Abstract
Background:
Disparities in disease incidence and outcomes are common in genitourinary cancers including renal cell carcinoma (RCC). As limited studies evaluate ethnic disparities, we used data from an equal access healthcare system to investigate differences between Hispanics and non-Hispanic whites with RCC.
Methods:
We performed a retrospective cohort study within the Kaiser Permanente healthcare system using records from RCC cases. Ethnicity was identified as Hispanic or non-Hispanic whites. Patient characteristics, comorbidities, tumor characteristics and treatment were compared. Overall survival and disease-specific survival was calculated and a Cox proportion hazard model estimated the association of ethnicity and survival.
Results:
2577 patients (2152 non-Hispanic whites, 425 Hispanic) were evaluated. Hispanics were diagnosed at a younger age (59.6 vs 65.3 years). Clear cell RCC was more prevalent while papillary RCC less common among Hispanics. Hispanics had a lower AJCC stage (I/II vs III/IV) than non-Hispanic whites (67.4% vs 62.2%). Hispanics were found to have a greater frequency of comorbidities such as chronic kidney disease and diabetes, but still were more likely to receive surgery. The presence of metastases, nodal involvement, increased tumor size, non-surgical management, increasing age, and Hispanic ethnicity were independent predictors of worse cancer specific outcome.
Conclusions:
Within an equal access healthcare system, Hispanics were diagnosed RCC at younger ages, had greater comorbidities, and more frequently had clear cell RCC. Despite lower stage and greater receipt of surgery, Hispanic ethnicity was an independent predictor of mortality. Further work is necessary to confirm these findings perhaps due to differences in disease biology or treatment.
Keywords: RCC, racial disparities, ethnic disparities, Hispanic
Condensed Abstract:
Even within a healthcare system, Hispanics with kidney cancer are younger, have greater comorbidities, and more frequently have clear cell RCC. Despite lower stage and greater receipt of surgery, Hispanic ethnicity is an independent predictor of worse outcome.
A. BACKGROUND
According to the 2014 United States (US) Census Bureau, 55 million Americans, or 17% of the total US population, were identified as having Hispanic ethnicity1, making people of Hispanic origin the nation’s largest ethnic minority. Approximately 37% of Hispanics are born outside the US and the largest populations of Hispanics are from Mexico (63%), Puerto Rico (9%), Central America (8%), South America (6%), and Cuba (4%)1 . Between 2000 and 2010 the Hispanic population grew by 43%, four times the growth of the total population. As certain ethnic and racial groups have specific cancer predispositions that are due to complex genetic and environmental factors, it is imperative we understand how different illnesses affect Hispanics in the US.
The cancer burden among Hispanics living in the US is generally similar to that seen in the countries of origin2. However, the descendants of Hispanic immigrants have cancer incidence that approaches that of non-Hispanic whites, presumably due to acculturation. Ethnic variations in cancer outcome may also widely vary depending on the specific type of cancer. For the most common cancers such as prostate, female breast, colorectal, and lung, the incidence and death rates are lower among Hispanics than among non-Hispanic whites. However for other cancers such as for stomach, cervix, liver, acute lymphocytic leukemia, and gallbladder, Hispanics have higher disease incidence and mortality2 . Despite renal cell carcinoma being a highly prevalent disease, there have been limited data comparing the outcomes of Hispanics with RCC to non-Hispanic whites1, 3, 4.
There has been a rising trend in the reported cases of RCC amongst all racial groups, with a disproportionate increase amongst several minority groups5. Studies have shown various epidemiologic risk factors predispose for RCC including male gender, chronic kidney disease (CKD), hypertension, and greater body mass index (BMI)6–10. The incidence of several of these risk factors differ between racial groups, which may partly explain some of the recent epidemiologic trends. Besides changing incidence, there has been concern that survival disparities also exist, partially explained by access to care. The largest increase in RCC incidence has occurred in the black population11, yet there are limited studies that compare whether racial disparities exist among Hispanics when they are being compared to other racial/ ethnic groups.
A thorough evaluation into racial disparities in kidney cancer survival requires understanding of comorbidities, tumor characteristics, and treatment. In the US, access to health care significantly differs by race12, which can influence outcomes due to barriers to treatment. Analyzing survival outcomes using a large database such as Surveillance, Epidemiology and End Results (SEER) program may not account for barriers to care. Therefore, it is reasonable to assume that survival disparities that are related to access to care and not tumor biology or treatment may disappear when evaluated in single health care system13, 14. By using a single health care payer database, we evaluated whether survival differences exist while controlling for demographics, tumor characteristics, comorbidities, and treatment in a system where all patients have comparable access to care.
B. MATERIALS AND METHODS
Study Design
To evaluate racial disparities between Hispanics and non-Hispanic whites in a single payer health care system, we performed a case-control study of renal cell carcinoma nested within a retrospective cohort of members of the Northern California Kaiser Permanente System 15. Study participants were included if they had been diagnosed with RCC (International Classification of Disease 9th edition, site code C64.9) between 1998–2008 and received care within this prepaid health care system. Data used to report demographics were obtained from membership databases which include age, race/ethnicity (self-described) and sex. The cancer data were obtained from the Kaiser Permanente Cancer Registry, which reports directly to the SEER program. Patient race and ethnicity are recorded in the Kaiser Permanente database and are based on self-reported patient records. Patient characteristics (age, ethnicity, gender), comorbidity data (BMI, smoking history, hypertension, diabetes, CKD, any renal disease, and anemia), pathologic tumor characteristics (histologic subtype, tumor size, T stage, grade, and AJCC stage (7th edition)), and follow-up outcome was recorded16. Vital status and date of last follow-up were available until December 31, 2013 and were used for survival analyses. The cause and date of death were assigned using a combination of cancer registry data and a mortality registry that concatenates state and federal mortality files, including primary cause of death from California death certificates.
Statistical Analysis
We evaluated differences in clinico-pathologic and comorbidity variables that might explain or confound the relationships between Hispanic ethnicity and adverse cancer outcomes. Continuous and categorical variables were analyzed with Kruskal-Wallis and Chi-Square tests, respectively. Disease-specific survival (DSS) and overall survival (OS) were calculated from the date of diagnosis to the date of last follow-up or death (based on the death certificate) using the Kaplan-Meier method and comparisons were made with log-rank testing. Cox proportional hazard models were performed to assess the influence of variables on OS and DSS. Only significant variables in the univariate analysis were included in the multivariable model. Statistical significance was considered for p values ≤ 0.05. Statistical analysis was performed using SAS (Cary, NC).
C. RESULTS
Patient Characteristics
A total of 2577 patients was included, of whom 2152 (83.5%) were non-Hispanic whites and 425 (16.5%) were Hispanic. Hispanics were diagnosed with RCC at a younger mean age compared to non-Hispanic whites (59 years vs 66 years, p <0.0001) (Table 1). Overall, Hispanics were found to have a higher prevalence of comorbidities such as chronic kidney disease (2.6% vs 0.7%, p <0.0001) and diabetes (23.8% vs 15.9%, p <0.001). The prevalence of obesity (BMI > 30) was also higher among Hispanics compared with non-Hispanic whites (57.9% vs 43.5%, p <0.0175), although BMI data were missing for most (2,013 patients missing BMI (1,695 non-Hispanic whites, 318 Hispanics)) of the patients included in this investigation. In contrast, non-Hispanic white patients were more likely to have a recorded history of smoking (24.6% vs 20.2%, p <0.0523). There were no significant differences between Hispanics and non-Hispanics for gender, hypertension, or anemia.
Table 1:
Demographic, clinical, pathologic, outcomes data of non-Hispanic white and Hispanic patients with RCC who received care with Kaiser Permanente Northern California from 1998–2008
| Variable | Hispanics (N=425) | Whites (N=2152) | p value |
|---|---|---|---|
| Age at diagnosis (years) | <.0001 | ||
| Median | 59.6 | 66.3 | |
| IQR | 51–70 | 57–74 | |
| SD | 13.21 | 12.06 | |
| Gender | 0.860 | ||
| Male | 273 (64.2%) | 1392 (64.7%) | |
| Female | 152 (35.8%) | 760 (35.3%) | |
| BMI* | 0.0175 | ||
| < 25 | 13 (12.2%) | 95 (20.8%) | |
| 25 to 30 | 32 (29.9%) | 163 (35.7%) | |
| > 30 | 62 (57.9%) | 199 (43.5%) | |
| *Missing data | 318 | 1695 | |
| Smoking History | 0.0523 | ||
| Yes | 86 (20.2%) | 530 (24.6%) | |
| No | 339 (79.8%) | 1622 (75.4%) | |
| Hypertension | 0.880 | ||
| Yes | 211 (49.6%) | 1077 (50.1%) | |
| No | 214 (50.4%) | 1075 (49.9%) | |
| Diabetes | <0.0001 | ||
| Yes | 101 (23.8%) | 343 (15.9%) | |
| No | 324 (76.2%) | 1809 (84.1%) | |
| CKD | <0.001 | ||
| Yes | 11 (2.6%) | 15 (0.7%) | |
| No | 414 (97.4%) | 2137 (99.3%) | |
| Any Renal Disease | 0.020 | ||
| Yes | 18 (4.2%) | 49 (2.3%) | |
| No | 407 (95.8%) | 2103 (97.7%) | |
| Anemia | 0.407 | ||
| Yes | 41 (9.6%) | 181 (8.4%) | |
| No | 384 (90.4%) | 1971 (91.6%) | |
| Tumor size | 0.14 | ||
| Median | 5.0 | 5.1 | |
| Histologic subtype | 0.004 | ||
| Clear cell | 378 (88.9%) | 1829 (85.0%) | |
| Papillary | 5 (1.2%) | 111 (5.2%) | |
| Chromophobe | 12 (2.8%) | 56 (2.6%) | |
| Others | 30 (7.1%) | 156 (7.3%) | |
| Tumor Grade (866 missing) | 0.297 | ||
| Grade 1 | 56 (17.9%) | 198 (14.2%) | |
| Grade 2 | 142 (45.4%) | 648 (46.4) | |
| Grade 3 | 96 (30.7%) | 441 (31.5%) | |
| Grade 4 | 19 (6.1%) | 111 (7.9%) | |
| T Stage (193 missing) | 0.181 | ||
| T1/T2 | 300 (75.4%) | 1424 (72.1%) | |
| T3/T4 | 98 (24.6%) | 551 (27.9%) | |
| N Stage (1 missing) | 0.382 | ||
| N0/Nx | 417 (98.1%) | 2095 (97.4%) | |
| N+ | 8 (1.9%) | 56 (2.6%) | |
| M Stage | 0.110 | ||
| M0/Mx | 346 (81.4.3%) | 1677 (77.9%) | |
| M+ | 79 (18.6%) | 475 (22.1%) | |
| AJCC Staging (15 missing) | 0.044 | ||
| Stage I/II | 285 (67.4%) | 1330 (62.2%) | |
| Stage III/IV | 138 (32.6%) | 808 (37.8%) | |
| Treatment Option | 0.004 | ||
| Surgery | 358 (84.2%) | 1677 (77.9%) | |
| No surgery | 67 (15.8%) | 475 (22.1%) | |
| Vital Status | 0.009 | ||
| Alive | 233 (54.8%) | 1031 (47.9%) | |
| Dead | 192 (45.2%) | 1121 (52.1%) | |
| Death from RCC | 0.033 | ||
| Alive | 233 (54.8%) | 1031 (47.9%) | |
| Dead from other cause | 109 (25.7%) | 626 (29.1%) | |
| Dead from RCC | 83 (19.5%) | 495 (23.0%) |
Cancer Characteristics
Substantial differences were found between Hispanics and non-Hispanic whites with respect to tumor characteristics. The histologic types differed between groups (p<0.044) with clear cell RCC more prevalent among Hispanics (88.9% vs 85%), while papillary RCC was more common among non-Hispanic whites (5.2% vs 1.2%). Hispanics were found to have lower AJCC stage at diagnosis (I/II vs III/IV) than non-Hispanic whites (67.4% vs 62.2%, p <0.044). No statistical difference was found when comparing tumor size and grade, or TNM staging.
Cancer Management and Outcomes
Hispanics were more likely than non-Hispanic whites to receive surgery (84.2% vs 77.9%, p <0.004) and overall RCC-related deaths were less frequent in Hispanic patients compared with non-Hispanic whites (19.5% vs 23.0%, p <0.033). On multivariate analysis of disease-specific survival (Table 2), tumor grade (P <0.001), AJCC III/IV staging (P <0.001), increased tumor size (P <0.001), non-surgical management (P <0.001), increasing age (P <0.001), and Hispanic ethnicity (P <0.03) were all independent predictors of worse outcome. The hazard ratio (HR) for Hispanic ethnicity was 1.38 (95% confidence interval 1.03–1.84). An unadjusted analysis removing tumor characteristics and treatment did not identify any differences in mortality among Hispanics (Supplemental Table 1).
Table 2:
Multivariate analysis of prognostic variables for Overall Survival (OS) and Disease Specific Survival (DSS) in patients with kidney cancer who received care with Northern California Kaiser Permanente General from 1998–2008
| Overall Survival | Disease-specific Survival | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | p value | Hazard Ratio | 95% CI | p value |
| Age | 1.04 | 1.03–1.05 | <0.0001 | 1.01 | 1.00–1.02 | 0.165 |
| Gender | 0.027 | 1.265 | ||||
| Male | 1.00 (ref) | 1.00 (ref) | ||||
| Female | 0.83 | 0.71–0.98 | 0.99 | 0.79–1.27 | ||
| Race | 0.113 | 0.031 | ||||
| White | 1.00 (ref) | 1.00 (ref) | ||||
| Hispanic | 1.18 | 0.96–1.45 | 1.38 | 1.03–1.84 | ||
| Any Renal Disease | 0.67 | 0.44–1.02 | 0.063 | 1.38 | 0.50–3.77 | 0.533 |
| Diabetes | 0.68 | 0.56–0.82 | <0.0001 | 1.03 | 0.76–1.40 | 0.853 |
| Tumor size (cm) | 1.04 | 1.027–1.047 | <0.0001 | 1.05 | 1.04–1.06 | <0.0001 |
| Tumor Grade | <0.0001 | <0.0001 | ||||
| Grade 1 | 0.45 | 0.33–0.62 | <0.0001 | 0.25 | 0.16–0.40 | <0.0001 |
| Grade 2 | 0.4 | 0.31–0.51 | <0.0001 | 0.26 | 0.19–0.36 | <0.0001 |
| Grade 3 | 0.53 | 0.41–0.68 | <0.0001 | 0.55 | 0.41–0.73 | <0.0001 |
| Grade 4 | 1.00 (ref) | 1.00 (ref) | ||||
| Histological Subtype | 0.364 | |||||
| Clear Cell | 1.00 (ref) | 1.00 (ref) | ||||
| Papillary | 0.92 | 0.62–1.38 | 0.695 | 0.9 | 0.42–1.92 | 0.78 |
| Others | 0.89 | 0.70–1.12 | 0.326 | 0.81 | 0.58–1.12 | 0.199 |
| AJCC Stage | <0.0001 | <0.0001 | ||||
| Stage 1 & 2 | 1.00 (ref) | 1.00 (ref) | ||||
| Stage 3 & 4 | 2.69 | 2.30–3.14 | 7.65 | 5.88–9.96 | ||
| Treatment Status | <0.0001 | <0.0001 | ||||
| Surgery | 1.00 (ref) | 1.00 (ref) | ||||
| No Surgery | 3.79 | 2.95–4.88 | 5.74 | 4.23–7.81 | <0.0001 | |
D. DISCUSSION
Racial and ethnic disparities in cancer incidence, disease characteristics, and mortality significantly differ in many cancers. These differences can be related to substantial differences in disease biology but can be the result of issues with access to care and the acceptance of screening or likelihood of early diagnosis for symptoms within specific groups. Over the next three decades, the number of Hispanics is expected to triple in the U.S. and will account for 30% of the population17. Therefore, it is imperative we understand the current state of cancer in the Hispanic population. While kidney cancer has received attention as being one of the cancers with significant differences by race/ethnicity in incidence and mortality, knowledge of potential differences in kidney cancer incidence, risk factors and outcomes in the Hispanic community have previously not been investigated.
In our evaluation of a large, diverse integrated health care system, we were able to examine the clinico-pathological characteristics and survival outcomes for Hispanic and non-Hispanic white patients with kidney cancer. There were significant ethnic differences in patient and tumor characteristics as well as outcome. Although Hispanic ethnicity was not associated with a higher overall risk of death, unadjusted for stage, Hispanic ethnicity was found to be an independent predictor of worse disease-specific survival when incorporating all prognostic variables. The difference between the stage-adjusted and unadjusted analysis appears to be related to the observation that Hispanics were more likely, for unclear reasons, to present with earlier-stage disease. There are multiple potential explanations for these findings, including underlying biology as well as differences in underlying risk factors, likelihood of presenting with symptoms such as pain or hematuria, disease biology, and differences in selection of post-diagnostic treatment. Understanding these differences may be necessary to close an observed stage-specific survival gap, in addition to improving traditionally recognized social issues, such as early diagnosis and access to care.
Other studies have tried to determine whether cancer biology differs for Hispanics compared to non-Hispanic whites. Hines et al. aimed to determine whether Hispanic and Non-Hispanic white women presented with differing proportions of breast cancer phenotypic subtypes18. Clinical and pathologic characteristics were compared by ethnic groups and Hispanic women showed a greater frequency of tumors that were ER-negative and HER2-positive18. In fact, the odds of HER2 positivity were 2.8 times higher in Hispanic patients, suggesting they may be more likely to benefit from therapy directed towards HER2 such as trastuzumab18. Altogether, these findings suggest that breast carcinoma in Hispanic women likely has a distinct underlying biology. Assessing these differences in disease biology and driver alterations between groups in other cancers, such as renal cell carcinoma, may yield results that help identify markers which lead to greater biologic aggressiveness or direct treatment.
In the Kaiser Permanente system, the availability of insurance, assigned primary care physicians, coordinated medical facilities, and clinical practice guidelines improve patients’ access to care and appropriate, guideline-based treatment. Unlike past studies where surgical treatment rates were more equivalent across racial groups in a single health care system, Hispanics were actually found to have higher surgical rates within the current study, compared to non-Hispanic whites5. Controlling for access to care, variation in the rates of surgery may be due to differences in surgical candidacy, such as earlier stage disease, which was more common among Hispanics in the current study. This may also correlate with the fact that the age of diagnosis of kidney cancer was significantly younger in Hispanics when compared to non-Hispanic whites. Another issue could be related to the cultural acceptance of newer non-surgical treatment modalities such as ablation or active surveillance. Recent work has suggested that minority groups may have differences in their decision-making regarding surgical treatment of early stage cancer19, and therefore decreased acceptance of active surveillance could account for an increased rate of treatment among Hispanics, although the current study cannot address the reasons for differences in treatment or why, despite aggressive surgical treatment, Hispanics had lower survival adjusted for stage.
Racial variation in histologic subtype distribution has recently come to light in kidney cancer as papillary kidney cancer is significantly enriched in the African American population. In our prior work involving the same Kaiser Permanente case-control study, the frequency of papillary RCC was nearly three times greater among black individuals than among whites with kidney cancer5. Our results demonstrate even compared to non-Hispanic whites, Hispanics have an even lower incidence of papillary histology, as it accounted for only 1.2% of overall kidney cancer cases. Complex genetic or environmental factors may be associated with the distribution of histology types present in specific ethnic or racial groups20. Established risk factors for RCC include male sex, African American race, family history of kidney cancer, obesity, hypertension and smoking7. Obesity has been suggested to be more strongly associated with clear cell RCC than with other subtypes in two clinical case series and an Italian case-control study21–23. Our study found that Hispanics were more likely to be diagnosed with clear cell RCC, and it has already been well documented that there is a direct link between Hispanic ethnicity and the risk of obesity24. These observations suggest that a combination of genetic predispositions and environmental factors may be largely responsible for our findings of worse Hispanic disease-specific survival.
To our knowledge, this is the first study that examines survival disparities among Hispanic patients with kidney cancer. By using a single regional healthcare system, we hoped to minimize extraneous variables such as access to care, which could influence results. Unlike studies based only on cancer registry data, we included comorbidity data such as CKD which may be critical to survival analyses as a recent paper suggests renal comorbidities account for much of the observed racial disparity in African-Americans.25 Besides influencing overall survival, comorbidities may influence specific treatment decisions and similarly affect cancer outcome.
Our analysis also has limitations including several categories where there was missing data. BMI was only available for patients enrolled from 2005 – 2008, and therefore is missing on a large number of patients. However, as observed in our study for those with available BMI, the prevalence of obesity is higher among Hispanics compared with non-Hispanic whites in the general U.S. population24. Paradoxically, obesity has been consistently associated with improved RCC survival26; as such, the observed association between Hispanic ethnicity and RCC survival is likely to be a conservative estimate, and we would expect the association to be stronger if we had been able to control for BMI. Additionally, although tumor stage was widely available, tumor grade also had a large number of missing values. Ethnicity is also a very complicated subject and there are blacks that have Hispanic ethnicity in the United States. We chose to exclude black Hispanics from the analysis as there are well-documented racial disparities in kidney cancer. While we had information on treatment we also did not have specifics on type of surgery and utilization of systemic therapy at time of recurrence- both of which could influence disease outcome.
Conclusion
Within a healthcare system with similar access to care, Hispanics with RCC were younger, had greater comorbidities, and more frequently had clear cell RCC. Despite a lower AJCC stage, increased receipt of surgery and similar overall survival, Hispanic ethnicity was an independent predictor of worse disease-survival. This study suggests there may be disparities in kidney cancer survival outcomes between Hispanics and non-Hispanic whites not resulting from lack of access to care but rather from disease biology or treatment. Further work is necessary to confirm this health disparity in other large datasets.
Supplementary Material
Supplemental Table 1: Multivariate analysis of prognostic variables for Disease-Specific Survival (DSS) unadjusted for tumor characteristics and treatment in patients with kidney cancer who received care with Northern California Kaiser Permanente General from 1998–2008
Figure 1:
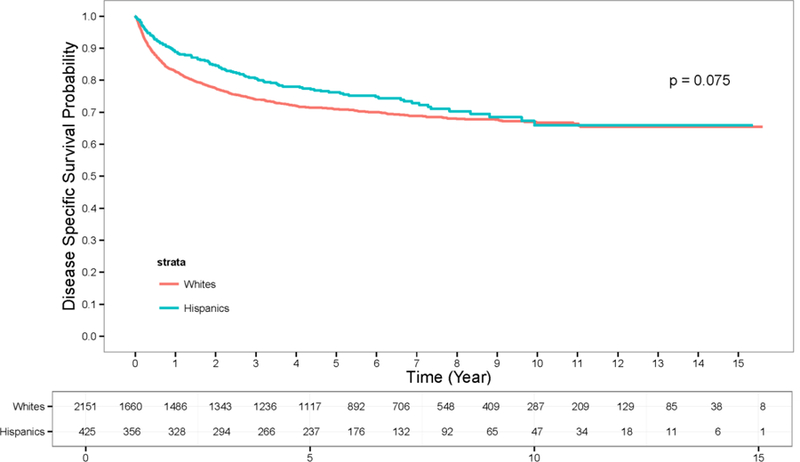
Disease-Specific Survival for Hispanic and Non-Hispanic White patients diagnosed with kidney cancer.
Figure 2:
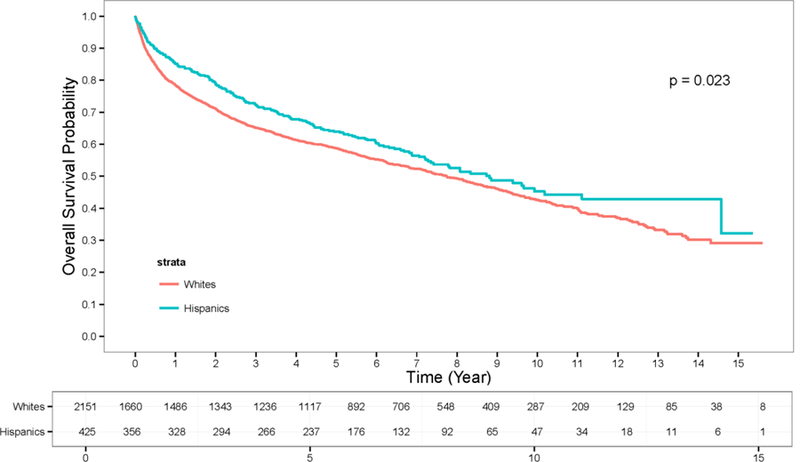
Overall Survival for Hispanic and Non-Hispanic White patients diagnosed with kidney cancer.
Abbreviations:
- RCC
Renal Cell Carcinoma
- BMI
Body Mass Index
- SEER
Surveillance, Epidemiology and End Results
- CKD
Chronic Kidney Disease
- AJCC
American Joint Committee on Cancer
- DSS
Disease-specific Survival
- OS
Overall Survival
RERERENCES
- 1.U.S. Census Bureau PD. Annual Estimates of the Resident Population by Sex, Age, Race, and Hispanic Origin for the United States: April 1, 2010 to July 1, 2014 In: U.S. Census Bureau PD, editor. Washington, D.C., 2014. [Google Scholar]
- 2.Society AC. Cancer Facts & Figures for Hispancis/Latinos 2012–2014 Atlanta, Ga: American Cancer Society, 2014. [Google Scholar]
- 3.Chao A, Gilliland FD, Hunt WC, Bulterys M, Becker TM, Key CR. Increasing incidence of colon and rectal cancer among Hispanics and American Indians in New Mexico (United States), 1969–94. Cancer Causes Control 1998;9: 137–144. [DOI] [PubMed] [Google Scholar]
- 4.Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev 2009;18: 2162–2169. [DOI] [PubMed] [Google Scholar]
- 5.Mafolasire A, Yao X, Nawaf C, et al. Racial disparities in renal cell carcinoma: a single-payer healthcare experience. Cancer Med 2016. [DOI] [PMC free article] [PubMed]
- 6.Chow WH DL, Devesa SS. Epidemiology and risk factors for kidney cancer. Nature Reviews Urology 2010;7: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purdue MP, Moore LE, Merino MJ, et al. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer 2013;132: 2640–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colt JS, Schwartz K, Graubard BI, et al. Hypertension and risk of renal cell carcinoma among white and black Americans. Epidemiology 2011;22: 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann JN, Schwartz K, Chow WH, et al. The association between chronic renal failure and renal cell carcinoma may differ between black and white Americans. Cancer Causes Control 2013;24: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann JN, Corley DA, Zhao WK, et al. Chronic kidney disease and risk of renal cell carcinoma: differences by race. Epidemiology 2015;26: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Fedewa SA, Miller KD, et al. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 2015;65: 457–480. [DOI] [PubMed] [Google Scholar]
- 12.Zini L, Perrotte P, Capitanio U, et al. Race affects access to nephrectomy but not survival in renal cell carcinoma. Bju International 2009;103: 889–893. [DOI] [PubMed] [Google Scholar]
- 13.Tsao CK, Small AC, Moshier EL, et al. Trends in the use of cytoreductive nephrectomy in the United States. Clin Genitourin Cancer 2012;10: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pui CH, Pei D, Pappo AS, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol 2012;30: 2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Zahm SH, Shriver CD, Purdue M, McGlynn KA, Zhu K. Survival among Black and White patients with renal cell carcinoma in an equal-access health care system. Cancer Causes Control 2015;26: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haile RW, John EM, Levine AJ, et al. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila) 2012;5: 150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hines LM, Risendal B, Byers T, Mengshol S, Lowery J, Singh M. Ethnic disparities in breast tumor phenotypic subtypes in Hispanic and non-Hispanic white women. J Womens Health (Larchmt) 2011;20: 1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Janisse J, Ruterbusch J, Ager J, Schwartz KL. Racial Differences in Treatment Decision-Making for Men with Clinically Localized Prostate Cancer: a Population-Based Study. J Racial Ethn Health Disparities 2016;3: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olshan AF, Kuo TM, Meyer AM, Nielsen ME, Purdue MP, Rathmell WK. Racial difference in histologic subtype of renal cell carcinoma. Cancer Med 2013;2: 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donat SM, Salzhauer EW, Mitra N, Yanke BV, Snyder ME, Russo P. Impact of body mass index on survival of patients with surgically treated renal cell carcinoma. J Urol 2006;175: 46–52. [DOI] [PubMed] [Google Scholar]
- 22.Lowrance WT, Thompson RH, Yee DS, Kaag M, Donat SM, Russo P. Obesity is associated with a higher risk of clear-cell renal cell carcinoma than with other histologies. Bju International 2010;105: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dal Maso L ZA, Tavani A, et al. Renal Cell Cancer and body size at different ages: an Italian multicenter case-control study. Am J Epidemiology 2007;166: 582–591. [DOI] [PubMed] [Google Scholar]
- 24.Ogden CL CM, Kit BK, Flegal KM. . Prevalence of obesity among adults: United States, 2011–2012 Hyattsville, MD: National Center for Health Statistics, 2013. [Google Scholar]
- 25.Schwartz K, Ruterbusch JJ, Colt JS, Miller DC, Chow WH, Purdue MP. Racial disparities in overall survival among renal cell carcinoma patients with young age and small tumors. Cancer Med 2015. [DOI] [PMC free article] [PubMed]
- 26.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 2013;105: 1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Multivariate analysis of prognostic variables for Disease-Specific Survival (DSS) unadjusted for tumor characteristics and treatment in patients with kidney cancer who received care with Northern California Kaiser Permanente General from 1998–2008


