Abstract
Background:
Imaging guidance for left atrial appendage (LAA) closure (LAAC), conventionally consists of transesophageal echocardiography (TEE) and fluoroscopy under general anesthesia (GA). Intracardiac echocardiography (ICE) can eliminate the need for GA, and expedite procedural logistics, and reduce the patient experience to a simple venous puncture.
Objective:
To define optimal ICE views and compare procedural parameters and cost of ICE vs TEE during LAAC with the Watchman device.
Methods:
Optimal ICE views of the LAA for Watchman implant were delineated using Carto-Sound and 3D rendition of the LAA in 6 patients. Procedural and financial parameters of 104 consecutive patients with standard indications for LAAC undergoing Watchman implant using ICE guidance through a single trans-septal puncture (n=53, 51%) were compared with those of TEE-guided implants (n=51, 49%) in 3 centers.
Results:
Clinical characteristics were similar between the two groups. Total in-room, turnaround and fluoroscopy times were all shorter using ICE (p<0.05) under local anesthesia compared to the TEE group. Implant success was 100% in both groups without peri-device leaks or procedural complications. Follow-up TEE showed no significant peri-device leak in both groups. Total hospital charges for ICE with local anesthesia vs TEE were similar as were total hospital direct and indirect costs. Professional fees were significantly lower with ICE and local anesthesia than with TEE as the charge of anesthesia staff was avoided.
Conclusion:
ICE-guided Watchman implant is safe, feasible, and comparable in cost to TEE during LAAC with a Watchman device while avoiding GA and expediting the procedure turnaround.
Keywords: Atrial fibrillation, Intracardiac echocardiography, Transesophageal echocardiography, Left atrial appendage occlusion, Watchman
Introduction:
Atrial fibrillation (AF) is one of the most common arrhythmias, affecting millions of people in the US each year 1. AF-related stroke is effectively prevented with oral anticoagulation (OAC) 2. A narrow therapeutic window, risk of bleeding, noncompliance, and the recognition of the left atrial appendage (LAA) as a major site of thrombus formation have led to the development of LAA closure (LAAC) techniques. Randomized trials have validated the Watchman device as a viable alternative to OAC 3, 4. In these trials, the Watchman device implant procedure was designed to be performed under transesophageal echocardiography (TEE) and fluoroscopy guidance. TEE guidance commonly leads to the need for general anesthesia (GA), and carries additional risks, including esophageal lesions, aspiration and others, along with patient inconvenience and increased complexity of procedural logistics. Intracardiac echocardiography (ICE) imaging has been used for over two decades in several interventional procedures including septal defect closure and arrhythmia ablations 5–7. ICE not only can obviate the need for GA but also reduce the patient experience to a simple venous puncture without compromising quality of care. Recently, it has been investigated in a few descriptive studies to guide through LAAC 8–11. None of these studies compared the use of ICE vs TEE in terms of both procedural outcomes and cost during LAAC using a Watchman device. Additionally, the optimal ICE imaging strategy and views have not been defined. Here we evaluated the optimal views to image the LAA with ICE during the Watchman procedure and delineated the impact of ICE in procedural parameters as well as hospital finances.
Methods:
Study Design and Patient Population:
Patients with nonvalvular AF, significant risk of stroke, and a history of bleeding or long-contraindication for anticoagulation, referred for LAAC (n=104) between April 2015 and January 2018 at 3 centers were chosen for a retrospective analysis from an IRB-approved registry. Data collection included medical history, procedural reports, and follow-up events. Informed consent was obtained from every patient prior to the procedure in accordance with the ethical standards of the Helsinki Declaration. All consecutive patients who underwent Watchman deployment using ICE guidance “ICE group” (n=53, 51%) were included, 41 (77%) of which underwent local anesthesia or monitored anesthesia care (MAC). We then selected a matched number of consecutive patients (n=51, 49%) using TEE “TEE group,” all of which underwent general anesthesia (GA), for comparison. The patients distribution among centers 1, 2 and 3 was 22, 29, 2 for the ICE group and 22, 29, 0 for the TEE group, respectively.
Defining the ICE imaging strategy to guide Watchman deployment
In six patients undergoing AF ablation, the ICE (SoundsStar, Biosense-Webster, Diamond Bar, CA) was inserted in the LA. A 3D geometry of the LA had been created with the Carto (Biosense-Webster) mapping system by roving a 20-pole radial catheter (Penta-Ray, Biosense-Webster) into the pulmonary veins, LA and LAA, including the LAA as a separate chamber. Guided by the Carto-Sound feature, the ultrasound beam was displayed in the LA geometry and the ICE catheter was maneuvered to direct the beam towards the LAA.
Intracardiac Echocardiography for Watchman deployment:
Two types of ICE ultrasound catheters were used in different patients to obtain echocardiographic images: ACUSON AcuNav™ (Siemens, Mountain View, CA) and ViewFlex™ Xtra (Saint Jude Medical, St. Paul, MN). The ICE catheter was inserted via a right femoral vein puncture in the same groin as the Watchman sheath. ICE was used in a systematic fashion in the following steps with their respective ICE catheter locations:
To exclude LAA thrombus, views from the right ventricular outflow tract (RVOT) were used 12
To guide the trans-septal puncture, views from the RA were obtained.
To guide the LAA pigtail cannulation, sheath advancement, and Watchman sizing, deployment, position, and peri-Watchman flow (“leak”), the ICE catheter was advanced trans-septally in the LA.
Watchman Device Implantation:
Vascular access was obtained with ultrasound-guided puncture of the right femoral vein. A Preface® (Biosense Webster) or SL1™ (Abbott) transseptal access sheath was then introduced. ICE ultrasound catheter (AcuNav™ or ViewFlex™) was advanced into the right atrium, self-guided by its images. Thrombus formation in the LAA was then excluded by placing the ICE catheter in the RVOT. After administering intravenous heparin to keep activated clotted time (ACT) between 250-300 s, the Preface or SL1 sheath was advanced to engage the fossa ovalis for a septal puncture, all under ICE guidance. The Preface sheath was then exchanged over the wire and a Watchman delivery sheath was inserted through the same trans-septal puncture, which was thus dilated to a 14F diameter. The Watchman delivery sheath was then retracted to the RA, allowing the ICE catheter to be advanced into the LA over the 14-F-dilated puncture. Then, the sheath was re-advanced into the LA. Thereafter, the sheath and the ICE catheter were both in the LA through the same trans-septal puncture. A pigtail catheter was then introduced to engage the LAA. After selecting an appropriate device size, it was loaded in the sheath and delivered in the LAA. ICE images were used to assess the device compression and stability. Peri-device leak was assessed with Doppler images for optimal seal. Partial device recaptures and deployment were done, if necessary, to achieve optimal position. The device was finally released after confirmation with ICE and angiography.
Statistical Analysis:
Quantitative data were expressed as mean ± SD and were compared using two-tailed t-test. P-values were considered statistically significant when p<0.05. Categorical data were expressed as percentages and compared using Chi-Square.
Results:
Defining Optimal ICE views
During each case, individual views were systematically evaluated to select a main “working view” for the procedure. After deployment, the Watchman was then evaluated by multiple views. The RVOT/PA view was used on every case as a screening view to rule out LAA thrombus prior to the trans-septal puncture. The CS view was not utilized in any case, since CS cannulation with the ICE catheter would have required additional instrumentation.
To demonstrate the anatomical basis of the ICE views of the LAA from different positions in the LA, six patients undergoing AF ablation were imaged using CARTO-Sound with the ICE catheter in the LA: they were 2 females, 65±12 year-old, with paroxysmal AF in 4, and persistent in 2, LA volumes of 94±25 ml. The optimal views to image the LAA included positioning the ICE catheter tip in the following positions, aiming the ultrasound beam towards the LAA:
LA body. Positioning the ICE catheter tip central in the LA geometry, aiming towards the LAA so that the edge of the LA ridge could be visualized, separating the LAA from the LSPV.
LSPV. Advancing the ICE catheter tip deeper into the LSPV a longitudinal view of the LAA could be obtained.
LIPV. Similar to LSPV, but with a slight angulation difference could display the LAA lobe structure.
Transmitral, deflecting the ICE catheter and advancing it across the mitral valve with the ultrasound beam pointing superiorly allowed visualization of the LAA.
Figures 1 and 2 show examples of these views.
Figure 1. ICE images of the LAA from different anatomical locations and 3D reconstruction of the LA. a,d,g.
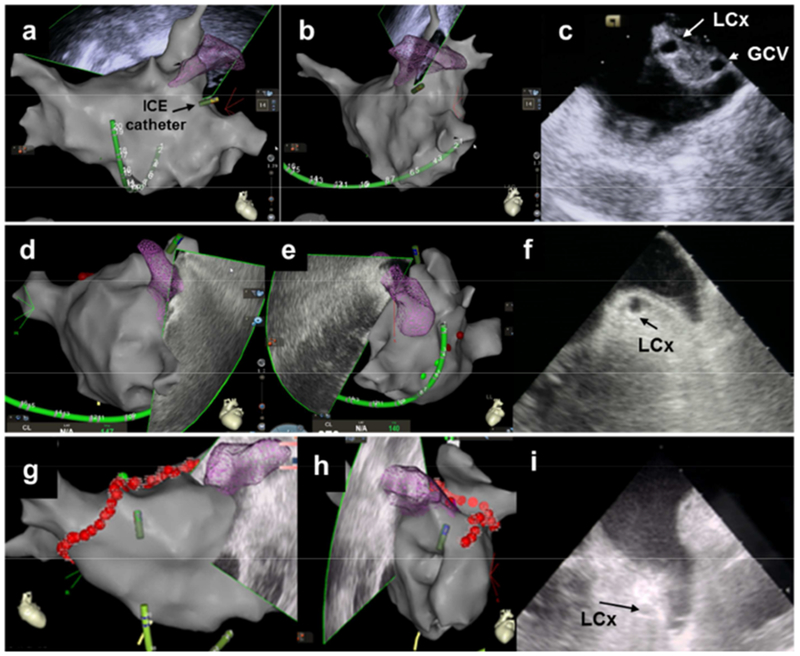
RAO view of 3D reconstruction of the LA. b,e,h. LAO view of 3D reconstruction of the LA. c. ICE view from the mitral annulus. f. ICE view from the LSPV. i. ICE view from the LA body.
ICE: Intracardiac echocardiography RAO: Right anterior oblique. LAO: Left anterior oblique. LSPV: left superior pulmonary vein. LA: left atrium. LAA: left atrial appendage LCx: left circumflex artery GCV: great coronary vein
Figure 2. ICE images of the LAA as viewed from different anatomical locations.
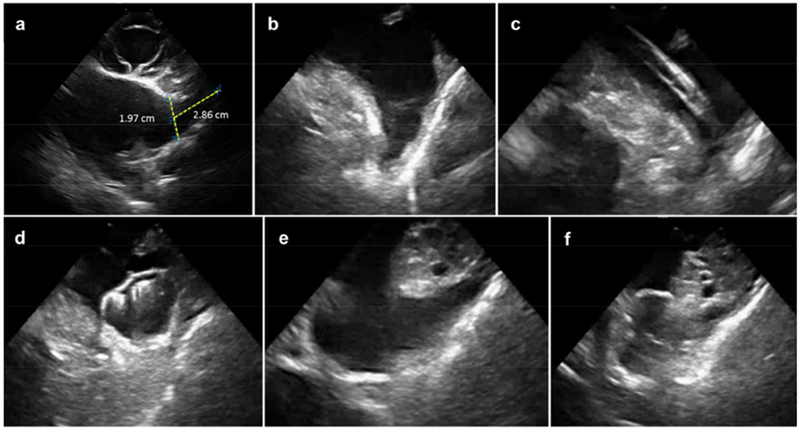
A. Right ventricular outflow tract (RVOT). B. LA body. C. LA body during cannulation of the LAA. D. LA body with a Watchman device in LAA. E. Trans-mitral. F. Trans-mitral with a Watchman device in LAA. ICE: Intracardiac echocardiography LAA: left atrial appendage.
Clinical and Procedural Characteristics:
In a total of 104 patients, the Watchman device was deployed using ICE (n=53, 51%, 77 ± 10 years, male/female: 33/20) of which 77% under local anesthesia or MAC (n=41, 77 ± 10 years, male/female: 25/16), or using TEE under GA (n=51, 49%, 76 ± 7 years, male/female: 31/20). Mean CHA2DS2-VASc score was elevated in both groups (4.5 ± 1.8 vs 4.5 ± 1.6, ICE vs TEE respectively) (Table 1).
Table 1.
Baseline Patient Characteristics
| ICE (n=53) | TEE (n=51) | P-value | |
|---|---|---|---|
| Age | 77 ± 10 | 76 ± 7 | 0.656 |
| Sex (male/female) | 33/20 | 31/20 | 0.963 |
| Mean CHA2DS2-VASC score | 4.5 ± 1.8 | 4.5 ± 1.6 | 1.0 |
| Congestive Heart Failure | 10 (19%) | 13 (25%) | 0.564 |
| Hypertension | 43 (81%) | 46 (90%) | 0.300 |
| Diabetes mellitus | 18 (34%) | 15 (29%) | 0.774 |
| Prior stroke or transient ischemic attack | 22 (42%) | 17 (33%) | 0.510 |
| Vascular disease | 21 (40%) | 19 (37%) | 0.963 |
Total fluoroscopy time was significantly lower with ICE than TEE (4.8 ± 2.7 vs 7.3± 4.7 minutes, p=0.004). LAA dimensions as measured intraoperatively by ICE or TEE were similar (Table 2). The number of device deployments and partial recaptures and the number of devices used per patient were also similar (Table 2). Device compression, however, was higher in the ICE group (19% ± 7% vs 15% ± 6%, p=0.003).
Table 2.
Procedure Parameters
| ICE (n=53) | TEE (n=51) | P-value | ||
|---|---|---|---|---|
| Fluoroscopy time | 4.8 ± 2.7 | 7.3 ± 4.7 | 0.004 | |
| LAA width | 21.27 ± 3.27 | 21.52 ± 4.68 | 0.762 | |
| LAA depth | 31.25 ± 6.50 | 28.47 ± 7.23 | 0.069 | |
| Device size | ||||
| 21 mm | 4 (8%) | 1 (2%) | 0.383 | |
| 24 mm | 2 (4%) | 15 (29%) | 0.001 | |
| 27 mm | 21 (40%) | 12 (24%) | 0.121 | |
| 30 mm | 14 (26%) | 8 (16%) | 0.272 | |
| 33 mm | 12 (23%) | 15 (29%) | 0.573 | |
| Device compression | 19% ± 7% | 15% ± 6% | 0.003 | |
| Number of deployments | 1.74 ± 1.29 | 2.04 ± 1.26 | 0.236 | |
| Devices needing recapture | 43% | 54% | 0.379 | |
| Number of recaptures (median/IQR) | 1/1 | 2/2 | N/A | |
| Number of devices/patient | 1.02 ± 0.14 | 1.08 ± 0.27 | 0.156 | |
| Peri-device flow on follow-up TEE† | None | 32 (72%) | 32 (70%) | 0.946 |
| Minimal (<5mm) | 12 (26%) | 13 (28%) | 0.949 | |
| >5 mm | 1 (2%) | 1(2%) | 1.000 | |
A total of 13 patients did not have a follow-up TEE on record due to: comorbidities precluding the use of TEE (n=2), patient refusal (n=2), done at an outside center (n=3), loss of contact (n=6).
When comparing ICE with local anesthesia and TEE, total in-room time was shorter with ICE (95 ± 28 min vs 116 ± 54 min, p=0.03). Turnaround time, measured as time from sedation start to puncture plus time from closure to out-of-room, was 45% shorter with ICE without GA than TEE (32 ± 13 min vs 59 ± 35 min, p<0.001). Total procedure time from puncture to closure was unchanged using ICE or TEE (46 ± 24 min vs 46 ± 30 min, respectively). (Figure 3).
Figure 3. Procedure and turnaround times.
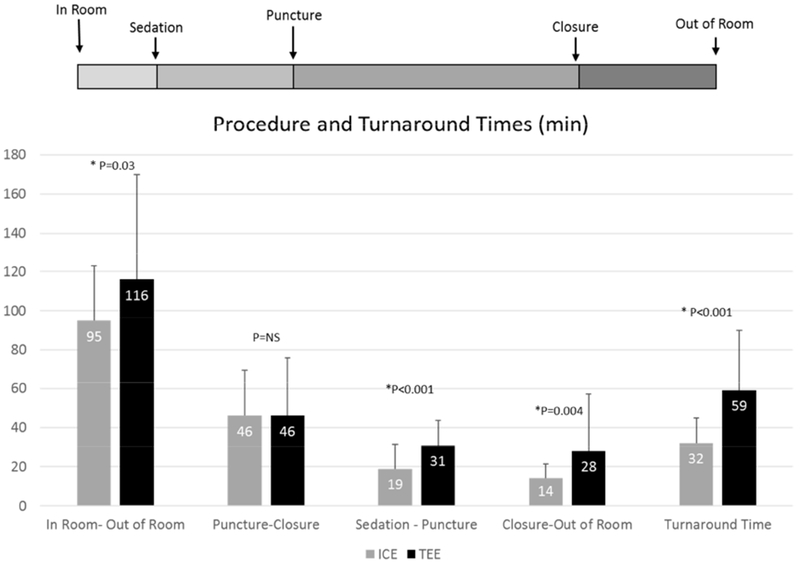
Turnaround time, time from sedation start to puncture plus time from closure to out-of-room.
Procedure Success and Follow-up:
Device implantation was 100% successful in both groups. No peri-device leak was detected in any patient at implant. Follow-up TEE was done after 45 days (in one center) and 120 days (in the other one) to assess for device thrombosis or leaks. In the ICE group, 32 patients (72%) had no peri-device flow, 12 (26%) had minimal flow (<5 mm), 1 patient (2%) had a flow of 6 mm. In the TEE group, 32 (70%) had no flow, 13 (28%) minimal flow, 1 patient had a flow of 8 mm (2%). No tamponade, procedural stroke, device embolization, puncture site hematoma, or procedure related deaths occurred in any group. Of note, ICE-guided patients had a single figure-eight suture deployed at the site of femoral vein punctures –encompassing both the Watchman delivery sheath puncture and the ICE-puncture. Adequate hemostasis was achieved in all cases and the suture was removed after 24 h without hematomas.
Cost and Charge Analysis:
Financial analysis was performed in 10 ICE-guided procedures done under local anesthesia and 10 TEE-guided patients performed under GA at the same center. When using ICE, moderate sedation was administered under the operator’s supervision without the presence of anesthesia staff. Total hospital charges and costs, and total professional fees were compiled. There were common charges between the two groups which included medical/surgical supplies during the procedure, clinical laboratory, ECGs, pharmacy, and telemetry room charges. Unique to the ICE group was the ultrasound catheter, ICE technical fees and moderate sedation charges. Unique to the TEE group included TEE and anesthesia technical fees, recovery room charges, and anesthesia professional fees. Total hospital charges were similar between the two groups ($128,275 ± $2,310 vs $129,733 ± $4,775, p=0.396; for ICE vs TEE, respectively) (Table 3). Total hospital costs were also similar between them ($32,290 ± $1,001 vs $31,482 ± $1,186, p=0.117; for ICE vs TEE respectively) (Table 4). Overall hospital ratio of cost-to-charge (RCC) was 25% and 24%, for ICE and TEE respectively. This was commensurate with the inpatient RCC of 22% reported by the Department of Health and Human Services of Texas at our center during 201713.
Table 3.
Hospital and Professional Charges: ICE with Local Anesthesia vs TEE
| ICE with Local Anesthesia | TEE | P-value | |
|---|---|---|---|
| Medical Supplies | $99,632 ± $1,587 | $97,520 ± $1,335 | 0.005 |
| Clinical Lab/ECG | $1,379 ± $772 | $2,112 ± $793 | 0.051 |
| Pharmacy | $2,080 ± $759 | $4,798 ± $2,739 | 0.007 |
| Telemetry Room | $2,333 ± $482 | $2,515 ± $0 | 0.244 |
| Intervention | $12,562 ± $0 | $12,562 ± $0 | 1.000 |
| ICE Technical Fees | $8,680 ± $0 | - | N/A |
| Moderate Sedation | $2,886 ± $919 | - | N/A |
| TEE Technical Fees | - | $2,753 ± $0 | N/A |
| Anesthesia Technical Fees | - | $6,305 ± $1,708 | N/A |
| Recovery | - | $1,719 ± $603 | N/A |
| Total hospital charge | $128,275 ± $2,310 | $129,733 ± $4,775 | 0.396 |
| Professional fee | $4,267 ± $84 | $11,736 ± $2,002 | <0.001 |
| Global (hospital and professional) charges | $132,202 ± $1,988 | $141,468 ± $6,229 | <0.001 |
Table 4.
Hospital Costs: ICE with Local Anesthesia vs TEE
| ICE with Local Anesthesia | TEE | P-value | |
|---|---|---|---|
| Cath Lab Supplies | $19,750 ± $553 | $19,158 ± $501 | 0.022 |
| Clinical Lab/ECG | $690 ± $327 | $157 ± $109 | <0.001 |
| Pharmacy | $516 ± $292 | $848 ± $433 | 0.059 |
| Telemetry Room | $1,260 ± $260 | $1,335 ± $57 | 0.385 |
| Intervention | $7,640 ± $0 | $7,640 ± $0 | 1.000 |
| ICE Technical Fees | $1,538 ± $0 | - | N/A |
| Moderate Sedation | $1,593 ± $582 | - | N/A |
| TEE Technical Fees | - | $140 ± $0 | N/A |
| Anesthesia Technical Fees | - | $284 ± $39 | N/A |
| Recovery | - | $1,948 ± $684 | N/A |
| Total hospital cost | $32,290 ± $1,001 | $31,482 ± $1,186 | 0.117 |
One of the major financial concerns with using ICE during LAAC is the cost of the single-use ultrasound catheter. Two types of ICE ultrasound catheters were used in our sample: ACUSON AcuNav™ (n=8) and ViewFlex™ Xtra (n=2). Their costs were $1,079 and $2,080 and charges $3,368 and $6,489, respectively. ICE technical charges were 3 times more expensive than TEE ones ($8,680 ± $0 vs $2,753 ± $0, respectively). However, this high difference was offset by the higher charges of anesthesia technical fees ($6,305 ± $1,708) and recovery room ($1,719 ± $603) in the TEE group. Total professional charges were significantly higher in the TEE group than ICE ($11,736 ± $2,002 vs $4,267 ± $84 p<0.001, respectively). This is due to the absence of anesthesia staff during ICE-guided procedures under local anesthesia. Overall, global (hospital and professional) charges were therefore lower with ICE-guided LAAC than with TEE ($132,202 ± 1,988 vs $141,468 ± $6,229, p=<0.001; respectively) (Figure 4).
Figure 4.
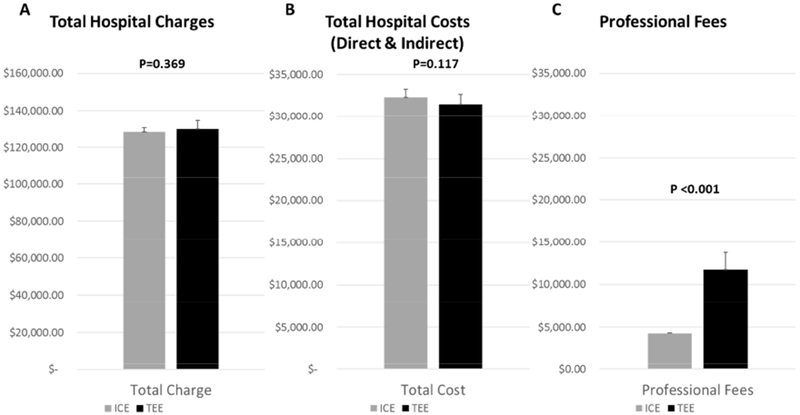
Total hospital costs and charges and professional fees.
Discussion:
The salient results of our study are that: 1) Complete imaging of the LAA to guide Watchman device implants can be obtained by ICE using a series of ICE catheter positionings; 2) Implant procedure is expedited by the use of ICE, with shorter in-room times, turn-around times, and fluoroscopy times; 3) Implant success is not compromised by ICE; and 4) Total costs and hospital charges are similar.
Our study aims primarily to compare the feasibility and safety of the procedure using ICE vs TEE irrespective of anesthesia type. Our second aim was to quantify the effect of avoiding general anesthesia during LAAC on the procedure cost and turnaround time, hence we only included patients undergoing ICE without GA for this sub-analysis.
Echocardiographic imaging is a requirement for Watchman-device implant procedures. TEE guidance has been an integral part of the procedure development and its teaching to new operators. A successful Watchman implant depends on a constellation of parameters: position, anchoring, stability and seal (“PASS criteria”) that rely heavily -if not exclusively- on TEE. The use of TEE entails two added logistical hurdles to the procedure. First, given the extensive imaging demands, it requires the continuous involvement of an experienced echocardiographist during the procedure. Second, given the potentially prolonged and unpleasant probe manipulation, it generates, in most settings, the need for GA, with the added risks of endotracheal intubation and mechanical ventilation. ICE circumvents these two hurdles, because it is under the control of the same operator that performs the implant and because, procedurally, it only entails an additional venous puncture –without altering sedation requirements.
ICE-Watchman imaging
Although it has been long used in various interventional procedures, only recently been reported for use during LAAC. Ho et al. used simultaneous TEE and ICE imaging during PLAATO device implantation (PLAATO System, MN)11. In 2011, McDonald et al. described, in a case report of a high-anesthetic risk patient, the first use of ICE under local anesthesia without any TEE imaging to guide through the Amplatzer cardiac plug (AGA Medical Corporation, Plymouth, MN) device implantation10. Imaging was done solely from the RVOT and PA without crossing the septum while placing the ICE catheter within a Mullins sheath. Accurate ICE-derived measurements of LAA dimensions and correct Amplatzer device selection have been reported.8,9 For Watchman devices, Matsuo et al. compared ICE and TEE in a series of 27 patients and first reported ICE as a feasible tool for such implants. In 2017, Frangieh et al. compared procedural outcomes in 76 patients undergoing LAAC with a Watchman device under the guidance of either ICE or TEE 14. In their study, ICE was as successful as TEE but with longer procedural time. LAA measurements were obtained with the help of TEE preoperatively even in patients undergoing LAAC with ICE. In our current study we have (1) shown that complete imaging of the LAA to guide Watchman device implant was possible using a series of ICE catheter positions without the use of preoperative TEE, (2) defined the optimal views to image the LAA using ICE, (3) verified that using ICE with local anesthesia can expedite the procedure with shorter in-room, turn-around and fluoroscopy times, (4) analyzed, for the first time, the total costs and hospital charges of LAAC with a Watchman device using ICE or TEE, (5) reconfirmed the safety and success of ICE compared to TEE during LAAC.
Despite its promising potential, a recent survey answered by 24 centers in Europe showed that only 4% use ICE during LAAC 15. It is possible that the lack of standardized ICE views –as opposed to the systematic multiplane TEE views may contribute to the lack of ICE use. The published strategies of LAA imaging with ICE have focused on its value for LAA thrombus exclusion. Pre-existing thrombus is a concern during LAAC. Ren et al have described a systematic way to rule out thrombus using ICE, when TEE was equivocal, by placing the transducer in the PA 12. The concordance between ICE and TEE for the presence or absence of thrombus was 97% in the LA and 92% in the LAA as described by Saksena et al 16. In our present series, a preoperative TEE was not obtained in the ICE group. Placing the probe in the RVOT or pulmonary artery (PA) before the septal puncture led to sufficient visualization to exclude thrombus in the LAA in all our patients. Once across the septum, images from the LA, LSPV and mitral annulus were adequate for confirmation. We also describe a series of systematic views that allow complete delineation of the LAA anatomy with ICE, not only for definitive exclusion of thrombus, but also to obtain all necessary Watchman assessments.
Impact of ICE on procedure parameters
The main advantage of using ICE during LAAC is the ability to avoid GA. In the ICE group, moderate sedation can be administered by the implanting physician without the requirement of dedicated anesthesia staff. The benefit was shorter in-room time (by 18%), turnaround times (by 45%), and total fluoroscopy time (by 1/3). Although not systematically measured in this study, there are obvious advantages for the subjective patient experience by eliminating endotracheal intubation and esophageal instrumentation and their risks (tooth-lip injuries, esophageal tears, aspiration pneumonia, ventilator dependence, etc).
The procedural disadvantages of using ICE included the need for an additional venous puncture and the additional trans-septal instrumentation required to insert the ICE catheter in the LA. These were acceptable: hemostasis was achieved with a single figure-eight suture and the added manipulation to cross the septum with the ICE did not compromise the reduced procedure and fluoroscopy times.
The Watchman device implantation success rate was 100% (n=104) in both groups. No peri-device leak was detected in any patient at implant. Optimal device position and stability was achieved in both groups with a similar number of device deployments. Follow-up TEE after 45 days or 120 days, showed no or minimal flow in 98% (n=44/45) and 98% (n=45/46) of the patients using ICE and TEE respectively. Device selection was based on LAA measurements obtained intraoperatively, which were similar between the two groups.
Financial impact of ICE-guidance
One of the major perceived concerns about the use of ICE during LAAC is the cost of the procedure. A large comparative study (n=4213) found similar hospital costs and charges when using ICE vs TEE during atrial septal defects closure 17. No published data is available to date, however, comparing the cost of ICE and TEE during LAAC. In our study, we randomly chose 10 patients who underwent ICE with local anesthesia and the same number from the TEE group for cost comparison. The single-use ultrasound catheter is of particular interest when considering the cost of the procedure, as opposed to the reusable TEE probes. In our center, we used two different types of ICE catheter: ACUSON AcuNav™ and ViewFlex™ Xtra. Their costs were $1,079 and $2,080, respectively. Other components that increased the cost of the procedure is ICE technical fees and moderate sedation, when used under local anesthesia. However, high TEE and anesthesia technical fees and recovery room charges in the TEE group offset the ICE charges. In fact, overall hospital costs and charges were similar between the two groups. Moreover, avoidance of GA when using ICE has dropped the professional fees by over 60%. Re-sterilization of the ICE catheter, which is only permitted in Germany and Eastern Europe 18, should further drop the cost of the procedure.
Study Limitations:
We admit to some limitations in our study. Our sample was small and nonrandomized. We chose all consecutive patients who underwent the procedure using ICE and then selected a similar number of consecutive patients using TEE for comparison. LAAC using ICE was done by operators with extensive experience using ICE, which might affect the reproducibility of our results.
Conclusion:
ICE is safe, feasible, and comparable in cost to TEE during LAAC with a Watchman device. Moreover, avoiding GA makes ICE a more convenient and less invasive option to high-risk patients.
Supplementary Material
Acknowledgment:
We thank the Lois and Carl Davis Centennial Chair, the Charles Burnett III endowment, the Antonio Pacifico fellowship fund and the NHLBI grant R01HL115003.
Disclosures:
MV has received consulting and speaking honoraria from Biosense-Webster, and Boston Scientific. VYR has served as a Consultant to manufacturers of the Watchman device (Boston Scientific) and the ICE catheter (Biosense-Webster); in addition, he has conflicts with other cardiovascular companies not related to this manuscript – a comprehensive list is in the Supplemental Appendix.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 3.Holmes DR Jr., Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 4.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P, Investigators PA. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 5.Ruisi CP, Brysiewicz N, Asnes JD, Sugeng L, Marieb M, Clancy J, Akar JG. Use of intracardiac echocardiography during atrial fibrillation ablation. Pacing Clin Electrophysiol 2013;36:781–788. [DOI] [PubMed] [Google Scholar]
- 6.Ponnuthurai FA, van Gaal WJ, Burchell A, Mitchell AR, Wilson N, Ormerod OJ. Safety and feasibility of day case patent foramen ovale (PFO) closure facilitated by intracardiac echocardiography. Int J Cardiol 2009;131:438–440. [DOI] [PubMed] [Google Scholar]
- 7.Chu E, Kalman JM, Kwasman MA, Jue JCY, Fitzgerald PJ, Epstein LM, Schiller NB, Yock PG, Lesh MD. Intracardiac echocardiography during radiofrequency catheter ablation of cardiac arrhythmias in humans. Journal of the American College of Cardiology 1994;24:1351–1357. [DOI] [PubMed] [Google Scholar]
- 8.Masson JB, Kouz R, Riahi M, Nguyen Thanh HK, Potvin J, Naim C, Salem R, Raymond JM. Transcatheter Left Atrial Appendage Closure Using Intracardiac Echocardiographic Guidance From the Left Atrium. Can J Cardiol 2015;31:1497 e1497–1497 e1414. [DOI] [PubMed] [Google Scholar]
- 9.Berti S, Paradossi U, Meucci F, Trianni G, Tzikas A, Rezzaghi M, Stolkova M, Palmieri C, Mori F, Santoro G. Periprocedural intracardiac echocardiography for left atrial appendage closure: a dual-center experience. JACC Cardiovasc Interv 2014;7:1036–1044. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald ST, Newton JD, Ormerod OJ. Intracardiac echocardiography off piste? Closure of the left atrial appendage using ICE and local anesthesia. Catheter Cardiovasc Interv 2011;77:124–127. [DOI] [PubMed] [Google Scholar]
- 11.Ho IC, Neuzil P, Mraz T, Beldova Z, Gross D, Formanek P, Taborsky M, Niederle P, Ruskin JN, Reddy VY. Use of intracardiac echocardiography to guide implantation of a left atrial appendage occlusion device (PLAATO). Heart Rhythm 2007;4:567–571. [DOI] [PubMed] [Google Scholar]
- 12.Ren JF, Marchlinski FE, Supple GE, Hutchinson MD, Garcia FC, Riley MP, Lin D, Zado ES, Callans DJ, Ferrari VA. Intracardiac echocardiographic diagnosis of thrombus formation in the left atrial appendage: a complementary role to transesophageal echocardiography. Echocardiography 2013;30:72–80. [DOI] [PubMed] [Google Scholar]
- 13.Texas Department of Health and Human Services. Inpatient Ratio of Cost to Charges. December 2017. https://rad.hhs.texas.gov/sites/rad/files/documents/hospital-svcs/2017/2017-12-inp-rcc.pdf. Last accessed on September 1st, 2018.
- 14.Frangieh AH, Alibegovic J, Templin C, Gaemperli O, Obeid S, Manka R, Holy EW, Maier W, Luscher TF, Binder RK. Intracardiac versus transesophageal echocardiography for left atrial appendage occlusion with watchman. Catheter Cardiovasc Interv 2017;90:331–338. [DOI] [PubMed] [Google Scholar]
- 15.Pison L, Potpara TS, Chen J, Larsen TB, Bongiorni MG, Blomstrom-Lundqvist C, Scientific Initiative Committee EHRA. Left atrial appendage closure-indications, techniques, and outcomes: results of the European Heart Rhythm Association Survey. Europace 2015;17:642–646. [DOI] [PubMed] [Google Scholar]
- 16.Saksena S, Sra J, Jordaens L, et al. A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: the intracardiac echocardiography guided cardioversion helps interventional procedures study. Circ Arrhythm Electrophysiol 2010;3:571–577. [DOI] [PubMed] [Google Scholar]
- 17.Alqahtani F, Bhirud A, Aljohani S, Mills J, Kawsara A, Runkana A, Alkhouli M. Intracardiac versus transesophageal echocardiography to guide transcatheter closure of interatrial communications: Nationwide trend and comparative analysis. J Interv Cardiol 2017;30:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartel T, Muller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J 2014;35:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


