Abstract
Objectives:
Urinary biomarkers of kidney injury may have potential to identify subclinical injury attributable to tenofovir disoproxil fumarate (TDF) toxicity.
Design:
This observational study included 198 HIV-infected participants from the Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study, who initiated TDF between 2009 and 2015 and had urine samples collected at baseline before and after TDF initiation.
Methods:
We used linear mixed effects models controlling for urine creatinine and time on TDF to evaluate the effects of TDF initiation on changes in fourteen urinary biomarkers.
Results:
Within 1 year after TDF initiation, concentrations of trefoil factor 3 (+78%; 95% CI: +38%, +129%), alpha-1 microglobulin (α1m) (+32%; 95% CI: +13%, 55%), clusterin (+21%; 95% CI: +6%, +38%), uromodulin (+19%; 95% CI: +4, +36%), and kidney injury molecule-1 (KIM-1) (+13%; 95% CI: +1%, +26%) significantly increased, whereas interleukin-18 (IL-18) significantly decreased (−13%, 95% CI: −7%, −25%). Subsequent to the first year of TDF use, biomarker concentrations stabilized, and these changes were not statistically significant. When stratifying by baseline viremia (HIV-1 RNA < vs. ≥ 80 copies/mL), concentration changes for most biomarkers during the first year of TDF use were greater among aviremic versus viremic participants, with significant differences in α1m (+80% vs. +22%), KIM-1 (+43% vs. +10%), beta-2 microglobulin (+83% vs. −10%), YKL-40 (+33% vs. −5%), and IL-18 (+20% vs. −27%).
Conclusions:
TDF initiation was associated with substantial changes in urinary biomarkers of kidney injury within the first year of use, particularly among aviremic participants. A urinary biomarker panel may be a clinically useful tool to detect and monitor the heterogeneous effects of TDF on the kidney.
Keywords: tenofovir disproxil fumarate (TDF), nephrotoxicity, HIV, biomarkers, kidney
Introduction
Tenofovir disoproxil fumarate (TDF) is widely utilized as part of first-line antiretroviral therapy (ART) for HIV treatment regimens worldwide.[1] While early trials reported a favorable safety profile, TDF is now recognized to be associated with increased risk of acute and chronic kidney disease.[2] However, serum creatinine and urine protein, the clinical standards for assessing kidney disease in HIV-infected persons,[3] are crude and insensitive measures of kidney damage that are not specific to etiology. This presents significant challenges to effective surveillance of TDF-associated nephrotoxicity, which is often detected late in the disease course when injury may be irreversible and cannot be distinguished from the numerous other kidney disease risk factors in the HIV-infected population.[4, 5]
Studies in the general population and in HIV-infected persons have shown that urinary biomarkers of kidney tubule injury can be useful for detecting early stages of kidney disease and predicting the onset of CKD and its complications. Because TDF toxicity is known to predominantly involve the proximal renal tubules,[6, 7] biomarkers of tubular pathology have the potential to detect subclinical kidney injury attributable to TDF[8] and would be more sensitive and specific than current indirect markers of proximal tubular dysfunction, such as fractional excretion of phosphate, uric acid, and glycosuria.[9] To date, however, only a limited number of biomarkers of kidney injury have been investigated in HIV-infected TDF-users, which have largely been in cross-sectional studies.[10-18]
Using stored urine samples from HIV-infected participants in the Multicenter AIDS Cohort Study (MACS) and Women Interagency HIV Study (WIHS) collected before and after TDF initiation, we conducted a longitudinal, observational study of new users of TDF. Our objective was to evaluate the association of TDF initiation with a panel of fourteen urinary biomarkers that is anatomically representative of the nephron and mechanistically diverse. Specifically, this panel included biomarkers of glomerular injury (albumin-creatinine ratio, osteopontin), proximal tubular injury (trefoil factor 3, clusterin, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, interleukin-18), tubular fibrosis and repair (monocyte chemoattractant protein-1, epidermal growth factor, chitinase-3-like protein-1), proximal tubular dysfunction (cystatin C, α1-microglobulin, β2-microglobulin), and loop of Henle function (uromodulin). We hypothesized that TDF initiation would be associated with higher concentrations of kidney injury biomarkers, particularly those associated with proximal tubular pathology.
Materials and Methods
Study Design and Population
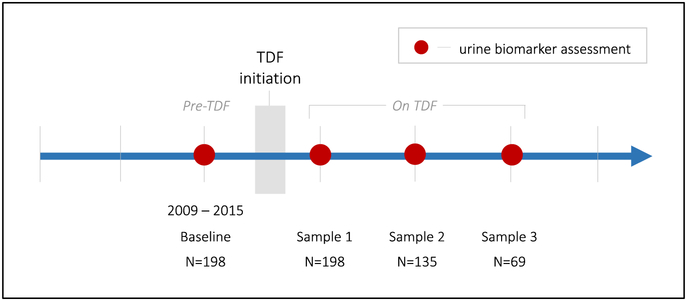
This study included 198 HIV-infected, initially TDF-naïve participants from MACS (n=87) and WIHS (n=111), who had a urine sample collected before TDF initiation (“baseline”) and at least one sample collected after TDF initiation while continuing to use TDF (Figure 1). Participants contributed a median of 3 samples (interquartile range [IQR]: 2-4). The MACS is an ongoing, prospective cohort study established in 1984 to describe the natural history of HIV infection among men who have sex with men.[19] Participants were enrolled from four sites in the United States: Baltimore, MD; Chicago, IL; Los Angeles, CA; and Pittsburgh, PA. The WIHS is a multicenter, prospective cohort study established in 1993 to investigate the progression of HIV in women with and at risk for HIV in the United States.[20, 21] Women were enrolled from eleven sites in the United States: Bronx, NY; Brooklyn, NY; Chicago, IL; Los Angeles, CA; San Francisco, CA; and Washington, DC (enrolled between 1994-1995, 2001-2002, and 2011-2012); and Atlanta, GA; Birmingham, AL; Jackson, MS, Chapel Hill, NC; and Miami, FL (enrolled between 2013-2015). The institutional review boards of participating institutions approved the study protocol, which was adherent to the Declaration of Helsinki.
Figure 1. Study design schematic of new TDF-users.
Study design schematic of simulated single-arm clinical trial of TDF initiation among HIV-infected men and women. Gray box denotes time of initiation of TDF-containing regimen. Red dots represent urine collections, which occurred at Women Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS) annual visits.
Exposure
ART use was ascertained for all participants at each semi-annual study visit. For each participant included in this analysis, a “pre-TDF” baseline sample collected between 2009 and 2015 was identified, and “on TDF” samples were identified at subsequent study visits. Median time from pre-TDF urine collection to TDF initiation was 0.99 years (IQR: 0.89-1.18), median time of TDF exposure at the first post-TDF initiation urine collection was 1.0 years (IQR: 0.9-1.4), and median follow-up time from the baseline sample was 2.9 years (range 0.9-4.7).
Urinary Biomarker Outcomes
The outcomes were changes in concentrations of fourteen urinary biomarkers: trefoil factor 3 (TFF3), alpha-1 microglobulin (α1m), clusterin, uromodulin (UMOD), kidney injury molecule-1 (KIM-1), beta-2 microglobulin (β2M), albumin-creatinine ratio (ACR), neutrophil gelatinase-associated lipocalin (NGAL), anti-chitinase-3-like protein 1 (YKL-40), monocyte chemoattractant protein-1 (MCP-1), cystatin C (CysC), osteoponin (OPN), epidermal growth factor (EGF), and interleukin-18 (IL-18). This biomarker panel included all novel biomarkers approved by the Food and Drug Administration (FDA), European Medicines Agency (EMA), and the Japanese Pharmaceutical and Medical Devices Agency (PMDA) for use in pre-clinical trials of drug-induced kidney toxicity.[22-24]
Urinary biomarkers were measured at the University of Vermont Laboratory for Clinical Biochemistry Research. Most biomarkers were measured using multiplex Meso Scale Discovery immunoassay kits (Meso Scale Diagnostics, LLC, Gaithersburg, MD), except for α1m, which was measured using the BN II Nephelometer assay (Siemens, Newark, DE). Urine creatinine, which was used to account for urine sample tonicity, was measured using a Cobas c311 clinical analyzer (Roche Diagnostics, Indianapolis, IN). Details regarding assay ranges, sensitivities, and coefficients of variation are shown in Supplementary Table 1. All urine specimens were in continuous storage without previous freeze-thaw until measurement. Laboratory personnel were blinded to clinical information about the participants, and specimens were evaluated in random order.
Statistical Analyses
We first compared within-participant characteristics before initiating TDF to those at the first post-TDF initiation urine measurement using Wilcoxon signed-rank test and McNemar’s test for continuous and categorical variables, respectively. Diabetes mellitus was defined as a fasting glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5%, or self-reported history of diabetes and diabetes medication use; and hepatitis C virus (HCV) infection was defined by either detectable HCV RNA and/or positive HCV antibody result. eGFR was calculated using the 2009 CKD Epidemiology Collaboration (CKD-EPI) creatinine equation.23
To examine the association of TDF initiation with changes in biomarker concentrations, we constructed separate linear mixed models for each biomarker, adjusting for urine creatinine and time on TDF. We used random intercepts and slopes for time on TDF and a linear spline for time on TDF with inflection point at 1 year. Given their right-skewed distributions, biomarker concentrations were log-transformed, and results were back transformed to produce estimated annual percentage changes. The detectable limit of the α1m assay was 0.5 mg/dL, and approximately 15% of urine α1m values were undetectable. Thus, we used a left-censored linear mixed model to estimate the change in α1m concentration for each participant and to impute values that were below the limit of detection. We then examined Spearman correlations among first-year biomarker changes and presented the results in a heat map for ease of comprehension.
Next, we compared biomarker concentration changes by baseline plasma viremia (detectable vs. undetectable). Undetectable viral load was defined as HIV-1 RNA < 80 copies/mL due to the assays in use during the study period. Given that viral load has been shown to be associated with kidney function decline,[25] we hypothesized that there would be differing impacts of TDF in aviremic participants (direct effects of TDF toxicity) vs. viremic participants (effects of both TDF toxicity and benefits of viral suppression of baseline viremia). Models were constructed separately for each biomarker, controlling for baseline viral load status, time on TDF, interaction by baseline viral load status, and urine creatinine. We estimated absolute biomarker concentrations at baseline and at 1 year after TDF initiation by baseline viremia status, using marginal means from linear mixed models, since samples were not collected at exact yearly intervals. Finally, we compared biomarker concentration changes by both ritonavir (RTV) and atazanavir (ATZ) usage (users vs. non-users, time-updated at each visit), as concurrent use of these medications has been associated with greater kidney function decline[26-28]; and by African American race, given the increased risk of kidney disease in this population.[29]
Although this study involved several biomarkers, we did not include formal adjustments for multiple comparisons as we hypothesized that TDF-associated biomarker changes would show a biologically coherent pattern. In particular, we hypothesized that the nephrotoxic effects of TDF would be reflected by increases in concentrations of all biomarkers with the exceptions of EGF[30] and UMOD,[31, 32] which have been shown to be protective. This biologically coherent pattern dictates that results should be mutually reinforcing, rather than a series of independent tests; therefore, formal multiple comparisons adjustments, such as the Bonferroni method, would not be appropriate.[33]
Results
Among the 198 HIV-infected participants in this study, the median age at baseline (pre-TDF) was 48 years, 56% were female, and approximately two-thirds were African American. The prevalence of kidney disease risk factors, including hypertension, diabetes, and Hepatitis C virus infection, did not significantly differ between the pre-TDF and first post-TDF time points (Table 1). After initiating TDF for a median of 1.0 year (IQR: 0.9-1.4), participants had significantly higher CD4 counts and serum albumin levels and a significantly greater proportion were aviremic. In addition, eGFR significantly decreased during the first year of TDF use. We initially compared participants included in our study based on the inclusion criteria of new TDF users with stored urine samples vs. participants who did not meet these criteria. Included participants were older with a smaller proportion having undetectable viral load and using antiretroviral treatments prior to their initiation of TDF. Baseline eGFR and CD4 counts were similar between included and excluded participants.
Table 1.
Summary of demographic and clinical characteristics of HIV-infected participants, before and after TDF initiation
| Pre-TDF (n=198) |
On TDF (~ year 1)* (n=198) |
P-value | |
|---|---|---|---|
| Age, years | 48 (41, 54) | 49 (42, 56) | — |
| Female | 111 (56%) | — | |
| Race | |||
| African American | 126 (64%) | ||
| White | 59 (30%) | — | |
| Other | 13 (7%) | ||
| Smoking | |||
| Current | 73 (37%) | 70 (35%) | 0.83 |
| Past | 62 (31%) | 66 (33%) | |
| Never | 62 (31%) | 62 (31%) | |
| Diabetes mellitus | 32 (17%) | 32 (17%) | 1.00 |
| Systolic BP, mmHg | 126 (114, 137) | 122 (113, 135) | 0.18 |
| Diastolic BP, mmHg | 77 (71, 86) | 77 (71, 85) | 0.47 |
| Antihypertensive use | 70 (35%) | 77 (39%) | 0.14 |
| History of CVD | 13 (7%) | 16 (8%) | 0.08 |
| Hepatitis C virus-infected | 33 (17%) | 34 (17%) | 0.32 |
| Serum creatinine, mg/dL | 0.85 (0.72, 0.95) | 0.91 (0.79, 1.0) | <0.001 |
| eGFR, ml/min/1.73m2 | 103 (88, 116) | 95 (79, 111) | <0.001 |
| LDL, mg/dL | 101 (79, 121) | 97 (71, 121) | 0.29 |
| HDL, mg/dL | 46 (38, 57) | 47 (38, 57) | 0.35 |
| TG, mg/dL | 113 (79, 172) | 114 (80, 167) | 0.95 |
| Serum albumin, g/dL | 4.2 (3.8, 4.4) | 4.3 (4.0, 4.5) | 0.01 |
| Current CD4, cells/mm3 | 483 (338, 682) | 587 (416, 743) | 0.003 |
| Nadir CD4, cells/mm3 | 347 (223, 471) | 340 (215, 458) | 0.68 |
| HIV RNA < 80 copies/mL | 56 (29%) | 162 (82%) | <0.001 |
| ART use | 79 (40%) | 198 (100%) | <0.001 |
| BMI, kg/m2 | 27 (23, 32) | 28 (24, 33) | 0.51 |
| Waist Circ., cm | 94 (83, 104) | 97 (85, 107) | 0.19 |
Data are presented as Median (IQR) or numbers (percent). P-values testing within-subject changes from baseline from Wilcoxon signed-rank test or McNemar’s test.
On TDF represents study visit corresponding to biomarker measurement closest to time at which participant had reached 1 year of TDF exposure. BP = blood pressure; eGFR = estimated glomerular filtration rate (CKD-EPI); LDL = low-density lipoprotein; HDL = high-density lipoprotein; TG = triglycerides; CVD = cardiovascular disease; BMI = body mass index; circ = circumference; ART = antiretroviral therapy.
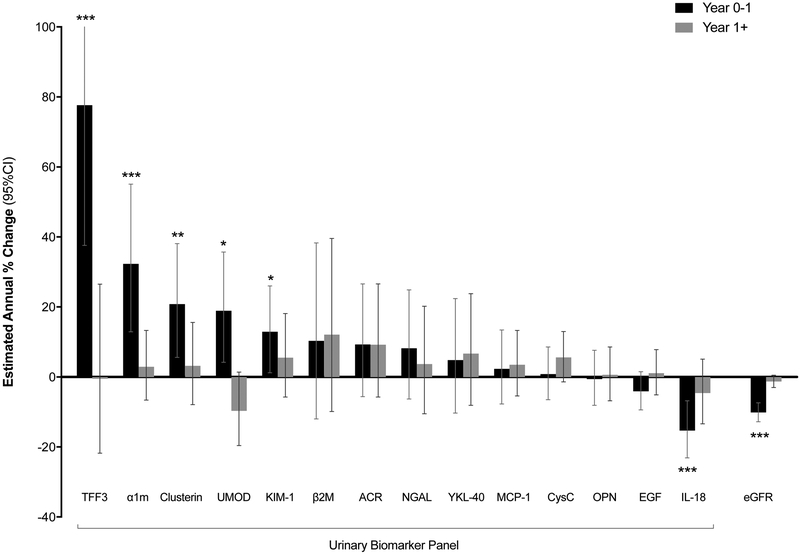
We first estimated the annual percent change in concentrations of the fourteen biomarkers after TDF initiation (Figure 2, Supplementary Table 2). During the first year after TDF initiation, we observed large changes in several urine biomarkers: TFF3 (+78%, 95% CI: +38%, +129%), α1m (+32%; 95% CI: +13%, +55%), clusterin (+21%; 95% CI: +6%, +38%), UMOD (+19%; 95% CI: +4%, +36%), and KIM-1 (+13%; 95% CI: +1%, +26%) concentrations significantly increased; β2M, ACR, NGAL, YKL-40, MCP-1, Cys C, OPN, and EGF concentrations showed smaller, non-statistically significant changes; and IL-18 (−13%; 95% CI: −7%, −25%) concentrations significantly decreased. These biomarker changes coincided with significant rises in serum creatinine (+10%; 95% CI: +7%, +13%) and declines in eGFR (−10%; 95% CI: −7%, −13%) within the first year. Subsequent to the first year of TDF use, annual changes in biomarker concentrations were substantially smaller and did not reach statistical significance. While the reduced number of urine samples after 1 year may have limited the power to detect differences, we found that changes in serum creatinine and eGFR after 1 year were also much smaller and did not reach statistical significance.
Figure 2. Association of TDF initiation with changes in urinary biomarker concentrations.
The black bars (“year 0-1”) denote the relative percent changes in concentrations of each biomarker that occurred during the first year of TDF use. The white bars (“year 1+”) denote the annual percent changes in concentrations of each biomarker that occurred after year 1. Error bars denote the 95% confidence intervals (CIs). Estimated annual percent changes were calculated from separate linear mixed models using all 198 participants, controlling for time on TDF (using linear spline with cutpoint at year 1) and urine creatinine. The y-axis is truncated at 100%. The 95% CI upper bound for change of TFF3 is truncated and extends to 129.1%. * p < 0.05. ** p < 0.01. *** p < 0.001. Numeric values of percent changes for each biomarker, 95% CI, and p-values are presented in Supplementary Table 2. Full names for each biomarker are as follows: trefoil factor 3 (TFF3), α1-microglobulin (α1m), clusterin, uromodulin (UMOD), kidney injury molecule-1 (KIM-1), β2-microglobulin (β2M), albumin-creatinine ratio (ACR), neutrophil gelatinase-associated lipocalin (NGAL), anti-chitinase-3-like protein 1 (YKL-40), monocyte chemoattractant protein-1 (MCP-1), cystatin C (CysC), osteoponin (OPN), epidermal growth factor (EGF), and interleukin-18 (IL-18). eGFR = estimated glomerular filtration rate.
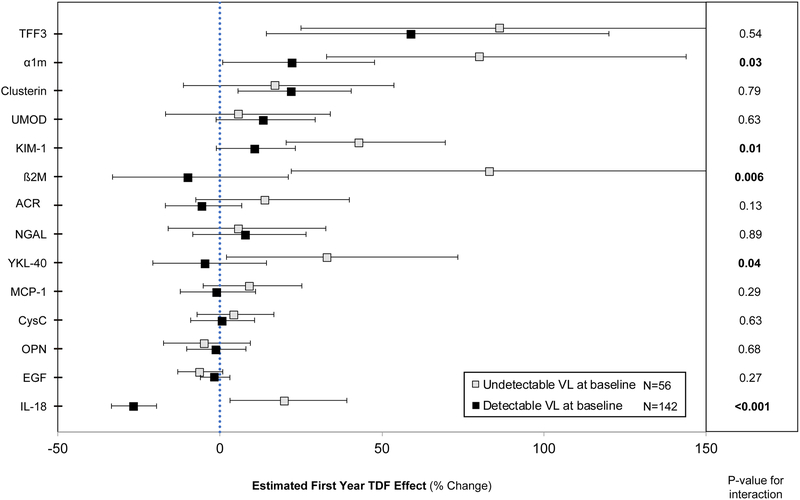
To distinguish the direct effects of TDF from those influenced by HIV viral suppression, we estimated first-year biomarker concentration changes for each biomarker, stratified by baseline viral load (undetectable vs. detectable) (Figure 3, Supplementary Table 3). For multiple biomarkers, viremic participants on average had attenuated increases or even decreases in biomarker concentrations, compared with aviremic participants. In particular, tests for interaction demonstrated statistically significant differences between viremic and aviremic participants for α1m, KIM-1, β2M, YKL-40, and IL-18. Notably, IL-18 concentrations significantly decreased from baseline among viremic participants (−27%; 95% CI: −20%, −33%) and significantly increased from baseline among aviremic participants (+20%; 95% CI: +3%, +39%). In addition, the magnitude of changes in biomarker concentrations from baseline was greater in the stratified analyses compared to the non-stratified analyses. In contrast, first-year eGFR declines did not differ by baseline viremia.
Figure 3. Association of TDF initiation with relative first-year changes in urine biomarker concentrations, stratified by baseline HIV RNA detectable status.
The unfilled boxes denote participants with undetectable baseline viral load (VL) (HIV RNA < 80 copies/ mL), N=56. The filled boxes denote participants with detectable baseline viral load (HIV RNA ≥ 80 copies/ mL), N=142. Estimates denote the relative percent changes in concentrations of each biomarker during the first year of TDF use. P-values are calculated from tests of time by viral load interaction for each marker. Error bars denote the 95% confidence intervals (CIs). The x-axis is truncated at 150%. The 95% CI upper bounds for changes in TFF3 and β2M in participants with baseline undetectable viral load are truncated and extend to 177.6% and 174.7%, respectively. Estimates were calculated from separate linear mixed models, controlling for baseline viral load status (HIV RNA detectable vs. undetectable), time on TDF (using linear spline with cutpoint at year 1), interaction by baseline viral load, and urine creatinine. Numeric values of percent changes for each biomarker, 95% CI, and p-values for interaction are presented in Supplementary Table 3. Full names for each biomarker are as follows: trefoil factor 3 (TFF3), α1-microglobulin (α1m), clusterin, uromodulin (UMOD), kidney injury molecule-1 (KIM-1), β2-microglobulin (β2M), albumin-creatinine ratio (ACR), neutrophil gelatinase-associated lipocalin (NGAL), anti-chitinase-3-like protein 1 (YKL-40), monocyte chemoattractant protein-1 (MCP-1), cystatin C (CysC), osteoponin (OPN), epidermal growth factor (EGF), and interleukin-18 (IL-18). eGFR = estimated glomerular filtration rate.
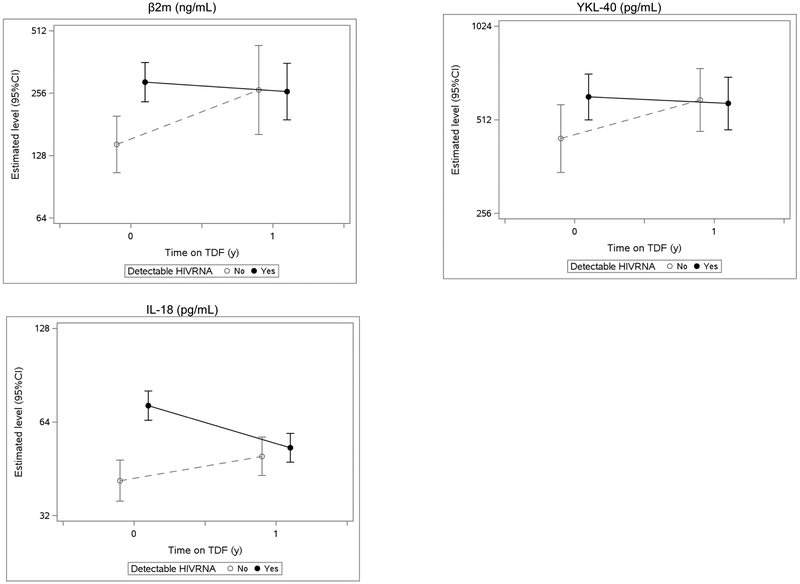
We next compared absolute biomarker concentrations at baseline and 1 year after TDF initiation, stratified by baseline viral load status. On average, most biomarker concentrations at baseline were higher among viremic participants, with statistically significant differences only for β2M, YKL-40, and IL-18 (Supplementary Table 4). After 1 year of TDF use, the absolute concentrations of most biomarkers, including β2M, YKL-40, and IL-18, converged between the subgroups by baseline viremia (Figure 4).
Figure 4. Estimated absolute biomarker concentrations before and after TDF initiation, stratified by baseline HIV RNA detectable status.
Estimated absolute biomarker concentrations at baseline (year 0) and year 1 are depicted for the three biomarker concentrations that significantly differed at baseline in viral load-stratified analyses. At baseline, β2M, YKL-40, and IL-18 levels were higher in participants with undetectable baseline viral loads (HIV RNA < 80 copies/ mL) relative to those with detectable viral loads (HIV RNA ≥ 80 copies/ mL). Marginal mean concentrations were calculated from separate linear mixed models, controlling for baseline viral load status (HIV RNA detectable vs. undetectable), time on TDF (using linear spline with cutpoint at year 1), interaction by baseline viral load, and urine creatinine. Full names for biomarkers above are as follows: β2-microglobulin (β2M), anti-chitinase-3-like protein 1 (YKL-40), and interleukin-18 (IL-18).
Finally, we evaluated for additional effect modifiers of the association of TDF initiation and first-year biomarker changes. We evaluated the impact of concurrent use of RTV boosting or co-administration of ATZ on the associations of TDF with biomarker changes. For RTV boosting, we observed significant interactions with larger elevations in IL-18 (+62.3% vs. −20.3%; p for interaction <0.0001) and OPN (+31.5% vs. −8.0%; p=0.017) among RTV users (N=50), compared with non-users. In contrast, ATZ users (N=27) had significant relative decreases of TFF3 (−45.3% vs. +93.5%; p=0.037) and UMOD (−32.0% vs. +18.3%; p=0.025), compared with non-users. When stratifying the first-year changes by race, African Americans (N=126) had significantly lower elevations or declines in several biomarkers, compared with non-African Americans: α1m (+17.8% vs. +72.1%; p=0.026), β2M (−20.4% vs. +120.5%; p<0.0001), and ACR (−12.2% vs. +27.8%; p=0.0015).
Finally, we evaluated the inter-relationships among the biomarker changes after TDF initiation. Overall, the first-year biomarker changes were only modestly inter-correlated (r<0.5), with the exception of TFF3 and β2M (r=0.6), and the vast majority of biomarker change pairs were weakly inter-correlated (r ≤0.3) (Supplementary Figure 1).
Discussion
In this observational study of longitudinally well-characterized HIV-infected men and women, we evaluated the evolution of kidney injury biomarkers among HIV-infected persons who initiated TDF. Overall, we found that TDF initiation was associated with substantial and distinct changes in biomarker concentrations within the first year that on average coincided with rising serum creatinine levels. To our knowledge, this is the first study to use a comprehensive panel of urinary biomarkers to characterize TDF-associated nephrotoxicity longitudinally. Our findings demonstrate the diverse impact of TDF on the kidney and suggest that TDF toxicity may not be adequately captured by our current clinical measures, nor by any single biomarker. Rather, a biomarker signature may be able to identify subclinical injury before the onset of overt, irreversible disease and to distinguish kidney injury attributable to TDF from the many other potential causes of rising creatinine levels in HIV-infected persons.[34]
Consistent with the literature and our hypothesis, we found that TDF initiation was predominantly associated with changes in biomarker concentrations that represent proximal tubular pathology (i.e., TFF3,[24, 35] clusterin,[24, 36] KIM-1,[37, 38] IL-18,[39] α1m,[18, 40] and β2M[24, 41]). In prior cross-sectional analyses of HIV-infected men in MACS, we found that each year of TDF exposure was independently associated with higher concentrations of α1m (7.6%), IL-18 (2.7%), KIM-1 (2.5%), and procollagen type III N-terminal propeptide (PIIINP) (2.0%),.[17, 18] Compared to these prior cross-sectional findings, effect sizes were as much as 10-fold larger for several biomarkers in this current longitudinal study. For example, participants on average had 78% increases in TFF3, 32% increases in α1m, and 13% increases in KIM-1 concentrations 1 year after TDF initiation.
Interestingly, we found a distinct decrease in IL-18 concentrations in the first year following TDF initiation, in contrast to the annual increase of IL-18 concentrations associated with TDF exposure in prior cross-sectional analyses. This discrepancy may be explained by the effect modification of biomarker changes by baseline viral suppression status, which was most striking in IL-18: initially viremic participants, who were the majority (71%) of the cohort, had significantly decreased IL-18 concentrations, while aviremic participants had significantly increased IL-18 concentrations. This divergence when stratifying by baseline viral suppression status is consistent with the strong association of HIV viral load with urinary IL-18 concentrations.[34] In the prior cross-sectional study, a large portion of HIV-infected participants were on ARVs for several years and were likely predominantly aviremic, which may explain the increases in IL-18 concentrations associated with TDF exposure.
In addition, stratification by viral load revealed associations of TDF initiation with changes of varying magnitudes in biomarkers of various nephron sites and functions. For example, in aviremic participants, TDF initiation was associated with significant increases in UMOD, a marker of Loop of Henle function,[31, 32] and YKL-40, a marker of renal fibrosis and repair.[42] Overall, aviremic participants had the largest biomarker changes, including over 80% increases in TFF3, α1m, and β2M after 1 year of TDF use. In contrast, baseline viremic participants consistently had attenuated biomarker increases or even declines, compared with aviremic participants. While the observed effect modification by viral load distinguished the relative differences in TDF toxicity, absolute biomarker concentrations in these two groups converged after 1 year of TDF use. One possible explanation for these findings is that the benefits of viral suppression induced by TDF-containing ART regimens offset or even outweighed the harms of TDF toxicity on the kidneys of viremic individuals. Notably, eGFR did not differ between the baseline viremia strata, demonstrating the potential of these biomarkers to distinguish etiologies of injury beyond that of current clinical paradigms.
We also observed that TDF initiation was associated with biomarker changes within the first year of initiation that neither significantly progressed nor recovered over a median of 3 years of TDF use. These findings are consistent with the ASSERT trial, which examined longitudinal urinary biomarker changes among 385 HIV-infected participants randomized to a TDF-containing ART regimen or an abacavir-containing regimen.[43, 44] Participants who received TDF-containing regimens had substantially higher increases of retinol binding protein (RBP) and β2M, markers of proximal tubular dysfunction, compared to users of abacavir-containing regimens. These relative biomarker concentration increases were noted at 6 months and remained stable through 2 years of follow-up. The observed kinetics of biomarker changes and kidney function decline within the first year following TDF initiation are also concordant with reported TDF-associated eGFRSCr declines that subsequently plateau at 6 months to 1 year.[45, 46] In another study using this cohort of new TDF users, we have demonstrated that 6 of these 14 biomarkers, including β2M, KIM-1, clusterin, UMOD, cystatin C, and IL-18, were independently associated with eGFR decline during follow-up.[47]
The recent introduction of tenofovir alafenamide fumarate (TAF), a less nephrotoxic alternative to TDF, has eased some concerns regarding tenofovir-associated nephrotoxicity. However, TAF has not completely replaced TDF nor the need for novel kidney function diagnostic methods. Only TDF-containing regimens are currently approved for pre-exposure prophylaxis, and use of TDF remains widespread for the treatment of HIV infection in resource-challenged areas.[1] It is projected that in 2020, TDF will hold 80% of the market share of first-line treatment regimens in low-to-middle income countries that disproportionately bear the burden of HIV infection.[48] Furthermore, the long-term kidney effects of TAF remain unknown and are critical to understand, as antiretroviral regimens are often life-long medications. While a recent meta-analysis of six trials reported that TAF use was associated with significantly lower declines in eGFRScr and lower β2M and RBP concentrations compared to TDF, TAF was still associated with modest kidney injury and change in eGFRScr.[49] In fact, a recent case report described the first documented instance of TAF-associated dysmorphic mitochondria on kidney biopsy, a histologic hallmark of tenofovir toxicity.[50] The use of novel diagnostics could potentially be useful and applicable to both TDF- and TAF-users to identify incident ART-related toxicities with long-term use.
Strengths of this study include the longitudinal, new-user design that captured incident TDF use and simulated the design of a single-arm, clinical trial.[51] This also allowed for a pre- vs. post-TDF initiation comparison in which individuals served as their own controls, reducing the bias of inter-individual variability in biomarker concentrations and time-invariant characteristics. In addition, we used a large, curated panel of urinary biomarkers supported by the literature[24, 52-55] and data from well-characterized cohorts of HIV-infected participants.
We also acknowledge several limitations. Although MACS and WIHS are national, multi-center cohorts, there may be important differences between study participants and the general HIV-infected population, including overall health and medical compliance. While we were unable to directly measure TDF adherence, viral load status served as a proxy and self-reported adherence was generally high for this once daily medication. Phosphaturia and hypophosphatemia have been traditionally considered the earliest clinical markers of renal tubular dysfunction but were not measured in our participants; however, prior studies have demonstrated that biomarkers representing tubular pathology may precede alterations in phosphate excretion in the setting of TDF use.[56] In addition, individuals who started TDF during the time period of our study but did not have urine samples were excluded, which may limit the generalizability of our findings. Our results may also have been attenuated by biomarker degradation in stored, frozen urine samples, particularly β2M, which has poor stability in acidic solutions.[57, 58] Because our study design only allowed for biomarker concentration measurements approximately annually, we may have also missed important changes in biomarker concentrations between these measurements, particularly those within a few months after initiation. Given the decrease in sample size in urine measurements following the first post-TDF initiation measurement, there may have been reduced power to detect subsequent changes. Finally, in our interpretation of the results, we considered both directions and magnitudes of estimates, instead of relying on p-values alone. However, we found that biomarker changes were only moderately inter-correlated, and several had highly significant p-values that would have remained significant even if adjustments for multiple comparisons were appropriate and performed.
Our study has several implications for clinical care and future studies. With validation in other diverse cohorts, uniform use of kidney injury biomarkers could be integrated into clinical decision-making as a novel diagnostic and surveillance strategy. For example, detection of a distinct signature of biomarker changes may help distinguish TDF-associated nephrotoxicity from the other causes of kidney disease among HIV-infected individuals that are currently indistinguishable based upon increases in serum creatinine. Recent studies in animal models have demonstrated that several of these biomarkers outperformed traditional measures of kidney function in detecting the onset and progression of histologically-confirmed TDF-associated tubular injury.[59] Future studies exploring this methodology should include comparisons of the long-term renal impact of TDF and TAF to inform risk stratification and judicious allocation of the newer formulation of tenofovir. To distinguish tenofovir toxicity from other etiologies of kidney injury, future studies should also characterize longitudinal biomarker profiles associated with other kidney disease risk factors among HIV-infected persons. By extension, urinary biomarker panels may better detect and characterize drug-induced nephrotoxicity and kidney disease in general.
In conclusion, we have demonstrated that TDF initiation is associated with a distinct profile of urinary biomarker concentration changes among HIV-infected men and women. If these findings are validated in future studies, a multi-biomarker panel may be an effective tool to detect and monitor TDF toxicity.
Supplementary Material
Acknowledgements:
William Zhang, Simon Ascher, Rebecca Scherzer, Michelle Estrella, and Michael Shlipak are responsible for the research idea and study design. Deborah Gustafson, Mary Young, Anjali Sharma, Mardge Cohen, Derek Ng, Frank Palella, Mallory Witt, Phyllis Tien, and Ken Ho are responsible for data acquisition. William Zhang, Simon Ascher, Rebecca Scherzer, Michelle Estrella, Michael Shlipak, Anthony Muiru, Vasantha Jotwani, Carl Grunfeld, Chirag Parikh, Deborah Gustafson, Mary Young, Anjali Sharma, Mardge Cohen, Derek Ng, Frank Palella, Mallory Witt, Phyllis Tien, and Ken Ho are responsible for data analysis and interpretation. Rebecca Scherzer is responsible for statistical analysis. Michael Shlipak is responsible for supervision and mentorship.
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). MACS (Principal Investigators): Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Oto Martinez-Maza), U01-AI35040; Harbor-University of California, Los Angeles (Mallory Witt), UL1-TR001881; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Gypsyamber D’Souza), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The MACS website is located at http://aidscohortstudy.org/.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR).
Conflicts of Interest and Source of Funding: Michael G. Shlipak is supported by the NIH/NIA (5R01AG034853). William R. Zhang is supported by the NIH/NCATS (TL1TR001871). Michael Shlipak is a Scientific Advisor and holds stock options in the following companies: TAI Diagnostics and Cricket Health, Inc. For the remaining authors, no disclosures were declared.
References
- 1.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach - Second edition. In; 2016. [PubMed] [Google Scholar]
- 2.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Niño MD, Izquierdo MC, Poveda J, et al. Tenofovir Nephrotoxicity: 2011 Update. AIDS Research and Treatment 2011; 2011:354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, et al. Clinical Practice Guideline for the Management of Chronic Kidney Disease in Patients Infected With HIV: 2014 Update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2014; 59(9):e96–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis 2010; 56(5):872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jotwani V, Li Y, Grunfeld C, Choi AI, Shlipak MG. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. American journal of kidney diseases : the official journal of the National Kidney Foundation 2012; 59(5):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest 2009; 89(5):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 2010; 78(11):1171–1177. [DOI] [PubMed] [Google Scholar]
- 8.Fiseha T, Gebreweld A. Urinary Markers of Tubular Injury in HIV-Infected Patients. Biochemistry Research International 2016; 2016:1501785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-Associated Kidney Toxicity in HIV-Infected Patients: A Review of the Evidence. American Journal of Kidney Diseases; 57(5):773–780. [DOI] [PubMed] [Google Scholar]
- 10.Oboho I, Abraham AG, Benning L, Anastos K, Sharma A, Young M, et al. Tenofovir use and urinary biomarkers among HIV-infected women in the Women's Interagency HIV Study (WIHS). Journal of acquired immune deficiency syndromes 2013; 62(4):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labarga P, Barreiro P, Martin-Carbonero L, Rodriguez-Novoa S, Solera C, Medrano J, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. Aids 2009; 23(6):689–696. [DOI] [PubMed] [Google Scholar]
- 12.Dauchy FA, Lawson-Ayayi S, de La Faille R, Bonnet F, Rigothier C, Mehsen N, et al. Increased risk of abnormal proximal renal tubular function with HIV infection and antiretroviral therapy. Kidney international 2011; 80(3):302–309. [DOI] [PubMed] [Google Scholar]
- 13.Maggi P, Montinaro V, Bellacosa C, Pietanza S, Volpe A, Graziano G, et al. Early markers of tubular dysfunction in antiretroviral-experienced HIV-infected patients treated with tenofovir versus abacavir. AIDS patient care and STDs 2012; 26(1):5–11. [DOI] [PubMed] [Google Scholar]
- 14.Post FA, Moyle GJ, Stellbrink HJ, Domingo P, Podzamczer D, Fisher M, et al. Randomized Comparison of Renal Effects, Efficacy, and Safety With Once-Daily Abacavir/Lamivudine Versus Tenofovir/Emtricitabine, Administered With Efavirenz, in Antiretroviral-Naive, HIV-1–Infected Adults: 48-Week Results From the ASSERT Study. JAIDS Journal of Acquired Immune Deficiency Syndromes 2010; 55(1):49–57. [DOI] [PubMed] [Google Scholar]
- 15.Takano M, Tanuma J, Tsukada K, Teruya K, Kikuchi Y, Nishijima T, et al. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: a diagnostic accuracy study. Journal of Infection and Chemotherapy; 19(5):850–857. [DOI] [PubMed] [Google Scholar]
- 16.Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-Associated Kidney Toxicity in HIV-Infected Patients: A Review of the Evidence. American Journal of Kidney Diseases 2011; 57(5):773–780. [DOI] [PubMed] [Google Scholar]
- 17.Jotwani V, Scherzer R, Estrella MM, Jacobson LP, Witt MD, Palella F, et al. Brief Report: Cumulative Tenofovir Disoproxil Fumarate Exposure is Associated With Biomarkers of Tubular Injury and Fibrosis in HIV-Infected Men. JAIDS Journal of Acquired Immune Deficiency Syndromes 2016; 73(2):177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jotwani V, Scherzer R, Estrella MM, Jacobson LP, Witt MD, Palella FJ, et al. HIV Infection, Tenofovir, and Urine α1-Microglobulin: A Cross-sectional Analysis in the Multicenter AIDS Cohort Study. American Journal of Kidney Diseases 2016; 68(4):571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo JCR. THE MULTICENTER AIDS COHORT STUDY: RATIONALE, ORGANIZATION, AND SELECTED CHARACTERISTICS OF THE PARTICIPANTS. American Journal of Epidemiology 1987; 126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women's Interagency HIV Study: an Observational Cohort Brings Clinical Sciences to the Bench. Clinical and Diagnostic Laboratory Immunology 2005; 12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women's Interagency HIV Study. Epidemiology 1998; 9(2):117–125. [PubMed] [Google Scholar]
- 22.Coons SJ. The FDA's critical path initiative: a brief introduction. Clin Ther 2009; 31(11):2572–2573. [DOI] [PubMed] [Google Scholar]
- 23.Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotech 2010; 28(5):486–494. [DOI] [PubMed] [Google Scholar]
- 24.Blank M, Felice AD, Goodsaid F, Harlow P, Hausner E, Jacobson-Kram D, et al. Review of Qualification Data for Biomarkers of Nephrotoxicity Submitted by the Predictive Safety Testing Consortium. In: Center for Drug Evaluation and Research US Food and Drug Administration 2009. [Google Scholar]
- 25.Longenecker CT, Scherzer R, Bacchetti P, Lewis CE, Grunfeld C, Shlipak MG. HIV viremia and changes in kidney function. AIDS (London, England) 2009; 23(9):1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, et al. Greater Tenofovir-Associated Renal Function Decline with Protease Inhibitor-Based versus Nonnucleoside Reverse-Transcriptase Inhibitor-Based Therapy. The Journal of Infectious Diseases 2008; 197(1):102–108. [DOI] [PubMed] [Google Scholar]
- 27.Cuzin L, Pugliese P, Allavena C, Rey D, Chirouze C, Bani-Sadr F, et al. Antiretroviral therapy as a risk factor for chronic kidney disease: Results from traditional regression modeling and causal approach in a large observational study. PLOS ONE 2017; 12(12):e0187517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose S, Nelson M, Phillips A, Chadwick D, Trevelion R, Jones R, et al. Improved kidney function in patients who switch their protease inhibitor from atazanavir or lopinavir to darunavir. AIDS 2017; 31(4):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, et al. Excess Risk of Chronic Kidney Disease among African-American versus White Subjects in the United States: A Population-Based Study of Potential Explanatory Factors. Journal of the American Society of Nephrology 2002; 13(9):2363–2370. [DOI] [PubMed] [Google Scholar]
- 30.Isaka Y Epidermal growth factor as a prognostic biomarker in chronic kidney diseases. Annals of Translational Medicine 2016; 4(Suppl 1):S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Achkar TM, Wu X-R. Uromodulin in Kidney Injury: An Instigator, Bystander, or Protector? American Journal of Kidney Diseases 2012; 59(3):452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 2017; 13(9):525–544. [DOI] [PubMed] [Google Scholar]
- 33.Bacchetti P Peer review of statistics in medical research: the other problem. BMJ 2002; 324(7348):1271–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxi SM, Scherzer R, Jotwani V, Estrella MM, Abraham AG, Parikh CR, et al. Changes in Urinary Biomarkers Over 10 Years Is Associated With Viral Suppression in a Prospective Cohort of Women Living With HIV. JAIDS Journal of Acquired Immune Deficiency Syndromes 2017; 74(5):e138–e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotech 2010; 28(5):470–477. [DOI] [PubMed] [Google Scholar]
- 36.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, et al. Urinary clusterin, cystatin C, [beta]2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotech 2010; 28(5):463–469. [DOI] [PubMed] [Google Scholar]
- 37.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62(1):237–244. [DOI] [PubMed] [Google Scholar]
- 38.Vaidya VS, Ozer JS, Frank D, Collings FB, Ramirez V, Troth S, et al. Kidney Injury Molecule-1 Outperforms Traditional Biomarkers of Kidney Injury in Multi-site Preclinical Biomarker Qualification Studies. Nature biotechnology 2010; 28(5):478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis1. American Journal of Kidney Diseases; 43(3):405–414. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Yanagisawa Y, Forbes MA, Cooper EH, Crockson RA, MacLennan IC. Alpha-1-microglobulin: an indicator protein for renal tubular function. Journal of Clinical Pathology 1983; 36(3):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaub S, Wilkins JA, Antonovici M, Krokhin O, Weiler T, Rush D, et al. Proteomic-Based Identification of Cleaved Urinary β2-microglobulin as a Potential Marker for Acute Tubular Injury in Renal Allografts. American Journal of Transplantation 2005; 5(4):729–738. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt IM, Hall IE, Kale S, Lee S, He C-H, Lee Y, et al. Chitinase-Like Protein Brp-39/YKL-40 Modulates the Renal Response to Ischemic Injury and Predicts Delayed Allograft Function. Journal of the American Society of Nephrology 2013; 24(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Post FA, Moyle GJ, Stellbrink HJ, Domingo P, Podzamczer D, Fisher M, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55(1):49–57. [DOI] [PubMed] [Google Scholar]
- 44.Moyle GJ, Stellbrink H-J, Compston J, Orkin C, Arribas J, Domingo P, et al. 96-Week results of abacavir/lamivudine versus tenofovir/emtricitabine, plus efavirenz, in antiretroviral-naive, HIV-1-infected adults: ASSERT study. 2013. [DOI] [PubMed] [Google Scholar]
- 45.Squillace N, Ricci E, Quirino T, Gori A, Bandera A, Carenzi L, et al. Safety and tolerability of Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil fumarate in a real life setting: Data from surveillance cohort long-term toxicity antiretrovirals/antivirals (SCOLTA) project. PLoS ONE 2017; 12(6):e0179254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laprise C, Baril J-G, Dufresne S, Trottier H. Association Between Tenofovir Exposure and Reduced Kidney Function in a Cohort of HIV-Positive Patients: Results From 10 Years of Follow-up. Clinical Infectious Diseases 2013; 56(4):567–575. [DOI] [PubMed] [Google Scholar]
- 47.Ascher SB, Scherzer R, Estrella MM, Zhang WR, Muiru AN, Jotwani V, et al. Association of Urinary Biomarkers of Kidney Injury with Estimated GFR Decline in HIV-Infected Individuals following Tenofovir Disoproxil Fumarate Initiation. Clinical Journal of the American Society of Nephrology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ARV Market Report: The State of the Antiretroviral Drug Market in Low- and Middle-Income Countries, 2015-2020. In: Clinton Health Access Initiative. Issue 7, October 2016 ed; 2016. [Google Scholar]
- 49.Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine 2016; 95(41):e5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Novick TK, Choi MJ, Rosenberg AZ, McMahon BA, Fine D, Atta MG. Tenofovir alafenamide nephrotoxicity in an HIV-positive patient: A case report. Medicine 2017; 96(36):e8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American Journal of Epidemiology 2016; 183(8):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotech 2010; 28(5):436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Endre ZH, Westhuyzen J. Early detection of acute kidney injury: Emerging new biomarkers (Review Article). Nephrology 2008; 13(2):91–98. [DOI] [PubMed] [Google Scholar]
- 54.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Critical Care Medicine 2008; 36(4):S159–S165. [DOI] [PubMed] [Google Scholar]
- 55.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of Acute Kidney Injury. Annual review of pharmacology and toxicology 2008; 48:463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maggi P, Montinaro V, Bellacosa C, Pietanza S, Volpe A, Graziano G, et al. Early Markers of Tubular Dysfunction in Antiretroviral-Experienced HIV-Infected Patients Treated with Tenofovir Versus Abacavir. AIDS Patient Care and STDs 2011; 26(1):5–11. [DOI] [PubMed] [Google Scholar]
- 57.Blumsohn A, Morris BW, Griffiths H, Ramsey CF. Stability of beta 2-microglobulin and retinol binding protein at different values of pH and temperature in normal and pathological urine. Clin Chim Acta 1991; 195(3):133–137. [DOI] [PubMed] [Google Scholar]
- 58.Donaldson MD, Chambers RE, Woolridge MW, Whicher JT. Stability of alpha 1-microglobulin, beta 2-microglobulin and retinol binding protein in urine. Clin Chim Acta 1989; 179(1):73–77. [DOI] [PubMed] [Google Scholar]
- 59.Gu Y-Z, Vlasakova K, Troth SP, Peiffer RL, Tournade H, Pasello dos Santos FR, et al. Performance Assessment of New Urinary Translational Safety Biomarkers of Drug-induced Renal Tubular Injury in Tenofovir-treated Cynomolgus Monkeys and Beagle Dogs. Toxicologic Pathology; 0(0):0192623318775023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.