Abstract
Objectives:
Fractures of the hip, distal radius, and proximal humerus are common in the Medicare population. This study’s objective was to characterize patterns and duration of opioid use, including regional variations in use, after both surgical and non-operative management.
Design:
Population-based cohort study
Setting and Participants:
A cohort of opioid-naïve community-dwelling United States (US) Medicare beneficiaries who survived a hip, distal radius, or proximal humerus fracture between January 1st, 2007 and December 31st, 2010. Cohort members were required to be opioid-naïve for 4 months prior to fracture.
Measures:
We analyzed the proportion of patients with an active opioid prescription in each month following the index fracture, and report continued fills at 12 months post-fracture. We also compared opioid prescription use in fractures treated surgically and non-surgically and characterized state-level variation in opioid prescription use at three months post-fracture.
Results:
There were 91,749 patients included in the cohort. Hip fracture patients had the highest rate of opioid use at 12 months (6.4%), followed by proximal humerus (5.7%), and distal radius (3.7%). Patients who underwent surgical fixation of proximal humerus and wrist fractures had higher rates of opioid use in each of the first 12 postoperative months compared to those managed non-operatively. There was significant variation of opioid use at the state level, ranging from 7.6% to 18.2% of fracture patients filling opioid prescriptions 3 months after the index fracture.
Conclusions/Implications:
Opioid-naïve patients sustaining fragility fractures of the hip, proximal humerus or distal radius are at risk to remain on opioid medications 12 months after their index injury, and surgical management of proximal humerus and distal radius fractures increases opioid use in the 12 months after the index fracture. There is significant state-level variation in opiate consumption after index fracture in non-vertebral geriatric fragility fractures. Opportunity exists for targeted quality improvement efforts to reduce the variation in opioid use following common geriatric fragility fractures.
Keywords: fragility fracture, opioids, surgery, non-operative treatment
Introduction
Fractures of the hip, distal radius and proximal humerus are the most common non-vertebral fragility fractures in patients over the age of 65.1–3 Although the incidence of these fractures varies among different demographic groups and geographic regions of the United States4–6, the incidence of these fractures is projected to increase.7–9 The morbidity, mortality, and costs associated with fragility fractures have been well-documented10–12, with one study noting that 30% of patients with a fragility fracture will enter a post-acute care facility for the first time.13
In the acute setting, pain management often assumes a central role after fracture. While pain management can take many forms depending on the clinical presentation of the patient, opioid pain medications are often used for analgesia. A recent study found that in Medicare patients with hip, distal radius, or proximal humerus fragility fractures, opiate use increased in a statistically significant manner after the fracture event.14 Though failure to manage pain for these patients in the acute setting can increase morbidity and delay discharge15,16, narcotic use comes with its own risks, including but not limited to dependence17; increased risk of balance disturbance and falling18; increased risk of secondary fracture at higher doses19; and multiple side effects including sedation, dizziness and balance problems, nausea, vomiting, constipation, and respiratory depression.20 These risks are especially pronounced in the US, which consumes upwards of 80% of the global opioid supply and up to 99% of the world’s oxycodone and hydrocodone.21,22 Indeed, on a societal level, US prescription opiate use and concomitant abuse has increased substantially over the past 2 decades.21,23–25 Additionally, in the “flood of opioids”26, deaths attributable to prescription opioids in the general population more than tripled between 1999 and 2014.27
Opioid prescribing patterns among US patients with fragility fractures is an important area for investigation. A recent study found that over 14% of opioid-naïve Medicare beneficiaries admitted to a hospital were given a new narcotic prescription at hospital discharge.28 This, along with data about the high prevalence of opioid use after fragility fractures in the Medicare population14, raises the concern that people with acute injuries resulting in fracture may be a population at particular risk of prolonged exposure to prescription opioids.
The purpose of this study was to characterize the patterns and duration of opioid use, including regional variations in use, after both surgical and non-operative management of fragility fractures in the hip, proximal humerus and distal radius in the US Medicare population. We hypothesized that a proportion of previously opioid-naïve patients would continue to use opioid medications 12 months after the fracture, that surgical treatment would lead to less opioid use, and that there would be significant state-level variation in opioid use.
Methods
Study Cohort
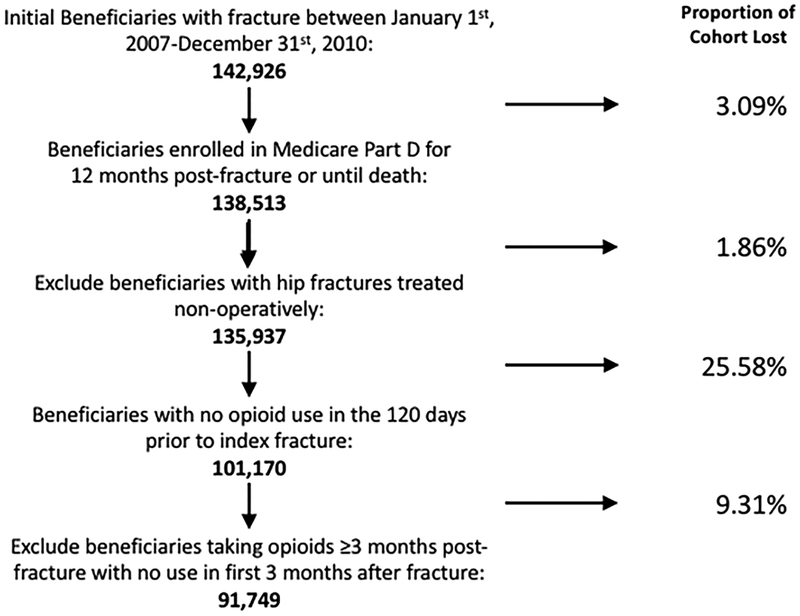
The study was approved by the Committee for the Protection of Human Subjects at XXX (STUDY0002338, last approval date 04/27/17). The cohort used in this study consisted of US Medicare beneficiaries who sustained a fracture of the hip, proximal humerus or distal radius between January 1st, 2007 through December 31st, 2010. Fractures were identified using International Classification of Diseases, Ninth Revision codes and had to be accompanied by an appropriate surgical procedure code (Current Procedural Terminology code) in the case of hip fracture or a code for a radiographic examination within 7 days of the diagnosis of a proximal humerus or distal radius fracture and immobilization or treatment. Beneficiaries sustaining a second fracture after the index fracture were excluded. Beneficiaries had to be enrolled in Medicare Parts A and B without managed care enrollment for one year before and one year after the fracture or until death. Beneficiaries were also required to be enrolled in Medicare Part D (prescription drug coverage) for four months prior to the index fracture and for 12 months after the fracture or until death. The beneficiaries were further required to be community dwelling at the time of the fracture and for at least 30 days within the four months following the fracture to ensure that retail pharmacy prescriptions would be captured in the database. Finally, beneficiaries were only included in the cohort if they were opioid-naïve prior to their index fracture. We defined opioid-naïve as no opioid prescription fills in the four months prior to fracture, excluding the immediate seven days prior to account for possible discrepancies between the date of diagnosis and the date a fracture occurred. Beneficiaries with hip fractures treated non-operatively were excluded because this small proportion of patients often are at the end of life and not appropriate for examination of long-term pharmaceutical use. A full description of the details regarding cohort selection, the database and the algorithm used to identify index fractures among beneficiaries has previously been published.29
Opioid Use
Pharmacy claims from the Part D Prescription Drug Event file were used to identify opioid prescriptions filled by patients in outpatient pharmacies. After the index fracture date, some beneficiaries may initiate opiates for reasons unrelated to the fracture. To reduce this possibility, measurement of chronic post-fracture opiate use was limited to those patients who had their first prescription fill within 3 months of the fracture.
Statistical Analysis
The demographic characteristics of the study population were summarized according to fracture type. Generalized linear mixed models were applied to binary indicators of individual opiate use in each month post fracture using a logit link function, and interaction terms were used to estimate monthly rates stratified by index fracture type. By modeling monthly rates, we estimated the use among survivors for each month in question. Beneficiary identifier numbers and indicators of state of residence were used as random effects. Age, gender, race, Charlson comorbidity score, operative vs. non-operative treatment and month post fracture were used as fixed effects. The adjusted percentage of fracture patients with opiate fills was then plotted over time, excluding the first month. This month had high missing data because prescription fills for patients in hospital or inpatient rehabilitation are not present in Part D files. To estimate the effects of surgery, separate models including the interaction of month and operative status were fit to obtain the adjusted percentage of fracture patients filling opiate prescriptions by operative status.
Regional variation in opiate use is presented by state. The adjusted percentage of opioid users at the third month post fracture were estimated for each state using empirical Bayes methods which adjust for demographic factors and the potential instability in raw rates due to variable population sizes. States were placed into population-weighted quintiles and the results were presented as a map. The third month post-fracture was chosen to avoid the problem of missing prescription data in the first month while also capturing a time point where a substantial proportion of beneficiaries would still be using opioids after their fracture.
Results
The cohort consisted of 91,749 patients who met the inclusion criteria for this study. The flowchart describing the cohort creation is shown in Figure 1. The demographics, comorbidities, and fracture types of the cohort are summarized in Table 1. Across all fracture types, the mean age was 80.4 years, 84.2% were women and 89.2% were white. The majority (65.3%) had zero or one Charlson comorbidity and 6.2% died within one year of their index fracture. Of the entire cohort, 61.1% were treated surgically, with 29.5% and 22.0% of distal radius and proximal humerus fractures treated surgically, respectively.
Figure 1:
Cohort selection
Table 1:
Baseline characteristics of opiate naïve Medicare beneficiaries who sustained a fragility fracture of the hip, distal radius or proximal humerus
| Total | HIP | Distal Radius | Proximal Humerus | |||||
|---|---|---|---|---|---|---|---|---|
| N= 91,749 | % (STD) | N= 42617 | % (STD) | N= 34385 | % (STD) | N= 14747 | % (STD) | |
| Female | 77276 | 84.23 | 34165 | 80.17 | 30515 | 88.75 | 12596 | 85.41 |
| Mean age | 80.36 | 7.64 | 82.37 | 7.32 | 78.95 | 7.48 | 78.48 | 7.49 |
| Age | ||||||||
| 66–74 | 23420 | 25.53 | 6958 | 16.33 | 11744 | 34.15 | 4718 | 31.99 |
| 75–84 | 39109 | 42.63 | 17967 | 42.16 | 14720 | 42.81 | 6422 | 43.55 |
| 85 and over | 29220 | 31.85 | 17692 | 41.51 | 7921 | 23.04 | 3607 | 24.46 |
| Race | ||||||||
| White | 81857 | 89.22 | 38144 | 89.50 | 30268 | 88.03 | 13445 | 91.17 |
| Black | 2391 | 2.61 | 1208 | 2.83 | 892 | 2.59 | 291 | 1.97 |
| Asian | 2048 | 2.23 | 939 | 2.20 | 895 | 2.60 | 214 | 1.45 |
| Hispanic | 4656 | 5.07 | 1931 | 4.53 | 2026 | 5.89 | 699 | 4.74 |
| Other | 797 | 0.87 | 395 | 0.93 | 304 | 0.88 | 98 | 0.66 |
| Number of Chronic Conditions | ||||||||
| 0 | 33307 | 36.30 | 11006 | 25.83 | 16435 | 47.80 | 5866 | 39.78 |
| 1 | 26642 | 29.04 | 12590 | 29.54 | 9707 | 28.23 | 4345 | 29.46 |
| 2 | 16332 | 17.80 | 9144 | 21.46 | 4749 | 13.81 | 2439 | 16.54 |
| 3 | 8674 | 9.45 | 5324 | 12.49 | 2173 | 6.32 | 1177 | 7.98 |
| 4 | 6794 | 7.40 | 4553 | 10.68 | 1321 | 3.84 | 920 | 6.24 |
| Died within 1 year of fracture | 5696 | 6.21 | 3562 | 8.36 | 774 | 5.25 | 1360 | 3.96 |
| Treated Surgically | 56022 | 61.06 | 42617 | 100.00 | 10151 | 29.52 | 3254 | 22.07 |
Opiate prescriptions after fracture
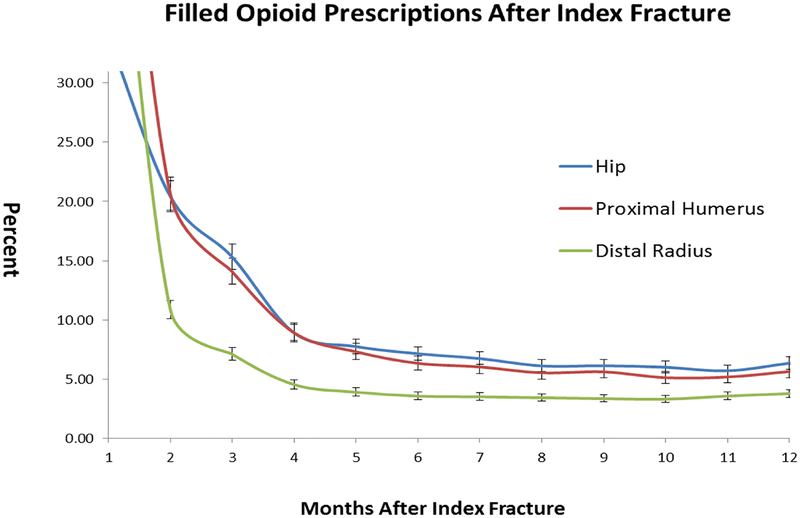
The percent of patients filling an opioid prescription per month after the index fracture based on the generalized linear mixed model is shown in Figure 2. In general, opiate use drops off quickly after a fracture but stops declining approximately six months post fracture, never going to less than 3.8% for any fracture type. At 12 months, hip fractures had the highest percentage of patients continuing to fill opiate prescriptions (6.4%), followed by proximal humerus fractures (5.7%) and distal radius fractures (3.8%). The hip and humerus fracture use rates were not significantly different from each other, while the rates of use after distal radius fracture were significantly lower than the hip and proximal humerus cohorts.
Figure 2:
Percent of patients with an active opioid prescription by month according to fracture type
Opiate exposure after surgical versus nonoperative treatment
For both proximal humerus and distal radius fractures, patients who were treated surgically had a higher rate of opiate use every month after the index procedure up to month 6, after which the differences gradually became less pronounced and often non-significant. Significantly higher opiate use among surgical patients persisted longer for proximal humerus (out to month 11) than for distal radius fracture patients. A proportion of patients in both the operative and non-operative cohorts remained on opioids after 12 months, ranging from a high of 6.0% in the operatively treated proximal humerus group to a low of 3.8% in the non-operative distal radius group.
Regional variation in opiate use
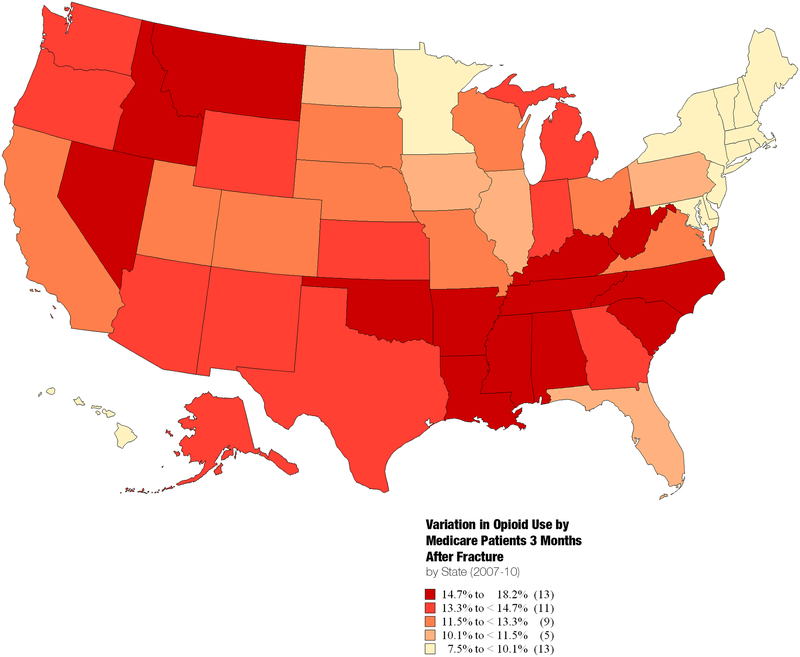
There was significant regional variation in opiate use after fracture among states (Figure 3). There was 2.4-fold variation in opiate use, ranging from a high of 18.2% (Oklahoma) to a low of 7.5% (Connecticut), with clustering of the highest-use states in the Southeast and Northwest regions.
Figure 3:
State-level variation in percentage of Medicare beneficiaries with fragility fractures filling prescription opioids in 3rd month after fracture
Adjusted associations with opiate use according to type of fracture
Adjusted associations with opiate use according to type of fracture are shown in Table 2. The multivariable model adjusted for age, sex, race, Charlson co-morbidity index, month post-fracture, and treatment type (surgical vs. non-surgical). In all fracture types, increasing age, female sex, and increasing number of Charlson Comorbidity Indices were significantly association with opioid use in the year following fracture.
Table 2:
Adjusted associations with opioid use according to type of fracture
| Proximal Humerus | Distal Radius | Hip | ||||
|---|---|---|---|---|---|---|
| OR | p-value | OR | p-value | OR | p-value | |
| Age | ||||||
| 66–74 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 75–84 | 0.75 | <0.01 | 0.80 | <0.01 | 0.69 | <0.01 |
| >85 | 0.56 | <0.01 | 0.67 | <0.01 | 0.51 | <0.01 |
| Sex | ||||||
| Male | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Female | 1.21 | <0.01 | 1.21 | <0.01 | 1.14 | <0.01 |
| Race/Ethnicity | ||||||
| White | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Black | 1.17 | 0.01 | 1.20 | <0.01 | 1.01 | 0.72 |
| Asian | 0.72 | <0.01 | 0.75 | <0.01 | 0.75 | <0.01 |
| Hispanic | 0.94 | 0.16 | 1.10 | 0.00 | 1.04 | 0.06 |
| Other | 1.65 | <0.01 | 1.04 | 0.59 | 1.08 | 0.09 |
| Charlson Comorbidity Score | ||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | |||
| 1 | 1.17 | <0.01 | 1.17 | <0.01 | 1.02 | 0.13 |
| 2 | 1.29 | <0.01 | 1.31 | <0.01 | 1.15 | <0.01 |
| 3 | 1.41 | <0.01 | 1.41 | <0.01 | 1.10 | <0.01 |
| 4 | 1.61 | <0.01 | 1.68 | <0.01 | 1.29 | <0.01 |
| Surgery | ||||||
| Non-operative | 1 (reference) | 1 (reference) | N/A | N/A | ||
| Operative | 1.15 | <0.01 | 4.50 | <0.01 | ||
Discussion
Our study has three primary findings. First, between 3.8–6.4% of opiate-naïve US Medicare beneficiaries who sustain fragility fractures of the hip, distal radius or proximal humerus continued to fill opioid prescriptions one year after their index fracture. While the percentage varies with the fracture type, all three fracture cohorts had some proportion of their initial opiate-naïve cohort still receiving opioids at one year. Second, surgical treatment of distal radius and proximal humerus fractures resulted in a statistically significant increase in the proportion of patients using opioids in the first six months after index fracture than non-surgically managed patients; this effect became less pronounced over the ensuing six months. Finally, there was significant regional variation at the state level in prescription opiate exposure following fragility fractures of the hip, distal radius and proximal humerus. While we cannot comment on the “correct” rate of opiate use following these fractures, a 2.5-fold variation suggests potential regional over- and under-prescribing patterns.
This paper contributes to the growing literature on opiate prescriptions for acute conditions that lead to long-term use. We examined persistence of opiate prescribing after non-vertebral fragility fractures in opiate-naïve elderly patients. Our study methodology and results are perhaps most consistent with those of Clarke et al,30 who found that 3.1% of their cohort of 39,140 opioid-naïve Canadian patients age 66 or older undergoing major elective surgery continued to receive opioids for more than 90 days after surgery. While this is lower than the rate at 3 months across all fracture types in our study, the authors did not specify how far beyond 90 days their follow-up continued. Important differences in the comorbidities of the patient populations and countries between the two studies also need to be understood. Despite the inherent difficulties of comparing studies in a fledgling field, our study is consistent with the idea that contact with the health system, whether represented by hospitalization, acute trauma, or surgery, is associated with long-term opioid exposure.
In our study, US Medicare beneficiaries who underwent surgical fixation of proximal humerus and distal radius fractures had significantly more prescription opiate use in the first six months after the index fracture than those who were treated non-operatively, although this difference became less pronounced in the ensuing six months. The persistent discrepancy seen after fracture between operative and non-operative patients is unlikely to be explained by acute postoperative pain alone. The difference may be the result of clinician prescribing behavior or increased frequency of interaction with the health care system after surgery, leading to more opportunities for opiate prescription renewals. However, these differences seem to be mitigated with the passage of time. Indeed, at one year there is minimal difference in the rate of opiate prescriptions between operatively and non-operatively treated patients. This provides an important new insight, as previous studies have not compared operatively to non-operatively treated patients with the same injury and have not examined rates of opiate prescriptions for up to one year after an index event.
While the geographic variation in hospital care, surgical intervention and medical costs has been well-documented31–33, relatively little is known about the variation in prescription opiate use in the United States, and even less is known about such variation after fracture. Studies have examined variation in prescription opiate use at the state level among Medicaid patients34, at the hospital level among Medicare patients28, and at the Hospital Referral Region (HRR) level among disabled Medicare beneficiaries.35 All studies found significant variation among prescribing patterns. Our study corroborates these findings. Our results must be interpreted with caution, however. The “correct” rate for opioid use after fractures in this population is unknown. Specifically, it is unknown whether the opioid prescribing was appropriate or inappropriate given that the experience of pain varies at the individual level36,37, according to demographic factors such as age38, sex39–41, and race42, and with co-morbidities such as anxiety and depression.43 Additionally, the influence of larger systemic factors such as hospital characteristics, culture and incentive measures related to the quality of inpatient pain management are difficult to measure and control.44–46 Nonetheless, the patients with fragility fractures, especially hip fractures, can be viewed as a population that is similarly ill across geographic regions.33,47 Moreover, there is documented variation in opioid prescribing between the US and other countries after fracture.48 In aggregate, these studies imply that variation in opioid prescribing is being driven by something in addition to differences in patient factors.
The strengths of our study include its large sample size and wide geographic representation of the study cohort. Additionally, we benefit from the comprehensive drug information available through Medicare Part D data. We acknowledge several limitations. First, our data do not permit a comparison of our cohort with opiate-naïve Medicare patients who did not sustain fragility fractures. Therefore, we are unable to ascertain whether the rate of opiate consumption is lower, higher, or similar among this comparison cohort. It is possible that patients in this age group have a baseline “risk” of opiate exposure given their comorbidities. Second, our follow-up is limited to one year. While this is a longer follow-up than seen in other studies examining long-term opioid use, analysis of opiate use beyond 1 year from index fracture may reveal trends that are not evident with our current follow-up time frame. Third, our cohort study design does not permit a meaningful description of possible predictive characteristics of the patients still receiving opiates at 12 months after their fracture. Presumably, the underlying reasons are a complex blend of physiological, psychological and social forces that we are not adequately able to capture with our data set. Similarly, we were not able to explore the characteristics of the providers prescribing the opiates. Fourth, by requiring patients in our cohort to be community dwellers for at least 30 days in the first 4 months after their index fracture, we necessarily excluded the chronically institutionalized from our analysis, biasing our cohort to a healthier population. Our data only permitted an analysis of filled opioid prescriptions as a proxy for the rate of opioid use among the cohort. This is well-accepted in the pharmacoepidemiology literature49–51 but does not permit an understanding of the exact rate of consumption or diversion of the prescriptions. Moreover, the limitations of our data precluded quantification of how many patients underwent post-acute care as well as their post-fracture treatment site (hospital or community). Finally, our data precludes an analysis of intensity of use as measured by the daily morphine equivalent dose (MED).
Conclusions/Relevance
A proportion of opiate-naïve US Medicare beneficiaries who sustain fragility fractures of the hip, proximal humerus or distal radius will remain exposed to opiates up to one year after the index fracture. Additionally, surgery for fractures of the distal radius and proximal humerus in these patients leads to significant increases in opiate prescriptions in the six months following surgery. Finally, there is significant variation in opiate prescribing at the state level after fragility fractures of the hip, proximal humerus and distal radius. This study adds to the small but growing literature showing an increased risk of long-term prescription opiate exposure after surgery, hospitalization, and acute injury. The results of this study suggest that targeted efforts by healthcare organizations to determine the mechanisms by which patients obtain opioids after acute medical events can result in actionable data to reduce the risks associated with excess opioid consumption. On a larger level, the aggregate of our data suggests the opportunity for targeted quality improvement to reduce the documented variation in opioid use after common geriatric fractures.
Acknowledgments
Funding/conflict of interest statements
The Multidisciplinary Clinical Research Center in Musculoskeletal Diseases is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P60-AR048094 and P60-AR062799), Anna N.A. Tosteson, ScD, PI.
Source of Funding: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P60AR062799). The funder played no role in the investigation or writing of the manuscript.
Footnotes
There are no conflicts of interest to declare.
References
- 1.Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the US Medicare population. Journal of clinical epidemiology. 1999;52(3):243–249. [DOI] [PubMed] [Google Scholar]
- 2.Clement N, Duckworth A, Aitken S, Biant L, McQueen M. The spectrum of fractures in the elderly. Bone Joint J. 2014;96(3):366–372. [DOI] [PubMed] [Google Scholar]
- 3.Sanders KM, Nicholson GC, Watts JJ, et al. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38(5):694–700. [DOI] [PubMed] [Google Scholar]
- 4.Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King AB, Tosteson AN, Wong JB, Solomon DH, Burge RT, Dawson-Hughes B. Interstate variation in the burden of fragility fractures. J Bone Miner Res. 2009;24(4):681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaioannou A, Kennedy CC, Ioannidis G, et al. The osteoporosis care gap in men with fragility fractures: the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2008;19(4):581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McQueen MM. Global forum: fractures in the elderly. JBJS. 2016;98(9):e36. [DOI] [PubMed] [Google Scholar]
- 8.Omsland TK, Magnus JH. Forecasting the burden of future postmenopausal hip fractures. Osteoporos Int. 2014;25(10):2493–2496. [DOI] [PubMed] [Google Scholar]
- 9.General OotS. Bone health and osteoporosis: a report of the Surgeon General. 2004. [PubMed]
- 10.Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):24–35. [DOI] [PubMed] [Google Scholar]
- 11.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 12.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6(2):99–105. [DOI] [PubMed] [Google Scholar]
- 13.Cooper C The crippling consequences of fractures and their impact on quality of life. The American journal of medicine. 1997;103(2):S12–S19. [DOI] [PubMed] [Google Scholar]
- 14.Munson JC, Bynum JP, Bell J-E, et al. Patterns of prescription drug use before and after fragility fracture. JAMA internal medicine. 2016;176(10):1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303–311. [DOI] [PubMed] [Google Scholar]
- 16.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(1):M76–M81. [DOI] [PubMed] [Google Scholar]
- 17.Ricardo Buenaventura M, Rajive Adlaka M, Nalini Sehgal M. Opioid complications and side effects. Pain physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 18.Dawson-Hughes B, National Osteoporosis Foundation Guide C. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008;93(7):2463–2465. [DOI] [PubMed] [Google Scholar]
- 19.Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. Journal of general internal medicine. 2010;25(4):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldini A, Von Korff M, Lin EH. A Review of Potential Adverse Effects of Long-Term Opioid Therapy: A Practitioner’s Guide. Prim Care Companion CNS Disord. 2012;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain physician. 2008;11(2 Suppl):S63–S88. [PubMed] [Google Scholar]
- 22.Hauser W, Petzke F, Radbruch L, Tolle TR. The opioid epidemic and the long-term opioid therapy for chronic noncancer pain revisited: a transatlantic perspective. Pain Manag. 2016;6(3):249–263. [DOI] [PubMed] [Google Scholar]
- 23.Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of OxyContin® and other opioid analgesics in the United States: 2002–2004. The Journal of Pain. 2005;6(10):662–672. [DOI] [PubMed] [Google Scholar]
- 24.Cone EJ, Fant RV, Rohay JM, et al. Oxycodone involvement in drug abuse deaths: a DAWN-based classification scheme applied to an oxycodone postmortem database containing over 1000 cases. J Anal Toxicol. 2003;27(2):57–67; discussion 67. [DOI] [PubMed] [Google Scholar]
- 25.Abuse S, Health UDo, Services H. Treatment episode data set (TEDS) highlights—2007. National admissions to substance abuse treatment services DASIS Series: S-45, DHHS Publication No.(SMA) 09–4360: Substance Abuse and Mental Health Services Administration; Rockville, MD; 2009. [Google Scholar]
- 26.Okie S A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981–1985. [DOI] [PubMed] [Google Scholar]
- 27.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. [DOI] [PubMed] [Google Scholar]
- 28.Jena AB, Goldman D, Karaca-Mandic P. Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med. 2016;176(7):990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bynum JP, Bell J-E, Cantu RV, et al. Second fractures among older adults in the year following hip, shoulder, or wrist fracture. Osteoporosis International. 2016;27(7):2207–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. Bmj. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wennberg JE, Gittelsohn AM. Small area variations in health care delivery. 1973. [DOI] [PubMed]
- 32.Birkmeyer JD, Sharp SM, Finlayson SR, Fisher ES, Wennberg JE. Variation profiles of common surgical procedures. Surgery. 1998;124(5):917–923. [PubMed] [Google Scholar]
- 33.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287. [DOI] [PubMed] [Google Scholar]
- 34.Zerzan JT, Morden NE, Soumerai S, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Medical care. 2006;44(11):1005–1010. [DOI] [PubMed] [Google Scholar]
- 35.Morden NE, Munson JC, Colla CH, et al. Prescription Opioid Use among Disabled Medicare Beneficiaries: Intensity, Trends and Regional Variation. Medical care. 2014;52(9):852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fillingim RB. Individual differences in pain responses. Curr Rheumatol Rep. 2005;7(5):342–347. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RR. Genetic predictors of acute and chronic pain. Curr Rheumatol Rep. 2006;8(6):411–417. [DOI] [PubMed] [Google Scholar]
- 38.Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20(4):227–239. [DOI] [PubMed] [Google Scholar]
- 39.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8(5):413–425. [DOI] [PubMed] [Google Scholar]
- 40.Wiesenfeld-Hallin Z Sex differences in pain perception. Gend Med. 2005;2(3):137–145. [DOI] [PubMed] [Google Scholar]
- 41.Vallerand AH, Polomano RC. The relationship of gender to pain. Pain Manag Nurs. 2000;1(3 Suppl 1):8–15. [DOI] [PubMed] [Google Scholar]
- 42.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–294. [DOI] [PubMed] [Google Scholar]
- 43.Meredith P, Strong J, Feeney JA. Adult attachment, anxiety, and pain self-efficacy as predictors of pain intensity and disability. Pain. 2006;123(1):146–154. [DOI] [PubMed] [Google Scholar]
- 44.Frasco PE, Sprung J, Trentman TL. The impact of the joint commission for accreditation of healthcare organizations pain initiative on perioperative opiate consumption and recovery room length of stay. Anesth Analg. 2005;100(1):162–168. [DOI] [PubMed] [Google Scholar]
- 45.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70–78. [DOI] [PubMed] [Google Scholar]
- 46.Gupta A, Daigle S, Mojica J, Hurley RW. Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298. [DOI] [PubMed] [Google Scholar]
- 48.Lindenhovious AL, Helmerhorts GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and The Netherlands. Journal of Trauma and Acute Care Surgery. 2009;67(1):160–164. [DOI] [PubMed] [Google Scholar]
- 49.Fredheim OMS, Skurtveit S, Breivik H, Borchgrevink PC. Increasing use of opioids from 2004 to 2007–pharmacoepidemiological data from a complete national prescription database in Norway. European Journal of Pain. 2010;14(3):289–294. [DOI] [PubMed] [Google Scholar]
- 50.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ. 2014;348:g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]