Abstract
OBJECTIVES:
Hypertension and obesity are common cardiometabolic risk factors in reproductive age women. The association of hypertensive disorders of pregnancy with later-life cardiovascular disease is well-established, however, it is unknown how obesity and hypertensive disorders of pregnancy converge to accelerate development of hypertension in the postpartum period. The aim of this study was to characterize rates of sustained hypertension at one year postpartum using the new American Heart Association /American College of Cardiology Guidelines among overweight and obese women with a normotensive pregnancy or hypertensive disorder of pregnancy.
STUDY DESIGN:
315 early pregnant women were enrolled prospectively and followed up to 12 months after delivery (mean 7.0 ± 1.8 months). At a postpartum research visit, we measured blood pressure and collected blood samples to measure cystatin C and high sensitivity C-reactive protein.
RESULTS:
A total of 254 women had a normotensive pregnancy, 39 had gestational hypertension (12.4%) and 22 had preeclampsia (7.0%). 91 women had hypertension at the postpartum study visit (28.9%). After adjustment for maternal age, BMI, lactation and time postpartum, preeclampsia was associated with an aOR 2.35 (95%CI 1.63–3.41) of development of sustained hypertension and an aOR 3.23 (95%CI 1.56–6.68) of hypertension with abnormal biomarkers compared to women with normotensive pregnancies.
CONCLUSIONS:
We demonstrate a high prevalence of hypertension and abnormal biomarkers associated with hypertensive disorders of pregnancy among overweight and obese women. Our findings support the need for structured follow up and risk reduction in overweight and obese women with hypertensive disorders of pregnancy as early as the first year postpartum.
Keywords: cardiovascular disease, chronic hypertension, cystatin C, gestational hypertension, hsCRP, preeclampsia
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in women worldwide. There is a linear relationship between increasing body mass index (BMI) and risk of CVD.[1,2] Of particular concern, along with the increase in prevalence of obesity in the United States is the increasing rate of CVD mortality in younger women.[3] The association of hypertensive disorders of pregnancy (including preeclampsia and gestational hypertension) with later-life cardiovascular disease has been well established and replicated in diverse populations across multiple studies and these complications may provide a sex-specific window to susceptability.[4–7] The biologic plausibility of this relationship is reinforced by the observation that the risk is amplified in women with recurrent preeclampsia, more severe forms, or early-onset disease.[8–11] While many hypotheses have been generated to explain this association, our understanding of the underlying mechanisms is quite limited. At the time of diagnosis, women with preeclampsia have profound disruption of the endothelium, activation of the coagulation cascade and systemic inflammation.[12] This disruption is manifested in abnormalities in markers of inflammation and renal function, such as high sensitivity C-reactive protein (hsCRP) and cystatin C at the time of diagnosis.[13–16]
HsCRP is an inflammatory marker that has been associated with increased risk of CVD, and has been incorporated into risk estimates for cardiovascular events, including the Reynolds Risk Score.[17,18] Cystatin C is a protease inhibitor used as an indicator of renal function and glomerular filtration rate. Elevated concentrations are also associated with cardiovascular disease and cardiovascular mortality.[19–21] Among women with preeclampsia, maternal levels of cystatin C are elevated in the first trimester and at the time of delivery. This has prompted investigation of cystatin C as a possible first trimester marker for subsequent development of preeclampsia.[22,23]
Women with a hypertensive disorder of pregnancy are at increased risk of chronic hypertension compared to women with normotensive pregnancies. Chronic hypertension is well recognized as a risk factor for CVD. The prevalence of chronic hypertension after a hypertensive pregnancy varies depending on multiple factors including maternal age, severity of the hypertensive disorder of pregnancy, and follow up time postpartum.[24,25] The American College of Cardiology (ACC) and the American Heart Association (AHA) recently revised the recommendations for the diagnosis of chronic hypertension in adults in the 2017 Task Force on Clinical Practice Guidelines.[26] Citing the strength of the evidence that incremental blood pressure increases impact the risk of clinical complications and death, the thresholds for the diagnosis of chronic hypertension were lowered to identify stage 1 hypertension as a systolic blood pressure between 130 mmHg and 139 mmHg or a diastolic blood pressure between 80 mmHg and 89 mmHg. However, these guidelines do not specifically address pregnancy or women with a history of hypertensive disorders of pregnancy. We sought to describe the rates of hypertension in overweight and obese women one year after delivery under the new ACC/AHA guidelines and to determine if pregnancy-associated hypertension is associated with an increased risk of abnormal biomarkers in the first year postpartum.
METHODS
The study population was derived from a cohort of women from the Prenatal Exposures & Preeclampsia Prevention (PEPP3) study, a prospective study of the impact of obesity on preeclampsia in women who received antepartum, delivery, and postpartum care at Magee-Womens Hospital of the University of Pittsburgh Medical Center. This study has been previously described in detail.[27] Women eligible for PEPP3 enrollment were 18–40 years of age and 6–16 weeks pregnant with a single fetus. Exclusion criteria included BMI <18 kg/m2, pre-existing hypertension, diabetes, seizure disorders, collagen vascular disorder, drug or alcohol abuse, and liver, heart, or kidney disease. Women were also excluded from participation after diagnosis of a major fetal anomaly or fetal demise. To examine mechanisms linking obesity to preeclampsia and gestational hypertension, overweight and obese women (BMI ≥ 25kg/m2) were preferentially recruited to comprise 85% of the study population; a small group of normal weight women were enrolled for comparison. As part of the study protocol, women were asked to attend a postpartum visit at least 3 months after delivery.
For this analysis, we included women who were overweight or obese before pregnancy (BMI≥25 kg/m2) who attended a postpartum study follow-up visit within one year postpartum, who had not become pregnant in the interim and completed a blood draw at the postpartum visit. We compared women with hypertension at the postpartum study visit, as defined by the 2017 American College of Cardiology (ACC) and the American Heart Association (AHA) Task Force on Clinical Practice Guidelines as systolic blood pressure ≥130mmHg or diastolic BP ≥80mmHg, on two measurements. Blood pressure measurements were taken by trained study staff using a standard research protocol. Briefly, patients were seated comfortably, with legs uncrossed after at least five minutes at rest. Aneroid sphygmomanometer instruments were used and multiple cuff sizes were available based on the size of the patient’s arm, which was measured at the midpoint of the upper arm. Two sitting blood pressures were obtained and if the first and second blood pressure differed by 10 or more mmHg, a third measurement was obtained. We excluded women who had a postpartum visit >12 months after delivery (n=50) as we sought to explore risk factors for hypertension in the first year postpartum. All women provided written informed consent and this study was approved by the University of Pittsburgh’s Institutional Review Board.
At enrollment (mean gestational age 8.9 weeks ± 2.6), participants completed a questionnaire which included demographic information as well as self-reported pre-pregnancy height and weight. Participants’ first study weight measure and self-reported pre-pregnancy weight were highly correlated (>0.97). Pre-pregnancy BMI (kg/m2) was calculated with self-reported weight and height and categorized based on World Health Organization (WHO) guidelines as normal weight (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), obese class I (BMI 30–34.9 kg/m2), class II (BMI 35 to 39.9 kg/m2) and class III (BMI ≥40 kg/m2). Postpartum BMI was based on height and weight measurement at the postpartum study visit and was categorized based on WHO guidelines. Delivery data abstracted from the electronic medical records of participants included gestational age at delivery, mode of delivery, and pregnancy complications including gestational diabetes, spontaneous or iatrogenic preterm delivery and pregnancy-associated hypertensive disorders. Gestational hypertension (two or more BP measurements ≥140/90 mmHg) and preeclampsia (gestational hypertension plus proteinuria) were defined based on American College of Obstetricians and Gynecologists (ACOG) guidelines in 2002 and adjudicated by the PEPP3 research team based on chart reviews.[28]
At the postpartum visit, demographic and clinical data were collected and participants completed a questionnaire. Fasting blood samples were collected from the participants at their postpartum visit. HsCRP was measured using 100µl of serum with reagents obtained from Beckman Coulter and analyzed on an Olympus AU400 Chemistry Analyzer from Olympus America, Inc at the Heinz Nutrition Lab at the University of Pittsburgh. The intra- and inter-assay coefficients of variation are 1.5% and 3.4%, respectively. Cystatin C was also measured turbidimetrically on an Olympus AU 400 in the same lab using 100µl of sample and reagents obtained from DakoCytomation N. America, Inc. The intra- and inter-assay coefficients of variation are 1.7% and 2.2%, respectively.
Statistical analyses were completed using Stata IC 15 software package (StataCorp LP, College Station, TX). Baseline characteristics were compared between women with hypertension and women who were normotensive at the postpartum study visit. Continuous variables were compared using Student’s t-tests and Wilcoxon-Mann Whitney tests as appropriate. Categorical variables were analyzed using Chi-square or Fisher’s exact, where appropriate. P-values <0.05 were considered statistically significant.
We examined the relationship between development of hypertension for women with (a) normotensive pregnancies, (b) preeclamptic pregnancies, or (c) gestational hypertensive pregnancies. We further explored the relationship of hypertensive disorders of pregnancy and abnormal biomarkers. We defined the most adverse cardiovascular profile as hypertension accompanied by both elevated hsCRP (hsCRP ≥3 mg/L)[29] and elevated cystatin C (in the highest quartile, ≥0.83 mg/L ). Moderate risk cardiovascular profiles were defined as hypertension with either elevated hsCRP or cystatin C. Multinomial logistic regression was used to adjust for potential confounding variables including maternal age, time since delivery, BMI at the postpartum visit and any breastfeeding as these varied by hypertension status or are known to be related to hsCRP or cystatin C. We performed sensitivity analyses restricting the sample to obese women only (BMI≥30 kg/m2) and in women followed up to 25 months postpartum to confirm that the observed relationships were consistent across these subgroups.
RESULTS
The PEPP3 cohort was comprised of 656 predominantly overweight or obese women. Of these, 439 attended the postpartum visit and 315 were included in this study (mean postpartum visit at 7.0 months, standard deviation (SD) 1.8 months postpartum). Women were excluded from this analysis if they had a pre-pregnancy BMI <25 kg/m2 (n=68), had a study visit >12 months postpartum (n=50) or did not have blood drawn or had missing data on pregnancy outcome (n=6) (Figure 1). Women who attended the postpartum visit were more likely to be of black race, lower income bracket, and to have Medicaid insurance compared with those who did not (Supplemental Table 1). Of the 315 women included in the study, 22 women had preeclampsia (7.0%) and 39 women had gestational hypertension (12.4%).
Figure 1.
Description of included cohort.
A total of 91 women had elevated blood pressure at the postpartum study visit (28.9%, 95%CI 24.3–34.4%). Of the women with elevated blood pressure, 81 women had stage 1 hypertension (89.0%) and 10 women had stage 2 hypertension (11.0%). Women with hypertension postpartum were older, had a higher pre-pregnancy and postpartum BMI, and underwent their study visits at a later time postpartum compared to normotensive women (Tables 1 and 2). There were no differences according to hypertensive status in race, education, insurance status, tobacco use or parity. Aside from hypertensive disorders of pregnancy, other pregnancy characteristics of the two groups were similar, with comparable rates of preterm birth and gestational diabetes and similar breastfeeding rates and duration. One patient reported taking anti-hypertensives at the postpartum study visit.
Table 1.
Cohort characteristics by presence of hypertension at postpartum study visit (n=315)
| Normotensive n=224 |
Hypertensive n=91 |
p-value | |
|---|---|---|---|
| Age (years) * | 23.5 ± 3.8 | 24.8 ± 4.7 | 0.03 |
| Race | |||
| Black | 157 (70.1) | 59 (64.5) | |
| White | 62 (27.7) | 29 (31.9) | 0.62 |
| Other | 5 (2.2) | 3 (3.3) | |
| Education | |||
| Less than High School | 15 (6.7) | 11 (12.1) | |
| High School or equivalent | 113 (50.7) | 46 (50.6) | 0.41 |
| College and above | 95 (42.4) | 34 (37.4) | |
| Insurance | |||
| Private | 24 (10.8) | 6 (6.6) | |
| Medicaid | 138 (62.2) | 58 (63.7) | 0.67 |
| None at enroll | 58 (26.1) | 27 (30.0) | |
| Smoking (in past 2 years) | 94 (42.2) | 39 (42.9) | 0.95 |
| Nulliparous | 170 (75.9) | 63 (69.2) | 0.22 |
| Pre-pregnancy BMI* | 32.3 ±5.4 | 38.0 ± 7.4 | <0.01 |
| Preterm Birth (<37wks) | 19 (8.5) | 13 (14.3) | 0.11 |
| Pregnancy-associated hypertension | 31 (13.8) | 30 (33.0) | <0.01 |
| Preeclampsia | 9 (4.0) | 13 (14.3) | <0.01 |
| Gestational hypertension | 22 (9.8) | 17 (18.9) | 0.03 |
| Gestational diabetes | 7 (3.1) | 4 (4.4) | 0.58 |
Mean ± standard deviation; BMI=body mass index
Table 2.
Postpartum visit characteristics by presence of hypertension at postpartum study visit (n=315)
| Normotensive | Hypertensive | ||
|---|---|---|---|
| p-value | |||
| n=224 | n=91 | ||
| Postpartum BMI* | 34.3 ± 7.1 | 41.3 ± 9.2 | <0.01 |
| Months Postpartum at Study Visit* | 6.9 ± 1.9 | 7.3 ±1.8 | 0.05 |
| Any breastfeeding | 141 (63.2) | 57 (63.3) | 1.0 |
| Months of breastfeeding* | 3.0 ± 1.8 | 3.2 ± 1.5 | 0.55 |
| hsCRP (mg/L)† | 3.7 (1.5–7.8) | 5.1 (2.9–11.6) | <0.01 |
| Cystatin C (mg/L)† | 0.77 (0.70–0.82) | 0.81 (0.74–0.87) | <0.01 |
Mean ± standard deviation
Median (interquartile range)
Women with pregnancy-associated hypertension were more likely to have hypertension at their postpartum study visit compared to women with a normotensive pregnancy (59.1%, 43.6%, and 24.0%, respectively; p<0.001). Among overweight and obese women with preterm preeclampsia, 80% (8/10) developed hypertension. After adjustment for maternal age, BMI, lactation and number of months postpartum, hypertensive disorders of pregnancy were associated with an aOR 1.86 (95%CI 1.37–2.52) of development of hypertension at one year in our cohort. Women with preeclampsia had an aOR 2.35 (95%CI 1.63–3.41) and women with gestational hypertension had an aOR 1.61 (95%CI 1.09–2.39) of development of hypertension at one year (Table 3).
Table 3.
Relative risk of progression to hypertension by 1 year postpartum in overweight and obese women (n=315).
| Unadjusted Model |
Multivariable model* |
|
|---|---|---|
| OR (95% CI) | aOR (95% CI) | |
| Normotensive pregnancy (n=254) | Referent | Referent |
| Any hypertensive disorder of pregnancy (n=61) | 2.05 (1.46–2.86) | 1.86 (1.37–2.52) |
| Gestational hypertension (n=39) | 1.82 (1.19–2.76) | 1.61 (1.09–2.39) |
| Preeclampsia (n=22) | 2.46 (1.63–3.71) | 2.35 (1.63–3.41) |
adjusted for maternal age, BMI, lactation, months postpartum
We then categorized women by the presence of abnormal biomarkers in the first year postpartum. A total of 190 women in our cohort (60.3%) had an abnormal hsCRP and 92 women (29.2%) had an abnormal cystatin C level. Preeclampsia was associated with an aOR 3.23 (95%CI 1.56–6.68) of the most adverse profile (hypertension, elevated hsCRP (≥3 mg/L) and cystatin C in the highest quartile) compared to women with normotensive pregnancies, adjusted for age, BMI and months postpartum. Similar findings were seen for the more moderate risk profiles (hypertension, elevated hsCRP (≥3 mg/L) or cystatin C in the highest quartile) in the first year postpartum. Gestational hypertension was associated with an aOR 2.03 (95%CI 1.07–3.85) of a moderate-risk profile at one year postpartum compared to women with normotensive pregnancies at one-year postpartum (Table 4).
Table 4.
Prevalence and multivariable model for prediction of various high-risk profiles at postpartum study visit in obese and overweight women (adjusted for maternal age, BMI, lactation, months postpartum).
| Normotensive Pregnancy |
Gestational hypertension | Gestational hypertension | |||
|---|---|---|---|---|---|
| Cardiovascular Profile | N=254 | N=39 | N=22 | ||
| n (%) | n(%) | aOR‡ (95%CI) | n(%) | aOR‡ (95%CI) | |
| Elevated hsCRP* | 146 (57.5%) | 27 (69.2%) | 1.11 (0.87–1.43) | 17 (77.3%) | 1.32 (1.03–1.69) |
| Elevated cystatin C† | 72 (28.4%) | 12 (30.8%) | 0.94 (0.58–1.52) | 8 (36.4%) | 1.20 (0.72–2.02) |
| Hypertensive with elevated hsCRP* and cystatin C† |
24 (9.4) | 3 (7.7) | 1.07 (0.40–2.85) | 6 (27.3) | 3.23 (1.56–6.68) |
| Hypertensive with either elevated hsCRP* or cystatin C† |
24 (9.4) | 10 (25.6) | 2.03 (1.07–3.85) | 5 (22.7) | 3.13 (1.44–6.78) |
Elevated hsCRP defined as hsCRP ≥ 3mg/L
Elevated cystatin C defined as cystatin C in highest quartile
Referent group is normotensive pregnancy
In sensitivity analyses, we performed the same analysis only in obese women (n=223, mean BMI 33.9 ± 6.6) and in all women with a postpartum study visit up to 25 months postpartum (n=365, mean 8.5±4.4 months) and found similar results (Supplemental Tables 2–5).
DISCUSSION
In this prospective cohort study of over 300 overweight and obese women, we report a high prevalence of hypertension as defined by the new ACC/AHA guidelines in the first year postpartum. While rates of hypertension within one year of delivery among overweight and obese women with normotensive pregnancies were quite high in our cohort (24.0%), this is consistent with recently published data, describing the prevalence of stage 1 hypertension among reproductive age women.[30] Further, rates of hypertension among overweight and obese women with preeclampsia were twice as high. Further, preeclampsia was also associated with an increased risk of abnormal biomarkers at one year postpartum compared to normotensive pregnancies. In our cohort, the number of overweight and obese women with preterm preeclampsia was small and yet 80% of them progressed to hypertension within a year, aligned with the seven-fold increased risk of later-life CVD that has been reported for this group.[31]
Tools for individualized risk estimates after a hypertensive disorder of pregnancy are currently lacking. Traditional risk prediction models for cardiovascular disease, such as the Framingham risk score, the ACC/AHA Pooled Cohort Equations, the Systematic Coronary Risk Evaluation (SCORE) and the QRISK score underestimate risk in young women.[32] The adverse cardiovascular profile variables we used in this analysis (chronic hypertension with elevated hsCRP and/or cystatin C) have not been previously studied in this population, however both hsCRP and cystatin C are associated with CVD risk and are currently being investigated for first-trimester prediction and late pregnancy diagnosis of preeclampsia. Given that preeclampsia is a multi-system disease with systemic inflammation and endothelial dysfunction in addition to hypertension, we sought to define adverse profiles that included not only sustained hypertension but also other subclinical biomarkers that may be disrupted by the disease process.
A strength of this study is the use of the new definitions of hypertension recently published by the ACC/AHA guidelines, including women with stage I hypertension, making it particularly relevant to current practice.[26] While the majority of women in this study with elevated blood pressure had stage 1 hypertension (89%), in addition to the long-term cardiovascular risk of stage 1 hypertension emphasized by the recently updated ACC/AHA guidelines,[26] our recent work suggests that among high-risk women, stage 1 hypertension is also associated with an increased risk of preeclampsia recurrence.[33] While we measured blood pressure using a standardized research protocol, we were limited by a single day of measurements, without additional time points or ambulatory blood pressure monitoring. A recent study by Benschop et al utilized ambulatory and in-office BP monitoring and found that 41.5% of women with severe preeclampsia had hypertension at one-year postpartum, with 17.5% of these cases only detected by APBM.[24] The rates of hypertension in the first year postpartum were slightly lower than the rates in our cohort, however, this study included mainly normal weight and overweight women with median BMI in their cohort of 25.5 (90% range 19.3–36.6) and did not use the new AHA/ACC definitions of hypertension, which may explain differences in findings. Additional strengths of our study include the prospective design, formal research adjudication of hypertensive disorders of pregnancy to ensure accurate distinction of preeclampsia and gestational hypertension, and the inclusion of biomarkers to characterize a high-risk profile.
This study had several limitations including the small number of participants with pregnancy-associated hypertension. As we are particularly interested in risk that can be identified in the first year postpartum, our follow up was limited to 12 months and thus we are unable to evaluate longer-term associations. In sensitivity analyses, we included participants followed up to 25 months postpartum (n=365) and demonstrated similar findings (Supplemental Tables 2, 3). We focused on hypertension developing in the first year postpartum as this is a time when women are more engaged with the healthcare system and may be a feasible time for transition of care and multidisciplinary follow up.[34] Our participants are from one study site, are all overweight and obese and are predominiantly African American. While this may limit the generalizability of these findings, this is a population who is at higher risk of hypertension, preeclampsia and cardiovascular disease.[35] We used the ACOG definitions of gestational hypertension and preeclampsia that were in place at the time of our study, however these definitions have since been updated.[28] The newer definition of preeclampsia now includes women without proteinuria who have other systemic findings consistent with preeclampsia.[36] This newer definition would likely include some women in our study who were classified as gestational hypertension. This study is also limited by our lack of information on traditional cardiovascular risk factors, such as family history and hyperlipidemia. Smoking status was not different between groups, and our results were unchanged when smoking status was incorporated into our model. Finally, there were significant differences in the severity of obesity between women who developed hypertension compared to those who were normotensive at one year postpartum, which we attempted to account for in our analysis. However, these groups are of particular importance given their excess burden of hypertension risk and the high prevalence of obesity in the United States.[37]
Cardiovascular disease (CVD) is the leading cause of death in women worldwide and despite declines in all other age groups, mortality rates from CVD are increasing in women aged 35 to 54 years.[3] The AHA and ACOG have identified hypertensive disorders of pregnancy as risk factors for later-life CVD with a magnitude of risk on par with smoking and diabetes. However, the mechanisms by which preeclampsia heralds an increased risk of CVD are still unclear. Whether pregnancy unmasks underlying subclinical CVD or whether preeclampsia causes vascular and metabolic damage that leads to future CVD is unknown. While ongoing efforts to understand mechanisms linking the two processes is imperative, attention also needs to be turned towards management of women with hypertensive disorders of pregnancy, as effective evidence-based interventions have not yet been adequately studied or implemented.[32,38] In addition, postpartum care after a hypertensive disorder of pregnancy is often fragmented, with no cohesive transition from the obstetrician to internist or cardiologist with few heath care providers carrying out postpartum CVD risk counseling or screening.[32] Nor is it clear when women should be screened for risk. Our results indicate that one-year postpartum might be too late to prevent development of hypertension for many overweight and obese women with hypertensive disorders of pregnancy. However, prior studies have shown that as many as 40% of women do not attend the traditional 6-week postpartum visit after delivery, underscoring the need for development of innovative models to care for these high-risk women.[39–41] Recognizing the underutilization of postpartum care, the American College of Obstetricians and Gynecologists recently updated its guidelines to reflect the importance of appropriate postpartum care and to propose a new paradigm for management in this period.[42] With the recommended changes in the scope of postpartum care and redefining this period as the “fourth trimester”, incorporation of innovative interventions for risk reduction in this population should be considered.
Because pregnancy occurs early in a woman’s life, often before the onset of clinically evident CVD, it serves as a unique opportunity for sex-specific screening to initiate primary prevention. If applied to this high-risk population early enough to avert cumulative damage of chronic disease, primary prevention may reduce cardiovascular disease incidence. Risk reduction in this group should focus on lifestyle interventions to reduce blood pressure and increase weight loss, which have been shown to impact future pregnancy outcomes as well as future CVD.[36,43]
CONCLUSIONS
In summary, our study demonstrates high rates of sustained hypertension as well as an adverse cardiometabolic profile associated with hypertensive disorders of pregnancy among overweight and obese women in the first year postpartum. To meaningfully impact rates of CVD in women, we may need to shift our focus towards structured postpartum follow up for these women and begin identifying interventions to reduce this risk.
Supplementary Material
Figure 2.
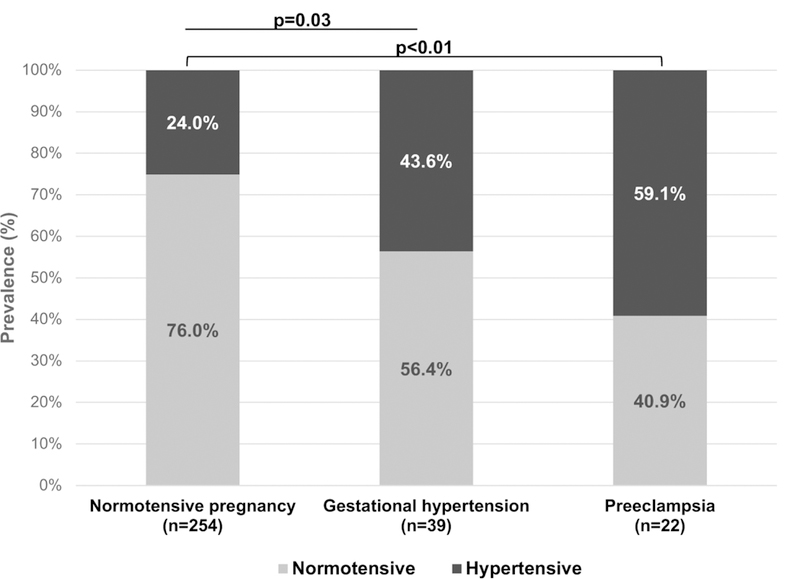
Proportion of women with hypertension at postpartum study visit by pregnancy outcome.
Highlights:
We demonstrate a high prevalence of development of sustained hypertension and abnormal biomarkers associated with hypertensive disorders of pregnancy among overweight and obese women as early as one year postpartum.
In line with the updated Committee Opinion from ACOG recommending changes in the scope of postpartum care and redefining this period as the “fourth trimester”, our findings suggest that incorporation of innovative interventions for risk reduction in this population should be considered.
Acknowledgments
SOURCE OF FUNDING: The PEPP3 study was funded by NICHD [P01 HD 30367] and University of Pittsburgh Clinical and Translational Research Center [UL1 TR000005]. This work is supported by the American Heart Association Go Red for Women Strategic Focused Research Network Grant [AHA16SFRN27810001] and [16SFRN28340000]
Footnotes
CONFLICTS OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, Ben-Ami Shor D, Tzur D, Afek A, Shamiss A, Haklai Z, Kark JD, Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood., N. Engl. J. Med 374 (2016) 2430–40. 10.1056/NEJMoa1503840. [DOI] [PubMed] [Google Scholar]
- [2].Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen W-K, Smith SC, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Society, 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society., Circulation 129 (2014) S102–38. 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Heart Disease and Stroke Statistics—2015 Update, Circulation 131 (2015) e29–e322. 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- [4].Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA, Preeclampsia and future cardiovascular health, Circ. Cardiovasc. Qual. Outcomes 10 (2017). 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- [5].Bellamy L, Casas J-P, Hingorani AD, Williams DJ, Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis, Bmj 335 (2007) 974–974. 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ, Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses, Am. Heart J 156 (2008) 918–930. 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- [7].Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P, Adverse pregnancy outcomes and future maternal cardiovascular disease, Clin. Cardiol (2018). 10.1002/clc.22887. [DOI] [PMC free article] [PubMed]
- [8].Ray JG, Booth GL, Alter DA, Vermeulen MJ, Prognosis after maternal placental events and revascularization: PAMPER study, Am. J. Obstet. Gynecol 214 (2016) 106. 10.1016/j.ajog.2015.08.021. [DOI] [PubMed] [Google Scholar]
- [9].Auger N, Fraser WD, Schnitzer M, Leduc L, Healy-Profitós J, Paradis G, Recurrent pre-eclampsia and subsequent cardiovascular risk, Heart 103 (2017) 235–243. 10.1136/heartjnl-2016-309671. [DOI] [PubMed] [Google Scholar]
- [10].Lykke JA, Langhoff-Roos J, Lockwood CJ, Triche EW, Paidas MJ, Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery, Paediatr. Perinat. Epidemiol 24 (2010) 323–330. 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- [11].Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ, Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother, Hypertension 53 (2009) 944–951. 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- [12].Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD, Chesley’s Hypertensive Disorders in Pregnancy, Fourth Edi, Elsevier, 2015. [Google Scholar]
- [13].Qiu C, Luthy DA, Zhang C, Walsh SW, Leisenring WM, Williams MA, A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia., Am. J. Hypertens 17 (2004) 154–60. http://www.ncbi.nlm.nih.gov/pubmed/14751658 (accessed February 21, 2018). [DOI] [PubMed] [Google Scholar]
- [14].Rebelo F, Schlüssel MM, Vaz JS, Franco-Sena AB, Pinto TJP, Bastos FI, Adegboye ARA, Kac G, C-reactive protein and later preeclampsia: systematic review and meta-analysis taking into account the weight status., J. Hypertens 31 (2013) 16–26. 10.1097/HJH.0b013e32835b0556. [DOI] [PubMed] [Google Scholar]
- [15].Lafayette RA, Druzin M, Sibley R, Derby G, Malik T, Huie P, Polhemus C, Deen WM, Myers BD, Nature of glomerular dysfunction in pre-eclampsia., Kidney Int 54 (1998) 1240–9. 10.1046/j.1523-1755.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- [16].Moran P, Baylis PH, Lindheimer MD, Davison JM, Glomerular ultrafiltration in normal and preeclamptic pregnancy., J. Am. Soc. Nephrol 14 (2003) 648–52. http://www.ncbi.nlm.nih.gov/pubmed/12595500 (accessed February 21, 2018). [DOI] [PubMed] [Google Scholar]
- [17].Ridker PM, Rifai N, Rose L, Buring JE, Cook NR, Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events., N. Engl. J. Med 347 (2002) 1557–65. 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- [18].Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Trial Study Group, Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial., Lancet (London, England) 373 (2009) 1175–82. 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- [19].Luo J, Wang L-P, Hu H-F, Zhang L, Li Y-L, Ai L-M, Mu H-Y, Wang Kun, Cystatin C and cardiovascular or all-cause mortality risk in the general population: A meta-analysis, Clin. Chim. Acta 450 (2015) 39–45. 10.1016/j.cca.2015.07.016. [DOI] [PubMed] [Google Scholar]
- [20].T.H.S. Dent, Predicting the risk of coronary heart disease. II: the role of novel molecular biomarkers and genetics in estimating risk, and the future of risk prediction., Atherosclerosis 213 (2010) 352–62. 10.1016/j.atherosclerosis.2010.06.021. [DOI] [PubMed] [Google Scholar]
- [21].van Holten TC, Waanders LF, de Groot PG, Vissers J, Hoefer IE, Pasterkamp G, Prins MWJ, Roest M, Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses., PLoS One 8 (2013) e62080 10.1371/journal.pone.0062080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang X, Wang H, Wang Z, Dong M, Alteration and significance of serum cardiac troponin I and cystatin C in preeclampsia., Clin. Chim. Acta 374 (2006) 168–9. 10.1016/j.cca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- [23].Strevens H, Wide-Swensson D, Grubb A, Serum cystatin C is a better marker for preeclampsia than serum creatinine or serum urate., Scand. J. Clin. Lab. Invest 61 (2001) 575–80. http://www.ncbi.nlm.nih.gov/pubmed/11763416 (accessed February 24, 2018). [DOI] [PubMed] [Google Scholar]
- [24].Benschop L, Duvekot JJ, Versmissen J, van Broekhoven V, Steegers EAP, Roeters van Lennep JE, Blood Pressure Profile 1 Year After Severe Preeclampsia, Hypertension 71 (2018) 10.1161/HYPERTENSIONAHA.117.10338. [DOI] [PubMed] [Google Scholar]
- [25].Berks D, Steegers EAP, Molas M, Visser W, Resolution of hypertension and proteinuria after preeclampsia., Obstet. Gynecol 114 (2009) 1307–14. 10.1097/AOG.0b013e3181c14e3e. [DOI] [PubMed] [Google Scholar]
- [26].Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT, 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Hypertension 71 (2018) e13–e115. 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- [27].Sween LK, Althouse AD, Roberts JM, Early-pregnancy percent body fat in relation to preeclampsia risk in obese women., Am. J. Obstet. Gynecol 212 (2015) 84. 10.1016/j.ajog.2014.07.055. [DOI] [PubMed] [Google Scholar]
- [28].ACOG Committee on Practice Bulletins--Obstetrics, ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002., Obstet. Gynecol 99 (2002) 159–67. http://www.ncbi.nlm.nih.gov/pubmed/16175681 (accessed February 24, 2018). [DOI] [PubMed] [Google Scholar]
- [29].Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention, American Heart Association, Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association., Circulation 107 (2003) 499–511. http://www.ncbi.nlm.nih.gov/pubmed/12551878. [DOI] [PubMed] [Google Scholar]
- [30].Smith G, Pudwell J, Saade G, Impact of the New American Hypertension Guidelines on the Prevalence of Postpartum Hypertension, Am. J. Perinatol (2018). 10.1055/s-0038-1669441. [DOI] [PubMed]
- [31].Riise HKR, Sulo G, Tell GS, Igland J, Nygård O, Vollset SE, Iversen AC, Austgulen R, Daltveit AK, Incident coronary heart disease after Preeclampsia: Role of reduced fetal growth, preterm delivery, and parity, J. Am. Heart Assoc 6 (2017). 10.1161/JAHA.116.004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Spaan J, Peeters L, Spaanderman M, Brown M, Cardiovascular Risk Management After a Hypertensive Disorder of Pregnancy, Hypertension 60 (2012) 1368–1373. 10.1161/HYPERTENSIONAHA.112.198812. [DOI] [PubMed] [Google Scholar]
- [33].Hauspurg A, Sutton EF, Catov JM, Caritis SN, Aspirin Effect on Adverse Pregnancy Outcomes Associated With Stage 1 Hypertension in a High-Risk Cohort., Hypertens. (Dallas, Tex. 1979) 72 (2018) 202–207. 10.1161/HYPERTENSIONAHA.118.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith GN, The Maternal Health Clinic: Improving women’s cardiovascular health, Semin. Perinatol 39 (2015) 316–319. 10.1053/j.semperi.2015.05.012. [DOI] [PubMed] [Google Scholar]
- [35].Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, Yancy CW, American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and, Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association., Circulation 136 (2017) e393–e423. 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- [36].Hypertension in Pregnancy, Obstet. Gynecol 122 (2013) 1122–1131. 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- [37].Ogden CL, Carroll MD, Kit BK, Flegal KM, Prevalence of Childhood and Adult Obesity in the United States, 2011–2012, JAMA 311 (2014) 806 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Graves CR, Davis SF, Cardiovascular Complications in Pregnancy: It Is Time for Action., Circulation (2018) 10.1161/CIRCULATIONAHA.117.031592. [DOI] [PubMed]
- [39].Bennett WL, Chang H-Y, Levine DM, Wang L, Neale D, Werner EF, Clark JM, Utilization of primary and obstetric care after medically complicated pregnancies: an analysis of medical claims data., J. Gen. Intern. Med 29 (2014) 636–45. 10.1007/s11606-013-2744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wilcox A, Levi EE, Garrett JM, Predictors of Non-Attendance to the Postpartum Follow-up Visit, Matern. Child Health J 20 (2016) 22–27. 10.1007/s10995-016-2184-9. [DOI] [PubMed] [Google Scholar]
- [41].Himes KP, Donovan H, Wang S, Weaver C, Grove JR, Facco FL, Healthy Beyond Pregnancy, a Web-Based Intervention to Improve Adherence to Postpartum Care: Randomized Controlled Feasibility Trial, JMIR Hum. Factors 4 (2017) e26 10.2196/humanfactors.7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].ACOG Committee Opinion No. 736, Obstet. Gynecol 131 (2018) e140–e150. 10.1097/AOG.0000000000002633. [DOI] [PubMed] [Google Scholar]
- [43].Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK, Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women−−2011 Update: A Guideline From the American Heart Association, Circulation 123 (2011) 1243–1262. 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


