC-specific survival | ||||
|---|---|---|---|---|---|---|---|
| recurrences, n (%)2 | HR (95% CI)3 | HR (95% CI)4 | deaths, n (%)2 | HR (95% CI)3 | HR (95% CI)4 | ||
| Low-grade endometrioid, N=97 | |||||||
| Absent (n=88) | 8 (9.1) | 1.00 | 1.00 | Absent (n=90) | 3 (3.3) | 1.00 | 1.00 |
| Present (n=7) | 1 (14.3) | 1.67 (0.21, 13.38) | 0.60 (0.06, 5.78) | Present (n=7) | 1 (14.3) | 4.61 (0.48, 44.31) | 0.49 (0.02, 10.77) |
| High-grade endometrioid, N=60 | |||||||
| Absent (n=49) | 14 (28.6) | 1.00 | 1.00 | Absent (n=55) | 13 (23.6) | 1.00 | 1.00 |
| Present (n=5) | 0 (0.0) | --- | --- | Present (n=5) | 0 (0.0) | --- | --- |
| Serous, N=40 | |||||||
| Absent (n=28) | 13 (46.4) | 1.00 | 1.00 | Absent (n=32) | 14 (43.8) | 1.00 | 1.00 |
| Present (n=4) | 4 (100.0) | 2.62 (0.84, 8.21) | 1.14 (0.20, 6.53) | Present (n=8) | 8 (100.0) | 3.19 (1.32, 7.72) | 3.38 (0.87, 13.13) |
| Mixed epithelial, N=57 | |||||||
| Absent (n=44) | 7 (15.9) | 1.00 | 1.00 | Absent (n=48) | 7 (14.6) | 1.00 | 1.00 |
| Present (n=6) | 0 (0.0) | --- | --- | Present (n=9) | 2 (22.2) | 1.73 (0.36, 8.34) | 1.10 (0.19, 6.49) |
| Carcinosarcoma, N=29 | |||||||
| Absent (n=21) | 11 (52.4) | 1.00 | 1.00 | Absent (n=25) | 13 (52.0) | 1.00 | 1.00 |
| Present (n=1) | 1 (100.0) | 4.73 (0.53, 42.35) | --- | Present (n=4) | 3 (75.0) | 2.78 (0.76, 10.14) | 0.52 (0.04, 7.65) |
| Clear cell, N=9 | |||||||
| Absent (n=8) | 2 (25.0) | 1.00 | 1.00 | Absent (n=8) | 1 (12.5) | 1.00 | 1.00 |
| Present (n=0) | --- | --- | --- | Present (n=1) | 1 (100.0) | --- | --- |
Women with stage IV disease excluded from model
row percentage
Unadjusted HRs and 95% CIs
HRs and 95% CIs adjusted for age (<55, 55–64, ≥65) and FIGO 2009 stage (I, II, III, IV)
Similar to the histological subtype-stratified models, we did not observe an association between ILTCs and RFS in the stage-stratified analyses (Table 3). In univariable models we observed lower EC-specific survival (HR=4.79, 95% CI=1.05–21.90) associated with ILTCs among women with stage I EC (Table 3); associations were attenuated after adjustment for age and histological subtype (HR=4.19, 95% CI=0.84–20.78, data not shown). Associations for stages II-IV ECs were not observed.
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between ILTCs, RFS, and endometrial cancer-specific survival by stage, N=295
| ILTCs | RFS1 | ILTCs | EC-specific survival | ||||
|---|---|---|---|---|---|---|---|
| recurrences, n (%)2 | HR (95% CI)3 | HR (95% CI)4 | deaths, n (%)2 | HR (95% CI)3 | HR (95% CI)4 | ||
| Stage I, n=183 | |||||||
| Absent (n=173) | 16 (9.3) | 1.00 | 1.00 | Absent (n=175) | 10 (5.7) | 1.00 | 1.00 |
| Present (n=8) | 2 (25.0) | 3.47 (0.79, 15.34) | 4.07 (0.85, 19.40) | Present (n=8) | 2 (25.0) | 4.79 (1.05, 21.90) | 4.19 (0.84, 20.78) |
| Stage II, n=23 | |||||||
| Absent (n=20) | 10 (50.0) | 1.00 | 1.00 | Absent (n=19) | 5 (26.3) | 1.00 | 1.00 |
| Present (n=3) | 0 (0.0) | --- | --- | Present (n=3) | 0 (0.0) | --- | --- |
| Stage III, n=59 | |||||||
| Absent (n=45) | 29 (64.4) | 1.00 | 1.00 | Absent (n=47) | 27 (57.5) | 1.00 | 1.00 |
| Present (n=12) | 4 (33.3) | 0.36 (0.13, 1.03) | 0.39 (0.12, 1.29) | Present (n=12) | 5 (41.7) | 0.56 (0.21, 1.46) | 0.75 (0.24, 2.29) |
| Stage IV, n=30 | |||||||
| --- | --- | --- | --- | Absent (n=17) | 9 (52.9) | 1.00 | 1.00 |
| --- | --- | --- | --- | Present (n=11) | 8 (72.7) | 1.71 (0.65, 4.50) | 3.33 (0.71, 15.59) |
Women with stage IV disease excluded from model
row percentage
Unadjusted HRs and 95% CI
HRs and 95% CIs adjusted for age (<55, 55–64, ≥65) and histology (low-grade endometrioid, high-grade endometrioid, serous, mixed epithelial, carcinosarcoma, clear cell)
We also compared EC-specific survival among 11 stage I or II women with ILTCs to 18 women with stage IIIA tumors to explore whether the addition of ILTC information resulted in similar survival between these two groups. EC-specific survival was significantly lower among women with stage IIIA compared to stage I or II tumors with ILTCs detected (61.1% vs. 18.2% died, log-rank p=0.05).
DISCUSSION
In this single institution retrospective study, we observed that detection of ILTCs on H&E stained sections was related to EC survival, particularly among women with serous EC or stage I disease. However, we did not detect significant relationships in multivariable models including other prognostic factors in this limited study set. We identified a higher frequency of ILTCs in women with aggressive tumor characteristics, including Type II EC, advanced stage, and LVSI, with multivariable analyses suggesting that stage and age are significantly associated with detection of ILTC. While preliminary, our results suggest that ILTCs may be a clinically relevant tumor characteristic that could identify subgroups of EC patients with poor prognosis.
Two studies of EC patients have evaluated fallopian tube specimens for ILTCs in relation to tumor characteristics (7, 8). In their analysis of 262 EC cases, over-sampled for women with high-grade EC (226 high-grade and 36 low-grade), Stewart and colleagues (7) reported a significantly higher prevalence of ILTCs among women with high-grade compared to low-grade EC (26.1% vs. 2.8%, respectively). Moreover, in the subgroup of 226 women with high-grade disease, ILTCs were significantly more likely to co-occur with other aggressive tumor features, including positive peritoneal cytology, peritoneal involvement, and lymph node involvement. In their case-series of 87 women with serous EC, Snyder and colleagues (8) reported an ILTC prevalence of 18% and a significant association with peritoneal spread: 100% of women with ILTCs present had evidence of peritoneal spread compared with only 13% of serous EC cases without ILTCs present.
Consistent with these studies, we also found associations of ILTCs with adverse tumor characteristics including advanced stage, LVSI, and Type II histology. In particular, we observed that 20% of women with serous tumors had ILTCs, which was similar to estimates reported by Snyder et al. (18%) and Stewart et al. (32%) (7, 8). This range of prevalence estimates likely reflects the varying assessments of the histological examination of the fallopian tubes. Results from our case-series along with Snyder et al. (8) likely reflect underestimates given the use of available H&E slides as opposed to Stewart et al. (7), where complete sectioning of the fallopian tubes was undertaken.
Our study further expands upon this literature by exploring relationships between ILTCs and survival. No univariable associations with recurrence were observed; however, we did note lower EC-specific survival in patients with ILTCs among women with serous histology or stage I disease. Our finding in the serous subgroup is not altogether surprising in light of studies demonstrating peritoneal spread in the absence of deep myometrial invasion or lymphatic involvement (8, 12, 13). Given that the fallopian tubes open directly into the peritoneal cavity and that some ECs, such as serous carcinomas may present with stage II/IV disease in the absence of substantial disease burden in the uterus, we hypothesize that a subset of aggressive carcinomas demonstrate a propensity for transtubal dissemination. On the other hand, our finding that women with stage I disease have worse EC-specific survival when ILTCs are detected was unexpected. Women with stage I disease have excellent survival; however, our study identified a subgroup of these patients at risk for poor outcomes. However, we also found that women with stage I or II ECs and ILTCs had improved survival compared with women with stage IIIA ECs, based on limited statistical power. Thus, it is unclear whether evaluation of ILTCs by evaluation of a limited number of H&E sections per tube could add information to established prognostic factors. Future studies including larger sample sets with more extensive sampling of the fallopian tube, such as with the Sectioning and Extensive Examination of the Fimbria protocol (SEE-Fim) and use of immunostains might inform this question (14). Further, if a negative examination for ILTCs provides added reassurance of safety, some women with stage I aggressive ECs might be spared more intensive therapy and follow-up.
Our study has several weaknesses including its retrospective, single-institution nature. Additionally, there were small numbers of women in several of the histological subtype categories making it difficult to draw conclusions about the association of ILTC detection and survival outcomes in these subgroups. Finally, our use of available H&E slides for ILTC detection likely resulted in an underestimate of ILTC prevalence. Strengths of this study include availability of robust clinical data for patients enrolled in the study. Moreover, we examined an area of research in which there is little available literature.
As we accumulate data on the clinical significance of EC patterns of spread, staging criteria will continue to be refined. Most recently, staging criteria were revised to remove peritoneal washings due to limited prognostic value (5). Detecting cells in the lumen of the fallopian tube may or may not be related to positive peritoneal cytology; we could not address this with our data although we would hypothesize an association exists. While our data suggest that ILTCs are associated with other aggressive tumor characteristics, we cannot, at this time, conclude that ILTCs are useful for staging or prognosis. Akin to studies that addressed the prognostic impact of peritoneal cytology, additional research, potentially aided by multi-institutional participation, is needed to more precisely understand the clinical significance of ILTCs in EC outcomes.
Footnotes
Conflict of Interest
Dr. Backes reports grants from Eisai Inc, grants from Clovis Oncology, grants from ImmunoGen, other from Tesaro, other from Advaxis. These grants are unrelated to the submitted work.
REFERENCES:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95 Suppl1: S105–143. [DOI] [PubMed] [Google Scholar]
- 3.Barakat RR, Berchuck A, Markman MM, Randall ME. Principles and Practice of Gynecologic Oncology. Sixth ed. New York: Wolters Kluwer and Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 4.Odicino F, Pecorelli S, Zigliani L, Creasman WT. History of the FIGO cancer staging system. Int J Gynaecol Obstet 2008;101: 205–210. [DOI] [PubMed] [Google Scholar]
- 5.Creasman W Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 2009;105: 109. [DOI] [PubMed] [Google Scholar]
- 6.Dahle T Transtubal spread of tumor cells in carcinoma of the body of the uterus. Surg Gynecol Obstet 1956;103: 332–336. [PubMed] [Google Scholar]
- 7.Stewart CJ, Doherty DA, Havlat M, Koay MH, Leung YC, Naran A, et al. Transtubal spread of endometrial carcinoma: correlation of intra-luminal tumour cells with tumour grade, peritoneal fluid cytology, and extra-uterine metastasis. Pathology 2013;45: 382–387. [DOI] [PubMed] [Google Scholar]
- 8.Snyder MJ, Bentley R, Robboy SJ. Transtubal spread of serous adenocarcinoma of the endometrium: an underrecognized mechanism of metastasis. Int J Gynecol Pathol 2006;25: 155–160. [DOI] [PubMed] [Google Scholar]
- 9.Joehlin-Price AS, Stephens JA, Zhang J, Backes FJ, Cohn DE, Suarez AA. Endometrial Cancer Insulin-Like Growth Factor 1 Receptor (IGF1R) Expression Increases with Body Mass Index and Is Associated with Pathologic Extent and Prognosis. Cancer Epidemiol Biomarkers Prev 2016;25: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix AS, Brinton LA, McMeekin DS, Creasman WT, Mutch D, Cohn DE, et al. Relationships of Tubal Ligation to Endometrial Carcinoma Stage and Mortality in the NRG Oncology/ Gynecologic Oncology Group 210 Trial. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lax SF. Pathology of Endometrial Carcinoma. Adv Exp Med Biol 2017;943: 75–96. [DOI] [PubMed] [Google Scholar]
- 12.Gehrig PA, Groben PA, Fowler WC Jr., Walton LA, Van Le L Noninvasive papillary serous carcinoma of the endometrium. Obstet Gynecol 2001;97: 153–157. [DOI] [PubMed] [Google Scholar]
- 13.Hui P, Kelly M, O’Malley DM, Tavassoli F, Schwartz PE. Minimal uterine serous carcinoma: a clinicopathological study of 40 cases. Mod Pathol 2005;18: 75–82. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 2006;30: 230–236. [DOI] [PubMed] [Google Scholar]


