Abstract
The objective of this study was to use qualitative methodology to tailor and refine an existing smoking cessation intervention for the population of people who use cigarettes and are diagnosed with schizophrenia, schizoaffective, or psychotic disorder. Successive cohort design methodology was used to iteratively modify the treatment in response to qualitative participant, therapist, and consultant feedback on the intervention. Qualitative methodology for participant feedback included analysis of semi-structured interviews with participants, visualization of app utilization data, and stakeholder feedback from study therapists and consultants. Using the successive cohort design, a tailored multi-component mobile health smoking cessation intervention was developed. The intervention included mobile contingency management (i.e., financial compensation for confirmed abstinence from smoking), pharmacotherapy for smoking cessation, cognitive-behavioral counseling sessions, and the StayQuit app for relapse prevention. Two cohorts (N = 13) were completed in the study; after each cohort, the treatment protocol was revised. The intervention is described, as well as the qualitative findings from each cohort and subsequent changes made to the intervention based upon patient and provider feedback. Metrics of patient engagement included treatment adherence (40% in Cohort 1 and 63% in Cohort 2). Both participants and therapists reported that the intervention was helpful. Over one third of participants self-reported abstinence at post-treatment. Since qualitative methodology is often underutilized in mental health treatment development, this study demonstrates the utility of the successive cohort design for treatment development of behavior change interventions for at-risk, vulnerable populations.
Keywords: schizophrenia, schizoaffective, psychotic disorders, cigarette smoking, cessation
Introduction
Among substance use disorders, nicotine dependence causes the most deaths per capita in the U.S., with tobacco-related mortality falling at over 480,000 annually (U.S. Department of Health and Human Services, 2014). However, tobacco use and its negative health consequences do not equally affect all segments of the population. Compared to the general population, individuals with schizophrenia are four times more likely to smoke, and subsequently suffer relatively greater adverse health consequences (de Leon & Diaz, 2005). The rate of cigarette smoking among people with schizophrenia ranges from 62–85% (Chapman, Ragg, & McGeechan, 2009; Ziedonis et al., 2008). Additionally, 29–46% of smokers with schizophrenia smoke heavily (> 20 cigarettes per day), which is far higher than the rate of heavy smoking among the general population of smokers (de Leon & Diaz, 2005). This elevated smoking rate is a potential cause of the observed increased risk among people with schizophrenia for cardiovascular disease, diabetes, and mortality (Hennekens, Hennekens, Hollar, & Casey, 2005; Olfson, Gerhard, Huang, Crystal, & Stroup, 2015).
Although smoking cessation benefits people with schizophrenia, long-term cessation is generally low among this population. Cessation results in a 90% reduction in 10-year risk of a cardiovascular event for smokers with schizophrenia (Bobes, Arango, Garcia-Garcia, & Rejas, 2010). In addition to the potential health benefits of cessation, there is empirical evidence that people with schizophrenia attempt to quit smoking at comparable rates to the general population (McClave, McKnight-Eily, Davis, & Dube, 2010). However, compared to the general population, smokers with schizophrenia are less likely to successfully stop smoking (de Leon & Diaz, 2005; McClave et al., 2010). The factors underlying the association between schizophrenia and continued smoking involve social, psychological, genetic, and neurobiological vulnerabilities (Aubin, Rollema, Svensson, & Winterer, 2012; Hu et al., 2018). Thus, there is a need to develop smoking cessation interventions for smokers with schizophrenia that optimize quit rates, reduce clinical/therapist burden, and increase reach and cost-effectiveness of evidence-based smoking cessation practices.
Although clinical trials have demonstrated the efficacy of various forms of pharmacotherapy for smoking cessation among people with schizophrenia, current approaches to smoking cessation among people with schizophrenia still yield low long-term abstinence rates (Cather, Pachas, Cieslak, & Evins, 2017). Current meta-analytic data indicate that for randomized control trials (RCT’s) of bupropion or bupropion plus nicotine replacement therapy (NRT), the odds of tobacco abstinence at 6-month are less than one in five participants (Tsoi, Porwal, & Webster, 2013). When examining longer-term outcomes (e.g., 12-months) among highly efficacious bupropion plus NRT, then abstinence rates are as low as 12% (Evins et al., 2007). Most trials involving pharmacotherapy also provide some form of behavioral counseling. However, there are few clinical trials to date that have combined pharmacotherapy with highly efficacious forms of behavioral treatment that go beyond standard-care counseling.
Contingency management (CM) is a form of intensive behavioral treatment that is understudied in the context of smoking cessation for people with schizophrenia. CM is an addiction treatment that involves providing monetary/prize incentives to individuals contingent on abstinence verification (Petry, 2011). Two previous trials examining CM for smokers with schizophrenia showed promise for short-term outcomes. One RCT compared CM with concurrent NRT, CM without NRT, and a minimal intervention (Gallagher, Penn, Schindler, & Layne, 2007). Follow-up quit rates based on exhaled carbon monoxide (CO) in the CM + NRT group (43%) were higher than in the CM-only group (37%) and the minimal intervention (8%) (Gallagher et al., 2007). However, cotinine-verified abstinence was much lower in all three groups (CM-only 7%, CM + NRT 2%, control 5%). Given that participants attended CM sessions less than weekly and verified abstinence via CO, the bioverification procedures would have only detected a short window (8 hours or less) of smoking. Considered with the cotinine-verified abstinence rates, results may suggest that participants smoked between CM sessions, resulting in reinforcement despite continued smoking. Another RCT demonstrated that among smokers with schizophrenia, CM reduced cotinine and cigarettes per day compared to NRT plus bupropion (Tidey, Rohsenow, Kaplan, Swift, & Reid, 2011). However, this RCT tested reductions in tobacco exposure rather than examining group differences in short- and long-term smoking abstinence. Several other studies examining CM interventions in various populations have found no difference between intervention and controls when examining long-term abstinence rates once the contingencies have been removed (Higgins, Davis, & Kurti, 2017).
Although there are concerns about the efficacy of CM once incentives are removed, it can help retain difficult-to-treat populations and assist them in achieving initial abstinence. Further, there is evidence that CM and associated reductions in smoking can lead to increased self-efficacy (Romanowich, Mintz, & Lamb, 2009). In this context, an ideal strategy would be to integrate CM with other evidence-based smoking cessation interventions, such as cognitive behavioral therapy (CBT) and smoking cessation pharmacotherapy. In conjunction with other treatment, extrinsic motivators can provide the initial stimulus for change and give individuals time to learn and practice long-term life skills for abstinence (Donatelle et al., 2004; Kollins, McClernon, & Van Voorhees, 2010; Petry, 2010).
CM research to date has not focused on the treatment development stage to ensure that the intervention is adequately adapted to populations with psychotic disorders. It has been increasingly suggested that both mental health and mobile health research should incorporate aspects of qualitative methodology during treatment development (Peters, 2010; Yardley, Morrison, Bradbury, & Muller, 2015). The use of qualitative methodology in this context can be used to iteratively modify the intervention in order to optimize the intervention from the patient’s perspective (Yardley et al., 2015). It is suggested that use of qualitative methodology may be especially helpful prior to testing the efficacy or effectiveness of an intervention in an RCT because qualitative data can help inform the design and delivery of the intervention (Peters, 2010).
The goal of the current treatment development study was to tailor and refine a smoking cessation intervention for the population of smokers diagnosed with schizophrenia, schizoaffective, or psychotic disorder. A successive cohort design was utilized, which enabled iterative improvements in the intervention design based on qualitative data (Epstein et al., 2007). Qualitative research involves an inductive, data-focused approach to analysis that may preclude hypothesis testing. As such, the original intervention is described, as well as iterative improvements to the intervention to maximize its efficacy.
Method
Design
The successive cohort design is an iterative process designed to use qualitative and quantitative data to systematically refine and modify behavioral treatments in the early stage of development (Epstein et al., 2007). It involves several steps: 1) identify a promising treatment based on current theoretical models; 2) identify relevant treatment elements; 3) develop initial treatment manuals, supporting materials, measures and procedures; and 4) conduct iterative revisions based on qualitative and quantitative data collected while providing the intervention to successive cohorts of participants (Epstein et al., 2007). In the current study, we have included two cohorts of participants. There is also a third cohort (not included in this study) that entails a currently ongoing pilot randomized controlled trial (ClinicalTrials.gov Identifier: NCT02420015).
Participants
Participants were recruited from several settings from 2015–2017 in a Southeastern metropolitan area, including outpatient psychiatric clinics, local clubhouses that provide recovery-oriented community and assistance for people with serious mental illness, Craigslist advertisements, and flyer advertisements. Inclusion criteria included: currently smoke at least ten cigarettes a day; smoking for at least one year; fluent speaking/writing in conversational English; between 18 and 70 years of age; willing to make a smoking cessation attempt; and met criteria for schizophrenia, schizoaffective disorder or another psychotic disorder as determined by the Structured Clinical Interview for DSM-5 Diagnosis (SCID-5; (First, Williams, Karg, & Spitzer, 2015). Participants were excluded from the study if they: had a history of myocardial infarction in the past 6 months; had a contraindication to NRT with no medical clearance from a primary care provider or the study physician; used other forms of nicotine such as cigars, pipes, or chewing tobacco with an unwillingness to stop other forms of nicotine; were pregnant; met criteria for current manic episode, as determined by the SCID-5; were currently enrolled in another smoking cessation trial; or were currently imprisoned or in psychiatric hospitalization. Twenty-one individuals were consented and completed screening procedures, resulting in 4 screen-outs due to not meeting criteria for a psychotic disorder. A total of 17 participants met criteria and were enrolled in two successive cohorts.
In Cohort 1, 7 participants were enrolled, and 2 participants withdrew (reasons for withdrawal were study was too much work, and moved out of the area). Ten participants were enrolled in Cohort 2, and 2 participants withdrew (reasons for withdrawal included not enough support for participants’ needs, and equipment was too difficult to use). This left 5 participants in Cohort 1 and 8 participants in Cohort 2. Two participants were lost to follow-up prior to post-treatment (one participant in Cohort 1 and one participant in Cohort 2). One other participant in Cohort 2 was lost to contact during the treatment phase, but re-initiated contact after the treatment phase, which allowed for post-treatment data to be collected. Data are included for patients lost to follow-up, but not those who requested to be withdrawn from the study.
Preliminary Intervention
The preliminary intervention design was based upon current empirical research, theoretical models, and preliminary data. Multi-Component Mobile-enhanced Treatment for Smoking Cessation (iCOMMIT) is a smoking cessation treatment that combines mobile technology with behavioral, cognitive-behavioral, and pharmacologic approaches shown to improve smoking cessation outcomes. The components of the intervention are described below.
mCM Smartphone Application.
iCOMMIT participants received monetary compensation daily based on reduced CO readings. The CM smartphone-based application (mCM) allowed participants to generate a side-profile video recording of themselves blowing into a small CO monitor. Using the application interface, they could log into a secure website, upload video recordings confirming abstinence, and receive compensation information. The criterion for abstinence was CO ≤ 6 parts per million (ppm). The mCM application automatically calculated compensation earned by each participant and displayed this information. Monetary payment was provided at the end of the treatment and monitoring phase (6 weeks). See Table 1 for the schedule of incentives. iCOMMIT participants received training in use of the smartphone and CO monitor. Smartphones were provided to participants (Apple iPhone or a Droid MAXX 2). The CO breath monitor was a hand-held battery-operated instrument that provided an LED reading of CO levels. For each video recording, participants were asked to 1) begin a recording using the iPhone or a Droid MAXX 2; 2) show the initial zero CO reading; 3) video record their face while holding their breath during the monitor’s countdown; 4) blow slowly into the CO monitor while on camera; 5) show the final CO reading to the camera; and 6) submit the video to the study’s secure server for review by study staff. Participants received mCM training in the baseline session and practiced for one week prior to their quit date. They were instructed to take 2 readings per 24-hour period, with at least 8 hours between each reading.
Table 1.
Reinforcement Schedule for mCM Monitoring and Abstinence
| Original Protocol |
Revised Protocol |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Week | Day | 1st CO | 2nd CO | Bonus | Weekly Totals |
1st CO | 2nd CO | Bonus | Weekly Totals |
| 3 | 1–7 | $1.00 | $1.00 |
Up to $14.00 |
$1.00 | -- | Up to $7 | ||
| 4 | 1 | $1.00 | $1.25 | $5.00 | $5.00 | $5.00 | |||
| 2 | $1.50 | $1.75 | $5.00 | $5.00 | $5.00 | ||||
| 3 | $2.00 | $2.25 | $5.00 | $5.00 | $5.00 | ||||
| 4 | $2.50 | $2.75 | $2.50 | $2.60 | |||||
| 5 | $3.00 | $3.25 | $5.00 | $2.70 | $2.80 | $5.00 | |||
| 6 | $3.50 | $3.75 | $2.90 | $3.00 | |||||
| 7 | $4.00 | $4.25 |
Up to $41.75 |
$3.10 | $3.20 |
Up to $72.80 |
|||
| 5 | 1 | $4.50 | $4.75 | $3.30 | $3.40 | ||||
| 2 | $5.00 | $5.25 | $3.50 | $3.60 | |||||
| 3 | $5.50 | $5.75 | $5.00 | $3.70 | $3.80 | $5.00 | |||
| 4 | $6.00 | $6.25 | $3.90 | $4.00 | |||||
| 5 | $6.50 | $6.75 | $4.10 | $4.20 | |||||
| 6 | $7.00 | $7.25 | $4.30 | $4.40 | |||||
| 7 | $7.50 | $7.75 |
Up to $90.75 |
$4.50 | $4.60 |
Up to $60.30 |
|||
| 6 | 1 | $8.00 | $8.25 | $5.00 | $4.70 | $4.80 | $5.00 | ||
| 2 | $8.50 | $8.75 | $4.90 | $5.00 | |||||
| 3 | $9.00 | $9.25 | $5.10 | $5.20 | |||||
| 4 | $9.50 | $9.75 | $5.30 | $5.40 | |||||
| 5 | $10.00 | $10.25 | $5.50 | $5.60 | |||||
| 6 | $10.50 | $10.75 | $5.00 | $5.70 | $5.80 | $5.00 | |||
| 7 | $11.00 | $11.25 |
Up to $144.75 |
$5.90 | $6.00 |
Up to $84.90 |
|||
| 7 | 1 | $11.50 | $11.75 | $6.10 | $6.20 | ||||
| 2 | $12.00 | $12.25 | $6.30 | $6.40 | |||||
| 3 | $12.50 | $12.75 | $6.50 | $6.60 | |||||
| 4 | $13.00 | $13.25 | $5.00 | $6.70 | $6.80 | $5.00 | |||
| 5 | $13.50 | $13.75 | $6.90 | $7.00 | |||||
| 6 | $14.00 | $14.25 | $7.10 | $7.20 | |||||
| 7 | $14.50 | $14.75 |
Up to $188.75 |
$7.30 | $7.40 |
Up to $99.50 |
|||
| 8–9 | 1–7 | $25.00 |
Up to $25.00 |
Up to $25.00 |
|||||
| TOTAL POSSIBLE mCM PAYMENT |
Up to $530.00 |
Up to $388.50 |
|||||||
Video recordings were uploaded to a secure server. The site was accessible via 512-bit SHA-2 hashed passwords. Study coordinators monitored validity and adherence on a daily basis and offered feedback to ongoing participants regarding compensation. The study team used AES-256 encryption protocols to ensure all video uploads and participant data were transferred through encrypted network connections. The web application passed checks for vulnerabilities including SQL injection, Code Injection, XSS, and RFI vulnerabilities.
Stay Quit Coach Smartphone Application.
The Stay Quit Coach app was loaded onto each participant’s study smartphone. The purpose of the app was to provide a cost-effective method to maintain abstinence over time. The app includes brainstorming reasons for quitting, interactive tools to help cope with smoking urges and lapses, motivational messages, and support contacts. A complete description can be found at https://itunes.apple.com/us/app/stay-quit-coach/id655892317?mt=8. The content of the study CBT counseling sessions corresponded to the app content. During counseling sessions, counselors assisted participants in using the app.
Pharmacotherapy.
Research shows that bupropion is associated with increased quit rates compared to placebo for smokers with schizophrenia (Tsoi, Porwal, & Webster, 2010) (Tsoi et al., 2013). Thus, all participants (who assented and for whom it was not contraindicated) were prescribed bupropion, which they started two weeks prior to their quit date – 150 mg daily for days 1–7 and then 150 mg twice daily for 6 months following the quit date. Contraindications for bupropion were assessed by the study physician.
Although there is little evidence that NRT alone improves long-term abstinence rates for smokers with schizophrenia (Tsoi et al., 2010), the study team included it as part of the multi-component treatment. NRT is recommended as a first line treatment in clinical practice guidelines (Fiore et al., 2008) and theoretically could minimize potential effects of nicotine withdrawal on neurocognitive functioning (Ziedonis et al., 2008). Smokers were offered transdermal nicotine patch and one rescue method (e.g., nicotine gum, lozenge, inhaler). If participants reported contraindications to NRT, study physician approval for NRT use was required prior to receiving NRT. Because the polycyclic aromatic hydrocarbons of tobacco smoke can affect the metabolism of some antipsychotic medications (Desai, Seabolt, & Jann, 2001), patients were closely monitored for increased medication side effects during the trial.
Cognitive Behavioral Counseling.
Clinical practice guidelines recommend the provision of smoking cessation counseling (Fiore et al., 2008). The study team developed a 5-session CBT smoking cessation therapist manual and a participant workbook. The content of the manual and workbook were adapted from the manual used in a large scale PTSD smoking cessation trial (McFall et al., 2010). Two therapists (one master’s-level counselor and one Ph.D.-level psychologist) provided the counseling across the two cohorts. Therapists attended a half-day training meeting, during which they were trained by a Ph.D.-level staff psychologist with specialized training in behavioral change psychology. Therapists also attended weekly group supervision meetings with the PI and treatment development team to review critical points in counseling sessions, discuss cases, and receive ongoing consultation.
Procedures
Cohort 1.
The first cohort completed the preliminary intervention and provided qualitative feedback during a semi-structured interview at a post-treatment visit. Field notes were taken by a master’s-level research staff member who conducted the interview. Then, responses were coded by their content area. Codes included a) intervention strengths, b) intervention weaknesses, and c) suggestions for improvement. These codes were also divided into sub-codes based on whether the participant’s feedback referred to the overall intervention or a specific intervention component. In addition to qualitative data, mCM app utilization, and participant outcomes (post-treatment self-reported abstinence and 3-month biochemically verified abstinence) were collected. App utilization was examined by graphing uploaded CO readings over the course of study participation for each participant. Informal qualitative data were also solicited from study therapists at weekly treatment team meetings. Detailed notes were retained from these meetings.
Cohort 2.
Following the completion of Cohort 1, revisions were iteratively made to the study protocol both before and during completion of a second cohort. All intervention refinements were approved by the Institutional Review Board of the medical center. The same data collection procedures from Cohort 1 were repeated for Cohort 2. Following the completion of Cohort 2, additional changes were made to the study protocol, and a finalized intervention package was generated.
Measures
Demographics.
Each participant was asked to report age, race, gender, education, and employment status.
Smoking.
The Fagerström Test of Nicotine Dependence (FTND; (Heatherton, Kozlowski, Frecker, & Fagerström, 1991) and number of cigarettes smoked were used to measure smoking behaviors.
Psychiatric Symptoms.
Psychiatric disorders were evaluated using the SCID-5 (First et al., 2015). Field trials of the SCID-5 indicate good test-retest reliability for diagnosis of schizophrenia (Regier et al., 2013). The Scale for the Assessment of Positive Symptoms (SAPS) was used to measure severity of positive symptomatology (Andreasen, Arndt, Miller, Flaum, & Nopoulos, 1995). The SAPS is a commonly used measure in schizophrenia research (Kumari, Malik, Florival, Manalai, & Sonje, 2017), and research of the performance of the SAPS shows its test-retest reliability (r = .49 over 24 months; Schuldberg, Quinlan, Morgenstern, & Glazer, 1990) and interrater reliability (ICC = 0.84; Norman, Malla, Cortese, & Diaz, 1996). The Clinical Assessment Interview for Negative Symptoms (CAINS) was used to measure negative symptomatology (Kring, Gur, Blanchard, Horan, & Reise, 2013). The CAINS is also a commonly used measure in schizophrenia research (Kumari et al., 2017), and has good internal consistency (α = 0.76), convergent validity, and discriminate validity (Kring et al., 2013). Both the SAPS and CAINS were administered at baseline and at immediate post-treatment to assess for changes in symptom severity over the course of treatment.
Practice Adherence. Practice adherence for the mCM app was measured as the percentage of days during the practice period when the participant uploaded a CO reading.
Treatment Adherence. Treatment adherence for the mCM app was measured as the percentage of possible readings for which the participant uploaded a CO reading. Perfect adherence (100%) would be indicated if the participant uploaded two videos per day for the entire duration of the 4-week mCM treatment period. Treatment adherence for counseling sessions was defined dichotomously as participating in at least 3 sessions (i.e., through the quit date).
Self-Reported Abstinence. At the post-treatment visit and 3-month follow-up, participants completed a timeline followback for tobacco use over the past 30 days (Sobell & Sobell, 1992). Self-reported abstinence was indicated by 7-day point prevalence abstinence (i.e., no cigarettes smoking within the past 7 days). Self-reported abstinence at 3-months post-treatment was biochemically verified by salivary cotinine with a cutpoint of ≤10 ng/ml indicating abstinence.
Quantitative Analysis
Quantitative analyses aimed at describing the data to aid with qualitative analysis. Descriptive statistics were generated for sample characteristics. Additionally, paired t-tests were used to examine change in positive and negative symptoms from baseline to post-treatment.
Qualitative Analysis
The goal of qualitative analysis was to identify elements for improvement within the intervention. Qualitative analysis was completed at the end of Cohorts 1 and 2. At the conclusion of each cohort, content analysis was used to identify high-frequency codes from the participant interviews. Participant outcomes and app utilization were also qualitatively analyzed using inductive thematic analysis (Braun & Clarke, 2006). This analysis focused on using participant data to identify characteristics of treatment utilization and smoking abstinence in the sample. In order to rapidly analyze data and make appropriate revisions to the intervention, the study team (PI, research assistants, study therapists, and study consultants) held weekly meetings to review participant data, examined emerging relevant empirical literature, and conduct ongoing discussion of potential intervention revisions. These weekly meetings were also used as an opportunity for the study therapists to provide qualitative feedback to the study team for intervention refinement. Power analysis is not possible with qualitative data collection. However, prior empirical work suggests that 97% of themes from a total of 60 interviews are generated after just the first 12 interviews (Guest, Bunce, & Johnson, 2006). Thus, it is likely that a sample of at least 12 participants will yield sufficient themes to interpret the data.
Results
Sample Characteristics
Baseline smoking characteristics and treatment outcomes are described for each participant in Table 2. There were a total of 5 participants in Cohort 1 and 8 participants in Cohort 2. Participants overall ranged in age from 26 to 63 (M = 47.8, SD = 11.0), and smoked on average 15.0 cigarettes per day at baseline (SD = 6.6). Scores on the FTND ranged from 2 (very low) to 8 (very high), with a mean score of 4.9 (SD = 1.8). Number of years smoked ranged from 7 years to 50 years (M = 28.2, SD = 13.7). Participants included 8 men and 5 women. The majority of participants (n = 10) identified as Black or African American; one participant identified as White, and two participants identified as Multiracial. Participants had a range of educational levels, including less than high school (n = 5), high school or GED (n = 4), and college experience/degree (n = 4). Eight of the study participants were U.S. military veterans. Two participants were working (both worked part-time), one participant was a student, one participant was currently unemployed, and nine participants reported that they were receiving disability benefits.
Table 2.
Participant Outcomes
| Participant | Age | Baseline Cigarettes per Day |
Number of Therapy Sessions |
mCM Practice Adherence (%) |
mCM Treatment Adherence (%) |
Prescribed NRT |
Prescribed Bupropion |
Lost to Follow-Up |
Post- Treatment Cigarettes per Day |
Post- Treatment Self- Reported Abstinence |
3-Month Biochemically Verified Abstinence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| COHORT 1 | |||||||||||
| 1 | 62 | 10 | 5 | 100% | 70% | Yes | No | No | 0 | Yes | Yes |
| 2 | 47 | 30 | 5 | 0% | 0% | Yes | Yes | No | 30 | No | No |
| 3 | 33 | 10 | 5 | 100% | 100% | No | Yes | No | 7 | No | No |
| 5 | 38 | 20 | 5 | 100% | 14% | Yes | Yes | Yes | Unknown | No* | No* |
| 7 | 63 | 20 | 5 | 43% | 2% | Yes | Yes | No | 0 | Yes | No |
| COHORT 2 | |||||||||||
| 1 | 48 | 10 | 5 | 100% | 71% | Yes | Yes | No | 2 | No | No |
| 2 | 46 | 20 | 4 | 43% | 2% | Yes | Yes | No | 2 | No | No |
| 3 | 55 | 10 | 1 | 0% | 0% | No | No | Yes | Unknown | No* | No* |
| 5 | 51 | 15 | 5 | 100% | 100% | Yes | No | No | 0 | Yes | Yes |
| 6 | 54 | 19 | 5 | 100% | 98% | Yes | No | No | 0 | Yes | No |
| 7 | 41 | 6 | 5 | 100% | 100% | Yes | No | No | 1 | No | No* |
| 9 | 57 | 10 | 5 | 100% | 98% | Yes | Yes | No | 0 | Yes | No* |
| 10 | 26 | 15 | 0 | 100% | 0% | No | No | Yes** | 20 | No | No* |
Coded as smoking due to missing data
Participant lost to follow-up during treatment phase, but reinitiated contact, which allowed for post-treatment data collection.
In terms of psychiatric diagnosis, 7 participants had a lifetime diagnosis of schizophrenia, 5 participants had a lifetime diagnosis of schizoaffective disorder, and 1 participant had a lifetime diagnosis of psychotic disorder, not otherwise specified. Among participants, 5 had psychotic symptoms that were in full or partial remission. The average score on the Motivation and Pleasure Scale of the CAINS was 11.6 (SD = 5.6), with scores ranging from 5 to 24. The mean score on the Expression Scale of the CAINS was 1.4 (SD = 1.5), with scores ranging from 0 to 4. The mean total score on the SAPS was 10.8 (SD = 9.8), with scores ranging from 0 to 34. Mean scores for SAPS subscales were as follows: Hallucinations (M = 4.9, SD = 6.3), Delusions (M = 3.7, SD = 4.6), Bizarre Behavior (M = 0.6, SD = 1.5), and Positive Formal Thought Disorder (M = 1.5, SD = 2.8). Regarding changes in positive and negative symptoms over time from baseline to post-treatment, there was a significant decrease over time in the CAINS Motivation and Pleasure Scale (11.6 at baseline vs. 7.1 at post-treatment; t [7] = 3.40, p = .01), which corresponds to an improvement in negative symptoms. There were no other significant increases or decreases within participants from baseline to post-treatment in their psychotic symptoms.
None of the participants reported any study-related adverse events (i.e., side effects from pharmacotherapy). Two study phones were lost due to lost contact with the participant.
Cohort 1 Findings
Among the 5 participants included in Cohort 1, two participants (40%) demonstrated high treatment adherence, as defined by engagement in both therapy sessions as well as mCM abstinence readings. At post-treatment, 2 participants (40%) reported abstinence, including one of the two participants with high treatment adherence. One participant who was a particularly heavy smoker (30 cigarettes per day) did not reduce their smoking at all. On average, participants in Cohort 1 were compensated $110.15 (out of $530 possible payment) for mCM.
In post-treatment qualitative interviews, all participants reported that the 5 counseling sessions were appropriate and helpful during their efforts to stop smoking. Most of the participants who were interviewed stated that the counselors and counseling sessions were the best part of the treatment package. Although participants generally noted the acceptability of the mCM app, some also identified barriers to using the mCM technology. Participant 7 had particularly low mCM treatment adherence, yet was able to achieve post-treatment abstinence. Participant 7 reported that s/he did not use the CO monitor because it was difficult to use a handheld CO monitor while also holding a cell phone to record his/her mCM abstinence readings. This difficulty resulted in Participant 7’s high adherence in the practice phase of mCM, followed by low adherence in the treatment phase (Table 2). Regarding the Stay Quit Coach app, self-reported app utilization for the app was low for this cohort. In qualitative interviews, most participants acknowledged that they did not use the app. One participant stated that the Stay Quit Coach app was difficult and frustrating to use. Only one of the five participants felt the Stay Quit Coach app was useful in their treatment.
Feedback was also elicited from study therapists. Therapists generally noted that the counseling sessions were appropriate in terms of content and timing. However, therapists specifically noted that participants often forgot their reasons for quitting during later therapy sessions. Therapists also noticed that participants were not using NRT rescue methods (lozenges or gum) daily for cravings. Furthermore, study therapists noted that completing a measure of medication adherence with each participant would be helpful for each therapy session. Therapists also shared that the Stay Quit Coach was difficult to incorporate into telephone sessions. Finally, therapists noted that participants had difficulty learning to use the mCM app. They also noted that it was important to give more leeway to clinicians who determine whether mCM abstinence readings were valid. In one specific case, a participant was noticeably exhaling lightly in order to continue smoking but still have readings indicating abstinence.
Cohort 1 Treatment Modifications
In response to Cohort 1 qualitative findings, changes were made to the contingency management app, pharmacotherapy treatment plan, and behavioral counseling plan (Table 3). First, given the modest post-treatment abstinence rate, there was a $100 bonus added for biochemically verified abstinence (CO ≤ 6) at 3-month follow-up. This practice was previously utilized to incentivize tobacco abstinence among people with schizophrenia (Gallagher et al., 2007). However, this is the first known treatment to date to use bonus incentives in conjunction with daily CM for smokers with schizophrenia. Additionally, since therapists had concerns regarding pharmacotherapy adherence, a messaging feature was added that enable therapists to send messages to participants. Messages consisted of feedback on mCM abstinence-confirmation videos, as well as encouraging messages about quitting (e.g., “Keep up the good work!”). A feature was also added to the mCM app that asked the participant daily about pharmacotherapy adherence. Given participant and therapist feedback that recording abstinence with the handheld CO monitor was unwieldy, mCM treatment equipment was switched to instead use the iCO™ Smokerlyzer®. This small device plugs directly into the cell phone, and the mCM app was reprogrammed to directly interface with the device. This enabled the participant to initiate a reading in the mCM app, and see their CO breath reading in the app as well. Additionally, procedures for mCM were altered to make clear to participants that their mCM readings had to include an audible exhalation that lasted for several seconds to prevent gaming. Other changes to the treatment included reviewing personalized reasons for quitting at every counseling session, as well as removing the Stay Quit Coach app from the treatment altogether.
Table 3.
Changes by Cohort
| Change Type | Change to Intervention | Reason for Change |
|---|---|---|
| COHORT 1 | ||
|
Contingency Management |
Added a $100 bonus for abstinence at 3 months after quitting |
Patient data and empirical evidence from other studies |
| Switched participants to use the iCO Smokerlyzer (plugs into the smartphone) |
Participant and therapist feedback | |
| Exhale for CO readings must be audible and last at least a few seconds |
Therapist feedback | |
| Messages within the mCM app provided feedback and encouragement to participants |
Therapist feedback | |
| Pharmacotherapy | Heavy smokers (30+ cigarettes per day) receive a 42mg nicotine patch |
Patient data and empirical evidence from other studies |
| Participants start using NRT on the quit date regardless of quit status |
Patient data and empirical evidence from other studies |
|
| Participants instructed to use 10 lozenges/ pieces of nicotine gum per day after quitting |
Participant data and therapist feedback |
|
| Participants answered daily prompts within the app measuring their use of pharmacotherapy |
Therapist feedback | |
| Psychotherapy | Review personalized reasons for quitting at every therapy session |
Therapist feedback |
|
Relapse Prevention |
Removed Stay Quit Coach smartphone app | Participant and therapist feedback |
| COHORT 2 | ||
|
Contingency Management |
Enhanced and extended practice video period | Therapist feedback |
| Increased reinforcement for smoking abstinence in the first 3 days of quitting |
Therapist feedback and patient data |
|
| If unable to achieve initial abstinence, participants encouraged to reset the quit day and restart CM |
Therapist feedback |
|
| Pharmacotherapy | Added varenicline for participants unable to stop within the first week of the quit date |
Patient data and empirical evidence from other studies [cite] |
| Psychotherapy | Sessions 1 and 3 held in-person sessions if preferred by participant |
Therapist feedback |
| New user-friendly therapist manual and accompanying patient workbook |
Consultant feedback | |
| Patients encouraged to repeat session 3 if they were unable to quit |
Participant data and consultant feedback |
|
Given the high rates of pharmacotherapy prescription, coupled with modest post-treatment abstinence, procedures for pharmacotherapy were also altered (Table 3). There is evidence for the efficacy of high-dose nicotine replacement therapy (42mg patch) for heavy smokers (Hughes, Cummings, & Hyland, 1999), and this practice has been shown to be safe for smoking cessation in the context of schizophrenia (J. M. Williams et al., 2007). Thus, the treatment protocol was altered to recommend 42mg nicotine patch to participants smoking 30 or more cigarettes per day. Additionally, participants were counseled to start using nicotine replacement therapy on their quit date even if they had not stopped smoking, which is a safe and effective practice (Fagerström & Hughes, 2002). Additionally, given low rates of rescue NRT use, the protocol was altered to provide counseling to participants that recommended using 10 lozenges or pieces of nicotine gum per day starting on the quit date (for those who were abstinent).
Cohort 2 Findings
Among the 8 participants included in Cohort 2, five participants (63%) demonstrated high treatment adherence for counseling sessions and mCM treatment monitoring (Table 2). At post-treatment, three participants (38%) reported abstinence. All three of the abstinent participants had high treatment adherence. One participant failed to reduce smoking at all; however, this participant was lost to contact during the treatment phase and did not complete any sessions or mCM treatment monitoring. On average, participants in Cohort 2 were compensated $212.96 for mCM (out of $530/$388 possible payment).
In post-treatment qualitative interviews, Cohort 2 participants noted that the telephone counseling and the mCM abstinence monitoring were the most helpful elements of the intervention. Some participants requested more assistance and support for efforts to reduce smoking prior to quitting, and additionally some requested more intensive counseling. Regarding mCM, one participant stated that they had difficulty remembering to initiate readings. App utilization for Cohort 2 indicated that while some participants’ utilization was consistently low, others fell off rapidly following the quit date, and some others had consistent utilization throughout (see Figure 1).
Figure 1. Example mCM App Utilization Graphical Data (Uploaded CO Readings over Time).
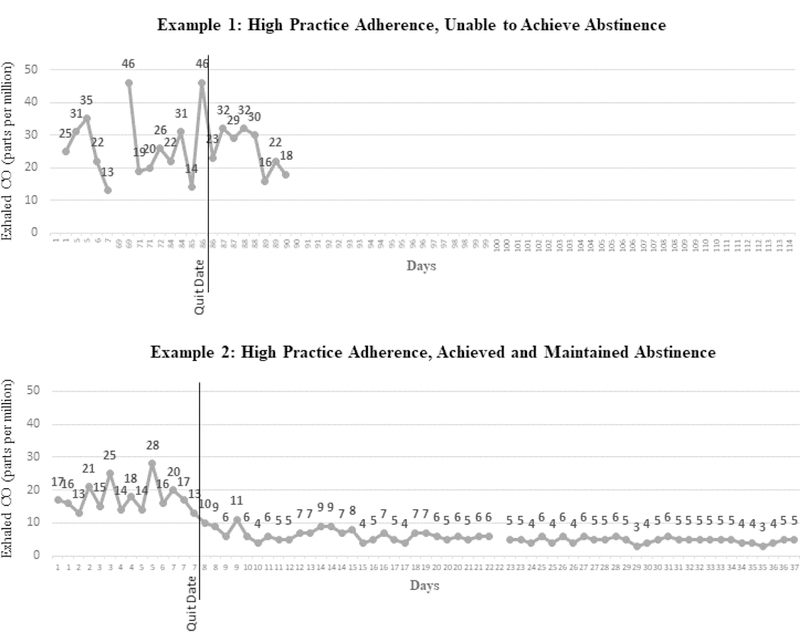
Figure 1. Example 1, Cohort 1 participant 5; Example 2, Cohort 2 participant 5. Exhaled carbon monoxide (CO) ≤ 6 ppm indicates abstinence.
Therapists also provided feedback for Cohort 2. They noted that the patient burden was high for mCM during the practice phase (prior to the quit date), since participants were asked to upload practice videos twice per day. Additionally, therapists noted that some patients forgot how to upload mCM monitoring videos at the beginning of the treatment phase; this occurred because there was sometimes a time gap between the end of the practice phase and the beginning of the treatment phase (i.e., quit date). Therapists also reported that many participants had difficulty achieving initial abstinence and also maintaining abstinence, even in the context of communication with the therapist and high motivation to quit. This was also observed in the patient data, which indicated that 40% of participants who had high treatment adherence were not fully abstinent at immediate post-treatment. Therapists also reported that some of the counseling sessions were difficult to administer over the telephone; they suggested that offering some in-person sessions (especially to establish rapport and to provide support around the quit date) might improve the effectiveness of counseling.
Given that therapists reported numerous patients had difficulty achieving initial abstinence, the study team sought additional input from a consultant who specialized in treating patients with SMI. The consultant made several suggestions for treatment modification. The first suggestion was to simplify all materials included in the participant workbook. For example, rather than asking participants to generate triggers and write them down, instead ask participants to check off their triggers from a list. Secondly, it was suggested that Session 3 (quit date) be repeated for participants who were not able to quit on their designated quit date.
Cohort 2 Treatment Modifications
Given findings from Cohort 2, several final changes were made to the treatment protocol (Table 3). First, during the mCM practice phase, participants were asked to only upload one video per day to reduce patient burden. The practice phase was also extended to last from the day the participant received their monitoring equipment until their quit date, allowing them to practice daily. The study therapist practiced mCM monitoring with the participant in person, and additionally offered follow-up telephone mCM coaching sessions if the participant had any difficulties with the technology. Second, the mCM app programming was modified to enhance rewards during the early quit period. To increase the odds of quit-day abstinence, CM incentives were increased for the first three days following the quit date. Whereas incentives previously started at $1 for the quit date and increased incrementally, this was changed to instead offer $5 for each reading indicating abstinence for the first 3 days post-quit. Additionally, the protocol was changed to more flexibly handle participants who were unable to achieve initial abstinence. Participants who were not abstinent on their quit day were encouraged to reset their quit day and were allowed to restart mCM (at $5 for quit day readings). In order to control the total cost of CM, the rate of reinforcement escalation was also changed from 25¢ to 10¢ per additional abstinent reading (Table 1). Since this change was completed during Cohort 2, three patients were enrolled after this change. Despite the change in compensation, average mCM compensation increased from $172.55 (SD = $208.73) for Cohort 2 prior to the change to $209.33 (SD = $189.30) for the three participants after the change.
There was also a major change made to the pharmacotherapy protocol. Because 40% of participants who adhered to treatment were not fully abstinent at post-treatment, the study team elected to enhance pharmacotherapy by adding varenicline, a highly efficacious smoking cessation medication (Kishi & Iwata, 2015). At the beginning of the study, there were some concerns about prescribing varenicline for smoking cessation for people with schizophrenia (Tsoi et al., 2013). However, during the course of the study, a number of trials were published indicating that varenicline is a safe and efficacious for smokers with schizophrenia (Jeon et al., 2016; Kishi & Iwata, 2015; Schuster et al., 2017). Hence, an adaptive design was added to the study. For participants that did not achieve abstinence during the first week after the quit date, bupropion and NRT were removed, and following a washout period, varenicline was offered.
There were also two changes made to the counseling protocol based upon the consultant’s feedback. First, Sessions 1 (Orientation to Therapy) and 3 (Quit Date) were held in person, preferably as a home visit. Holding sessions in-person was theorized to enhanced rapport and provide opportunities to observe barriers to abstinence in the home. Finally, the counseling participant workbook was completely redesigned for an audience with SMI. This included shortening and simplifying exercises, reorganizing some content, and contracting a graphic designer to create engaging visuals throughout the workbook. These changes were completed and constituted the treatment provided in a subsequent, ongoing randomized clinical pilot trial.
Discussion
The current study utilized a successive cohort design to iteratively refine a smoking cessation intervention for people with schizophrenia. This is the first known study to use the successive cohort design to elicit qualitative feedback from smokers with schizophrenia regarding their treatment, and to subsequently use this data to refine treatment. Results indicated that participants highly valued behavioral treatments, including both CM and CBT counseling. Furthermore, participant data underscored the importance of combined treatment with both behavioral and pharmacologic approaches.
The protocol for participant mCM incentives underwent a number of changes as a result of participant data and feedback gathered as part of the successive cohort design. Given feedback regarding the salience of incentives, one of the most dramatic changes was to incentives for the first 3 days post-quit. Since many participants were not able to achieve initial abstinence, incentives were increased to $5 per reading for the first 3 days. Additionally, participant data indicated that multiple quit attempts were necessary for participants. Thus, the incentive protocol was further adjusted to allow for $5 incentives even if the quit date was delayed.
Another meaningful observation from the current study was the extent to which participants engaged with the treatment and modified their smoking behavior. In Cohort 2, the majority of participants had high adherence with both the app and the counseling sessions in the treatment phase. Given that the treatment is intensive (with weekly sessions and daily CO readings), this finding is encouraging. Additionally, the participants who were abstinent at 3-months had high adherence with the mCM app and counseling sessions. Among those who did not achieve abstinence, nearly all the participants reduced their cigarettes per day, which indicates that smoking reduction may be a meaningful outcome to examine in future studies.
Additionally, given participant feedback and emerging empirical data from other studies, varenicline was deemed appropriate for this study population. When the study was first initiated, there were concerns among both mental health professionals and smoking cessation experts regarding the safety of varenicline for smokers with schizophrenia (Tsoi et al., 2010; J.M. Williams et al., 2012). However, during the course of the study, new evidence emerged that varenicline is efficacious and safe to prescribe as a stop smoking aid for people with schizophrenia (Kishi & Iwata, 2015). Additionally, new evidence emerged that a graduated approach to smoking cessation pharmacotherapy may be appropriate, such that smokers with difficulty achieving initial abstinence may benefit from switching to varenicline (Rose & Behm, 2013). Given that participants had difficulty achieving initial quit status, the study team decided to introduce varenicline as an option for participants that were unable to achieve abstinence after the quit date. This change was made after Cohort 2’s completion, so measurement of varenicline utilization and acceptability are currently being tested in an ongoing RCT.
Regarding refinements to CBT counseling, several changes were made in order to tailor the counseling sessions to the study population. Important considerations included that the participants highly valued time spent with the study therapist. As such, in-person sessions were added in order to enhance rapport and effectiveness of counseling. Furthermore, the option to repeat the quit date session allowed for participants who struggled with quitting to receive additional support. Finally, the counseling workbook and sessions were restructured in order to more fully accommodate participants with difficulties in learning and executive functioning.
This treatment development study was not designed to measure whether changes (and which changes) to the iCOMMIT intervention may improve abstinence outcomes, or whether the iCOMMIT intervention is superior in efficacy to other smoking cessation approaches. However, we may hypothesize that increased face-to-face interaction with participants and the option to repeat quit date sessions may particularly improve abstinence outcomes, especially given evidence of the strong dose-response association between behavioral intervention intensity and abstinence likelihood (Fiore et al., 2008). Additionally, the adaptive approach to pharmacotherapy (participants may switch to varenicline) could also affect abstinence outcomes (Rose & Behm, 2013), especially given promising results on the efficacy of varenicline for smoking cessation among people with serious mental illness (Roberts, Eden Evins, McNeill, & Robson, 2016). There is limited empirical literature regarding what proportion of smokers with serious mental illness are willing to take varenicline (or bupropion). If there is a considerable proportion of smokers who are unwilling to take a medication, then the current study’s multi-component, flexible, and adaptive approach could prove particularly useful.
Observations and suggestions from study therapists and the study consultant were also valuable to the study design. Study therapists provided vital feedback that led to changes in the mobile health equipment used for the intervention, psychotherapy content, contingency management schedule of incentives and training procedures, and methods for targeting pharmacotherapy adherence. The study consultant was instrumental in drawing attention to the ways in which the psychotherapy materials could be simplified and thus better suit the cognitive needs of the people diagnosed with schizophrenia.
The current study is not without limitations. Since the goal of the study was to iteratively improve and develop an intervention, the design does not include random assignment to a control or comparison condition. However, an RCT is ongoing which tests the intervention’s effect on 3- and 6-month smoking abstinence compared to telehealth CBT for smoking cessation plus patch and bupropion. Another limitation of the current study was the relatively small size of each cohort of participants. However, there is evidence that after 12 qualitative interviews analyzed using thematic analysis, there are diminishing returns on new themes emerging; and by the 17th participant, the majority of themes have been identified (Guest et al., 2006).
Conclusions
In conclusion, this paper describes successive cohort methodology for refining a multi-component smoking cessation intervention tailored for people with schizophrenia. It addresses the need for more modifications to behavioral treatments for this population, as well as the need to study combined effects of behavioral and pharmacological interventions for smoking cessation. This study also highlights the importance of using standardized methodology for treatment development, such as the successive cohort design. This methodology allows for treatment development decisions to be made based upon a mixture of theoretical considerations, qualitative data, and emerging empirical evidence. This initial treatment development step is often overlooked in mental health research, and can provide important information about an intervention prior to efficacy or effectiveness testing. Although the efficacy of pharmacological treatments for smoking cessation among people with schizophrenia has been established, relatively few studies have focused on methodology for developing behavioral treatments that compliment or enhance the efficacy of smoking cessation pharmacotherapy in this population. The successive cohort design enabled rich and patient-centered intervention development. This process provided some essential insights into the needs of smokers with schizophrenia, including the need for high-quality behavioral treatment, adaptable approaches to smoking cessation, and assistance with adherence to efficacious pharmacotherapy.
Acknowledgement
This work was supported by NIH grant R34DA038272 from the National Institute of Drug Abuse. It was also supported by the VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center, the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment (Dr. Wilson), a VA Career Development Award from VA Health Services Research and Development (IK2HX002398) (Dr. Wilson), a VA Research Scientist Award from the VA Office of Research and Development (ORD) Clinical Sciences Research and Development Service (lK6CX001494) (Dr. Beckham), a VA Career Development Award from VA ORD CSR&D (IK2CX000718) (Dr. Dedert), and NIH grant R25MH083620 from the National Institute of Mental Health (Dr. Wilson).
References
- Andreasen NC, Arndt S, Miller D, Flaum M, & Nopoulos P (1995). Correlational studies of the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Postivie Symptoms: An overview and update. Psychopathology, 28, 7–17. doi: 10.1159/000284894 [DOI] [PubMed] [Google Scholar]
- Aubin H, Rollema H, Svensson TH, & Winterer G (2012). Smoking, quitting, and psychiatric disease: A review. Neuroscience & Biobehavioral Review, 36, 271–284. doi: 10.1016/j.neubiorev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, & Rejas J (2010). Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: An analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophrenia Research, 119(1), 101–109. doi: 10.1016/j.schres.2010.02.1030 [DOI] [PubMed] [Google Scholar]
- Braun V, & Clarke V (2006). Using thematic analysis in psychology. Qualitative Research in Psychology, 3(2), 77–101. doi: 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- Cather C, Pachas G, Cieslak K, & Evins A (2017). Achieving smoking cessation in individuals with schizophrenia: Special considerations. CNS Drugs, 31(6), 471–481. doi: 10.1007/s40263-017-0438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Ragg M, & McGeechan K (2009). Citation Bias in Reported Smoking Prevalence in People with Schizophrenia. Australian & New Zealand Journal of Psychiatry, 43(3), 277–282. doi: 10.1080/00048670802653372 [DOI] [PubMed] [Google Scholar]
- de Leon J, & Diaz FJ (2005). A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research, 76, 1351–1357. doi: 10.1016/j.schres.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Desai HD, Seabolt J, & Jann MW (2001). Smoking in patients receiving psychotropic medications: A pharmacokinetic perspective. Central Nervous System Drugs, 15(6), 469–494. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, & Oswald K (2004). Incentives in smoking cessation: Status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research, 6, S163–S179. doi:10.1080114622200410001669196 [DOI] [PubMed] [Google Scholar]
- Epstein EE, McCrady BS, Morgan TJ, Cook SM, Kugler G, & Ziedonis D (2007). The successive cohort design: A model for developing new behavioral therapies for drug use disorders, and the application to behavioral couple treatment. Addictive Disorders and Their Treatment, 6, 1–19. doi: 10.1097/01.adt.0000210707.49703.2c [DOI] [Google Scholar]
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, … Goff DC (2007). A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Pharmacology, 27(4), 380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa [DOI] [PubMed] [Google Scholar]
- Fagerström KO, & Hughes JR (2002). Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: A review. Nicotine & Tobacco Research, 4(2), S73–S79. doi: 10.1080/1462220021000032753 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker T, Bailey WC, Benowitz NL, Curry SEEA, ... & Henderson PN (2008). Treating tobacco use and dependence: 2008 update Rockville, MD: US Department of Health and Human Services. [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured Clinical Interview for DSM-5 (SCID-5 for DSM-5) Arlington, VA: American Psychiatric Association. [Google Scholar]
- Gallagher SM, Penn PE, Schindler E, & Layne W (2007). A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. Journal of Psychoactive Drugs, 39, 487–497. doi: 10.1080/02791072.2007.10399888 [DOI] [PubMed] [Google Scholar]
- Guest G, Bunce A, & Johnson L (2006). How many interviews are enough? Field Methods, 18(1), 59–82. doi: 10.1177/1525822X05279903 [DOI] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, & Casey DE (2005). Schizophrenia and increased risks of cardiovascular disease. American Heart Journal, 150(6), 1115–1121. doi: 10.1016/j.ahj.2005.02.007 [DOI] [PubMed] [Google Scholar]
- Higgins ST, Davis DR, & Kurti AN (2017). Financial incentives for reducing smoking and promoting other health-related behavior change in vulnerable populations. Policy Insights from the Behavioral and Brain Sciences, 4(1), 33–40. doi: 10.1177/2372732216683518 [DOI] [Google Scholar]
- Hu Y, Fang ZH, Yang YC, Rohlsen-Neal D, Cheng F, & Wang J (2018). Analyzing the genes related to nicotine addiction or schizophrenia via a pathway and network based approach. Scientific Reports, 8, 10. doi: 10.1038/s41598-018-21297-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Cummings KM, & Hyland A (1999). Ability of smokers to reduce their smoking and its association with future smoking cessation. Addiction, 94, 109–114. doi: 10.1046/j.1360-0443.1999.9411097.x [DOI] [PubMed] [Google Scholar]
- Jeon D, Shim J, Kong B, Moon J, Seo Y, Kim S, … Jung D (2016). Adjunctive varenicline treatment for smoking reduction in patients with schizophrenia: A randomized double-blind placebo-controlled trial. Schizophrenia Research, 176(2), 206–211. doi: 10.1016/j.schres.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Kishi T, & Iwata N (2015). Varenicline for smoking cessation in people with schizophrenia: systematic review and meta-analysis. European Archives of Psychiatry and Clinical Neuroscience, 265(3), 259–268. doi: 10.1007/s00406-014-0551-3 [DOI] [PubMed] [Google Scholar]
- Kollins SH, McClernon FJ, & Van Voorhees EE (2010). Monetary incentives promote smoking abstinence in adults with attention deficit hyperactivity disorder (ADHD). Experimental Clinical Psychopharmacology, 18, 221–228. doi: 10.1037/a0019565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. The American Journal of Psychiatry, 170(2), 165–172. doi: 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Malik M, Florival C, Manalai P, & Sonje S (2017). An Assessment of Five (PANSS, SAPS, SANS, NSA-16, CGI-SCH) commonly used Symptoms Rating Scales in Schizophrenia and Comparison to Newer Scales (CAINS, BNSS). Journal of addiction research & therapy, 8(3). doi: 10.4172/2155-6105.1000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClave AK, McKnight-Eily LR, Davis SP, & Dube SR (2010). Smoking characteristics of adults with selected lifetime mental illnesses: Results from the 2007 National Health Interview Survey. American Journal of Public Health, 196, 116–121. doi: 10.2105/AJPH.2009.188136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon A, Malte C, Chow B, Bailey S, Baker D, … Lavori PW (2010). Integrating tobacco cessation into mental health care for posttraumatic stress disorder: A randomized controlled trial. Journal of the American Medical Association, 304(22), 2485–2493. doi: 10.1177/1740774507076923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RM, Malla AK, Cortese L, & Diaz F (1996). A study of the interrelationship between and comparative interrater reliability of the SAPS, SANS and PANSS. Schizophrenia Research, 19, 73–85. doi: 10.1016/0920-9964(95)00055-0 [DOI] [PubMed] [Google Scholar]
- Olfson M, Gerhard T, Huang C, Crystal S, & Stroup T (2015). Premature mortality among adults with schizophrenia in the united states. JAMA Psychiatry, 72(12), 1172–1181. doi: 10.1001/jamapsychiatry.2015.1737 [DOI] [PubMed] [Google Scholar]
- Peters S (2010). Qualitative research methods in mental health. Evidence Based Mental Health, 13(2), 35–40. doi: 10.1136/ebmh.13.2.35. [DOI] [PubMed] [Google Scholar]
- Petry NM (2010). Contingency management treatments: Controversies and challenges. Addiction, 105, 1507–1509. doi: 10.1111/j.1360-0443.2009.02879.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM (2011). Contingency management: What it is and why psychiatrists should want to use it. Psychiatrist, 35, 161–163. doi: 10.1192/pb.bp.110.031831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, & Kupfer DJ (2013). DSM-5 field trials in the United States and Canada, Part II: Test-retest reliability of selected categorical diagnoses. American Journal of Psychiatry, 170(1), 59–70. doi: 10.1176/appi.ajp.2012.12070999 [DOI] [PubMed] [Google Scholar]
- Roberts E, Eden Evins A, McNeill A, & Robson D (2016). Efficacy and tolerability of pharmacotherapy for smoking cessation in adults with serious mental illness: a systematic review and network meta‐analysis. Addiction, 111, 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, Mintz J, & Lamb RJ (2009). The relationship between self-efficacy and reductions in smoking in a contingency management procedure. Experimental Clinical Psychopharmacology, 17(3), 139–145. doi: 10.1037/a0015842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, & Behm F (2013). Adapting smoking cessation treatment according to initial response to precessation nicotine patch. American Journal of Psychiatry, 170(8), 860–867. doi: 10.1176/appi.ajp.2013.12070919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldberg D, Quinlan DM, Morgenstern H, & Glazer W (1990). Positive and negative symptoms in chronic psychiatric outpatients: Reliability, stability, and factor structure. Psychological Assessment, 2, 262. doi: 10.1037/1040-3590.2.3.262 [DOI] [Google Scholar]
- Schuster R, Cather C, Pachas G, Zhang H, Cieslak K, Hoeppner S, … Eden Evins A (2017). Predictors of tobacco abstinence in outpatient smokers with schizophrenia or bipolar disorder treated with varenicline and cognitive behavioral smoking cessation therapy. Addictive Behaviors, 71, 89–95. doi: 10.1016/j.addbeh.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported alcohol consumption. In Litten R & Allen J (Eds.), Measuring Alcohol Consumption: Psychosocial and Biochemical Methods (pp. 41–72). Totowa: The Humana Press, Inc. [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, & Reid N (2011). Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology, 217(2), 279–287. doi: 10.1007/s00213-011-2282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi DT, Porwal M, & Webster AC (2010). Efficacy and safety of bupropion for smoking cessation and reduction in schizophrenia: Systematic review and meta-analysis. British Journal of Psychiatry, 196, 346–353. doi: 10.1192/bjp.bp.109.066019 [DOI] [PubMed] [Google Scholar]
- Tsoi DT, Porwal M, & Webster AC (2013). Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews, 2, CD007253. doi: 10.1002/14651858.CD007253.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2014). The Health Consequences of Smoking —50 Years of Progress: A Report of the Surgeon General Atlanta, GA: U.S. Department of Health and Human Services. [Google Scholar]
- Williams JM, Anthenelli RM, Morris CS, Treadow J, Shompson JR, Yunis C, & George TP (2012). A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry, 73, 654–660. doi: 10.4088/JCP.J12-lcx07908 [DOI] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Foulds J, Steinberg M, Lu S, Masumova F, … Ziedonis D (2007). No advantage for high dose compared to regular dose nicotine patch on short-term abstinence rates in schizophrenia. Conference abstract of the Society for Research on Nicotine and Tobacco 13th Annual Meeting.
- Yardley L, Morrison L, Bradbury K, & Muller I (2015). The person-based approach to intervention development: application to digital health-related behavior change interventions. Journal of Medical Internet Research, 17(1). doi: 10.2196/jmir.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, … Riley WT (2008). Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research, 10(12), 1691–1715. doi: 10.1080/14622200802443569 [DOI] [PubMed] [Google Scholar]


